LINAC-based Fractionated Stereotactic Radiotherapy for Residual and Recurrent Nasopharyngeal Carcinoma in the Era of Intensity-modulated Radiotherapy: A 10-year Experience
ORIGINAL ARTICLE
LINAC-based Fractionated Stereotactic Radiotherapy for Residual and Recurrent Nasopharyngeal Carcinoma in the Era of Intensity-modulated Radiotherapy: A 10-year Experience
TTS Lau1, LL Chan1, ELM Yu2, JWY Lai1, KT Yuen1, ACK Cheng1
1 Department of Oncology, Princess Margaret Hospital, Laichikok, Hong Kong
2 Clinical Research Centre, Princess Margaret Hospital, Laichikok, Hong Kong
Correspondence: Dr Lau, Department of Oncology, Princess Margaret Hospital, Laichikok, Hong Kong. Email: tracylts@ha.org.hk
Submitted: 14 Sep 2018; Accepted: 22 Oct 2018.
Contributors: TTSL designed the study; TTSL acquired the data; TTSL, LLC and ELMY analysed the data; TTSL drafted the manuscript.
All authors critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study,
approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: The authors have no conflict of interest to declare.
Funding/Support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics Approval: This retrospective study was approved by the Kowloon West Cluster Research Ethics Committee (Ref KW/EX-18-069(123-
08)).
Abstract
Introduction
We reviewed the use of frameless linear accelerator–based fractionated stereotactic radiotherapy
(FSRT) in a single centre as salvage treatment for patients with nasopharyngeal carcinoma with local failure.
Methods
We retrospectively reviewed the data of all patients with residual or recurrent nasopharyngeal carcinoma
who had undergone salvage therapy with FSRT at our institution between 2008 and 2017. Survival data were analysed
by the Kaplan-Meier method. Univariate analyses for survival outcomes were performed using the Cox proportional
hazards model. Severe late radiation toxicities were assessed.
Results
Of the 49 patients included, 44 (90%) had previously received intensity-modulated radiotherapy as primary
treatment. The median FSRT dose was 18 Gy in three fractions for residual disease, and 48 Gy in six fractions for
recurrent disease. Median follow-up was 41.1 months. The 3-year local control rate, progression-free survival (PFS),
disease-specific survival, and overall survival (OS) for patients with residual disease (n = 34) were 78.9%, 66.2%,
82.2%, and 74.0%, respectively. Those for patients with recurrent disease (n = 15) were 68.2%, 40.0%, 58.7%,
and 46.7%, respectively. Using FSRT, a gross tumour volume of ≤16 mL of residual disease was associated with
longer PFS and OS. N3 nodal staging status was associated with poorer PFS in the residual disease group. Severe
late complications occurred in 12 patients (24%), including one patient from the residual disease group and four
patients from the recurrent disease group with fatal haemorrhage (10%).
Conclusion
Using this less-invasive and resource-friendly technique, the clinical outcomes from our centre were
comparable to those in the literature.
Key Words: Dose fractionation, radiation; Nasopharyngeal carcinoma; Neoplasm, residual; Salvage treatment
中文摘要
調強放療年代的殘存及復發性鼻咽癌的直線加速器分段立體定向放射治療:10年經驗回顧
劉芷珊、陳瓏、余洛汶、黎詠宇、袁錦堂、鄭志堅
引言
回顧無框架式直線加速器分段立體定向放射治療(FSRT)在一所本地醫院作為鼻咽癌局部失敗挽救性治療的療效和安全性。
方法
回顧2008年至2017年在本院接受FSRT治療的殘存及復發性鼻咽癌患者,評估腫瘤反應和放療毒性。使用Kaplan-Meier法計算生存數據,以Cox比例風險回歸模型分析存活率風險因子。
結果
共納入49名患者,當中44名 (90%)接受調強放療作為首次治療手段。殘存性鼻咽癌(34名)FSRT中位劑量為18Gy/3次,復發性鼻咽癌中位劑量為48Gy/6次。中位隨訪期為41.1個月。殘存性鼻咽癌(15名)3年局部控制率為78.9%,無惡化存活率為66.2%,無瘤存活率為82.2%,總存活率為74%。復發性鼻咽癌3年局部控制率為68.2%,無惡化存活率為40%,無瘤存活率為58.7%,總存活率為46.7%。在殘存性鼻咽癌患者中,腫瘤體積少於16 mL與較長無惡化存活期和總存活率期有關聯,而N3則與較短無惡化存活期有關聯。12名病人(24%)出現嚴重後期併發症,當中包括一名殘存性鼻咽癌患者和四名復發性鼻咽癌患者出現致命性出血(10%)。
結論
作為相對低入侵性及節省資源的放射治療方法,本研究患者的治療效果和文獻記載相若。
INTRODUCTION
Nasopharyngeal cancer (NPC) is common in Southeast
Asia, especially Southern China. Despite a continued
decreasing trend, the latest reported incidence in Hong
Kong is 12 per 100 000, ranking tenth among the
commonest cancers in the region.[1]
Intensity-modulated radiotherapy (IMRT) allows
remarkable improvement in dose conformity compared
with two-dimensional or three-dimensional conformal
radiotherapy (RT), and IMRT use is associated with
better survival outcomes and less treatment toxicity in
NPC.[2] In modern series using IMRT, the reported local
failure rate is 5% to 15% for earlier stages, but is much
higher, 15% to 45%, for T4 disease.[3] [4] The Hong Kong
Nasopharyngeal Cancer Study Group (HKNPCSG) 1301
study evaluated more than 3000 patients treated with
primary IMRT. Despite a low overall local recurrence
rate of 3.9%, the 8-year actuarial local failure-free
survival was only 71.6% for T4 disease, in contrast to the
satisfactory outcomes of T1 to T3 disease (87%-92%).[5]
Management of local failure requires consideration of
multiple factors, including location and extent of disease,
availability of modality and expertise, as well as the
patients’ preferences and co-morbidities. An operative approach with nasopharyngectomy has been reported to
offer favourable local control, but is often challenging due
to the complex anatomy. A high level of surgical expertise
is required, and such surgery is only feasible for patients
with lower rT stages (rT1 to limited rT3).[6] [7] [8] It may also
be less favoured for patients with significant concerns to
operative risks and cosmetic result especially for open
surgery. Nonoperative approaches include reirradiation
with IMRT, stereotactic radiotherapy (single or multiple
fractions), brachytherapy (intracavitary or interstitial),
and photodynamic therapy, with the latter two only for
T1 to early T2 lesions. Chemotherapy may be added as
a component of salvage treatment but should not be used
alone if long-term control is pursued.[6] [7] [8]
Stereotactic single-fraction radiotherapy, also known
as stereotactic radiosurgery (SRS), was first used
as a treatment option for recurrent NPC in the late
1990s. However, the use of stereotactic frames with
neurosurgery expertise was almost always required,
which caused logistic challenges in practice, and the
frames themselves were uncomfortable. Fractionated
stereotactic radiotherapy (FSRT) adopts the concept
of precision in SRS but delivers the dose in multiple
fractions, resulting in a better therapeutic ratio based
on radiobiology principles. A matched cohort analysis showed better 3-year local failure-free survival rates
with FSRT than with single-fraction SRS, especially
for recurrent and non-T1 disease.[9] The concerns of
FSRT being more resource-intense and the issue
of interfractional reproducibility have been tackled
by advancements in treatment delivery speed and
in stereotactic systems. LINAC-based frameless
stereotactic systems are now commercially available,
allowing FSRT to be delivered in a less-invasive and
resource-friendly manner.
METHODS
Patients
The data of all patients with NPC local failure treated
with reirradiation by FSRT in our institution between
2008 and 2017 were retrospectively reviewed. Patients
with residual disease (residual tumour or relapse within
6 months of primary RT completion) and recurrent
disease (relapse beyond 6 months of primary RT
completion) were analysed.
Primary Treatment Methods
The primary RT treatment was a course of high-dose
IMRT, delivering 70 Gy to the gross tumour,
lymphadenopathy and nasopharynx, 60 Gy to the high-risk
subclinical sites and lymphatic regions, and 54 Gy
to the low-risk lymphatic regions using simultaneous
integrated boosts, one fraction per day on weekdays to
total 33 to 35 fractions. Concurrent chemotherapy was
administered to patients with stage III-IV and T2N1
disease (as an option for high-risk stage II).
Assessment of Residual or Recurrent Disease
For the residual disease group, all patients had
completed radical RT as the primary treatment of NPC.
Their tumour response was assessed by two fibreoptic
nasopharyngoscopy sessions performed 8 and 10 weeks
after completion of RT. A systematic six-site (bilateral
roofs, lateral walls, and posterior walls) mapping biopsy
of the nasopharynx was performed during each session,
and remission was defined as two consecutive negative
biopsies at each site. A positive biopsy at any one site in
any of these two sessions warranted an additional session
12 weeks after RT. If the repeated biopsy was positive,
the patient was considered to harbour persistent residual
disease and salvage treatment (FSRT) was initiated,
accounting for the residual disease group in this study.[10]
All patients considered free from persistent disease
were followed up routinely with clinic visits and
physical examinations. Investigations, such as fibreoptic nasopharyngoscopy and magnetic resonance imaging
(MRI) were arranged on symptom presentation or
abnormal physical findings. In the recurrent disease
group, systemic re-staging was mandatory, with most
patients undergoing positron-emission tomography and
computed tomography (CT) scan to allow planning of
FSRT.
Fractionated Stereotactic Radiotherapy
Technique, Planning and Treatment Delivery
FSRT was performed using an Eclipse IMRS Planning
System (Varian Medical Systems; Palo Alto [CA],
United States), capable of delivering both cone and
multileaf collimation–based IMRT by the Varian
Clinac® iX linear accelerators.
Gross tumour volume (GTV) was contoured on the CT
images using all available imaging data gathered by CT,
MRI and positron-emission tomography/CT, as well as
by endoscopic mapping. The planning target volume was
generated by adding a 2-to-3-mm margin to the GTV.
No elective reirradiation of regional lymph nodes was
performed. Planning organ-at-risk (OAR) volumes were
routinely contoured for critical neurological structures,
including the brainstem, optic chiasm, optic nerves, and
spinal cord by adding 3-mm margins to the optic chiasm,
optic nerves and brainstem, and 5-mm margins to the
spinal cord. In cases where the high-dose region was in
close proximity to these OARs, smaller planning OAR
volume margins (1-2 mm) would be used, where stringent
image-guided treatment verification would be practised.
The patients were informed of the relatively higher risk.
Two treatment fractions per week, with an interfractional
interval of at least 24 hours, were administered.
Different total doses and fractionations of FSRT were
chosen based on the type of relapse (with residual
disease considered more radiosensitive than recurrent
disease), tumour extent, cumulative radiation dose for
critical structures, and time interval from previous RT.
For patients whose time interval between two RT courses
was >1 year, a 33% to 50% dose tolerance recovery
of central nervous system structures from the initial
treatment course was assumed.[11] No patient received a
cumulative lifetime biologically equivalent dose in 2-Gy
fractions (EQD2) of >60 Gy to the brainstem[12] or optic
chiasm, or 50 Gy to the spinal cord.[13]
Follow-up
After FSRT, tumour response was assessed by
nasopharyngoscopy (with biopsy) and MRI. Regular follow-up included clinical examination and toxicity
assessment. Further imaging or endoscopy was arranged
if clinically indicated.
Data Collection and Statistical Analyses
The cut-off date for data collection was 15 August 2018.
The duration of follow-up was calculated from the date
of completion of reirradiation to either the day of death
or the day of the last follow-up.
The data were analysed to determine the pathological
complete response rate to FSRT, local control rate,
progression-free survival (PFS), disease-specific survival
(DSS), and overall survival (OS).
Actuarial rates were calculated using the Kaplan-
Meier method, and differences were compared using
the log-rank tests. Univariate analyses using the Cox
proportional hazards regression model were utilised
to test the significance of different prognostic factors.
Patient factors (age, sex, histological type, relapse-free
interval for recurrent disease, time from pathological
diagnosis of local failure to completion of treatment),
tumour factors (initial T and N stage, recurrent T and
N stage, FSRT GTV), and treatment factors (cumulative
concurrent cisplatin dose, dose of FSRT [biologically
effective dose with α/β = 10 Gy; BED10]) were included
in univariate analyses. Time-dependent receiver
operating characteristic analysis was used to determine
the cut-off values of FSRT GTV in predicting survival
outcome.
All analyses were performed using SPSS (Windows
version 16.0; SPSS Inc, Chicago [IL], United States) and
R (Version 3.5.1; www.r-project.org). The criterion for
statistical significance was set at p < 0.05.
RESULTS
Patient Characteristics
All 49 patients with NPC local failure treated
with reirradiation by FSRT in our institution were
retrospectively reviewed. The median follow-up for
the entire cohort was 41.1 (range, 3.7-118.1) months.
Among them, 34 had residual disease and 15 had
recurrent disease.
Overall, most tumours (>90%) were of the
undifferentiated subtype (World Health Organization
type III). For the residual tumour group, more than half
of the patients had had locally advanced (T3/4) tumours
at presentation. All patients had salvage FSRT completed within 6 months after residual disease was confirmed.
In the recurrent disease group, most tumours were
rT3 (53.3%). Five patients with recurrent disease had
concomitant nodal recurrences (all N1-2); two were in
the retropharyngeal region, which were treated with the
same course of FSRT; three were in the cervical region
and had undergone radical neck dissection. The median
time from completion of primary treatment to recurrence
was 21.5 months (range, 7.2-182.3 months). Before
FSRT, 14 of 15 patients had had histological proof of
recurrent carcinoma, except for one with an inaccessible
site (retropharyngeal region), and the diagnosis was
made by imaging. Detailed patient characteristics are
shown in Table 1.
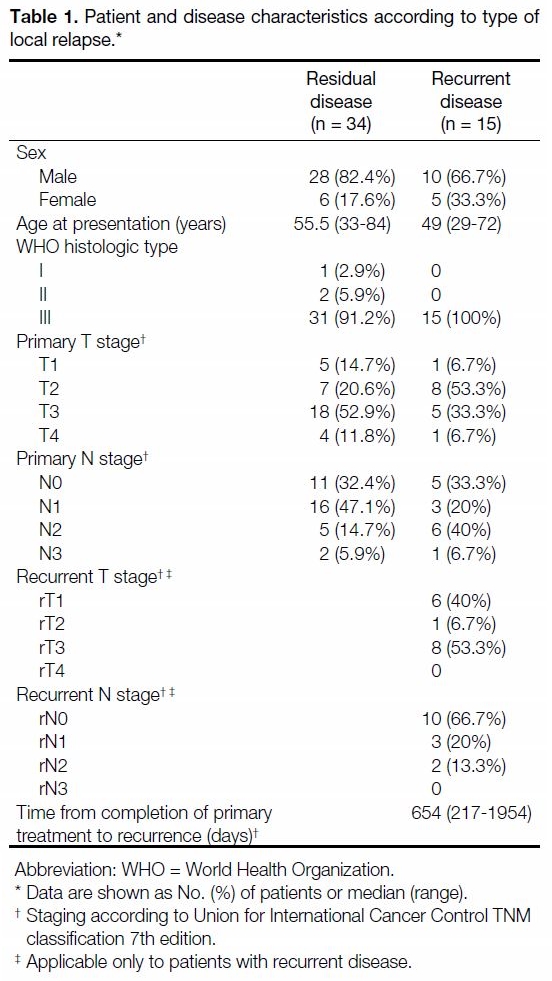
Table 1. Patient and disease characteristics according to type of local relapse.
During the primary treatment course, 53% of patients
received concurrent chemotherapy (mostly cisplatin-based), with a median cumulative cisplatin dose of
160 mg/m2 (range, 100-300 mg/m2); 12% of patients
received adjuvant chemotherapy with cisplatin and
5-fluorouracil (PF), while 10% of patients received
induction chemotherapy (PF). Table 2 summarises the
details of the primary treatment courses.
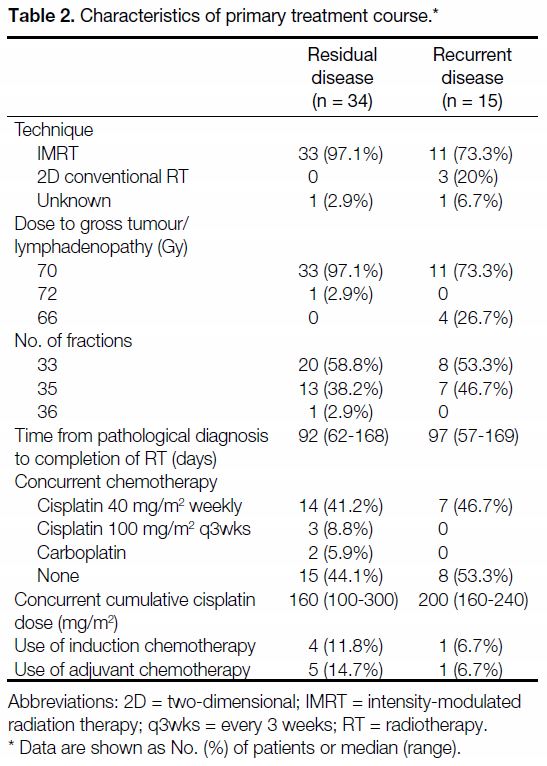
Table 2. Characteristics of primary treatment course.
For residual disease, the median prescribed FSRT dose
was 18 Gy (range, 12-18 Gy), delivered in a median
of three fractions (range, 2-3 fractions), with a median
fractional dose of 6 Gy (range, 5-6 Gy). The median
BED10 was 28.8 Gy10 (range, 19.2-28.8 Gy10) and that
of EQD2 was 24 Gy (range, 16-24 Gy). For recurrent
disease, when FSRT was used alone, the median
prescribed dose was 48 Gy (range, 14-48 Gy), delivered
in a median of 6 fractions (range, 2-6 fractions), with a
median fractional dose of 8 Gy (range, 6-8 Gy). In two
patients, FSRT was used as a tumour boost on top of
a long conventional fractionated reirradiation IMRT of
50 to 60 Gy. The median BED10 was 86.4 Gy10 (range,
48-120 Gy10) and that of EQD2 was 72 Gy (range,
40-100 Gy). Figure 1 illustrates details of the setup
and planning of FSRT. Table 3 summarises the FSRT treatment parameters.
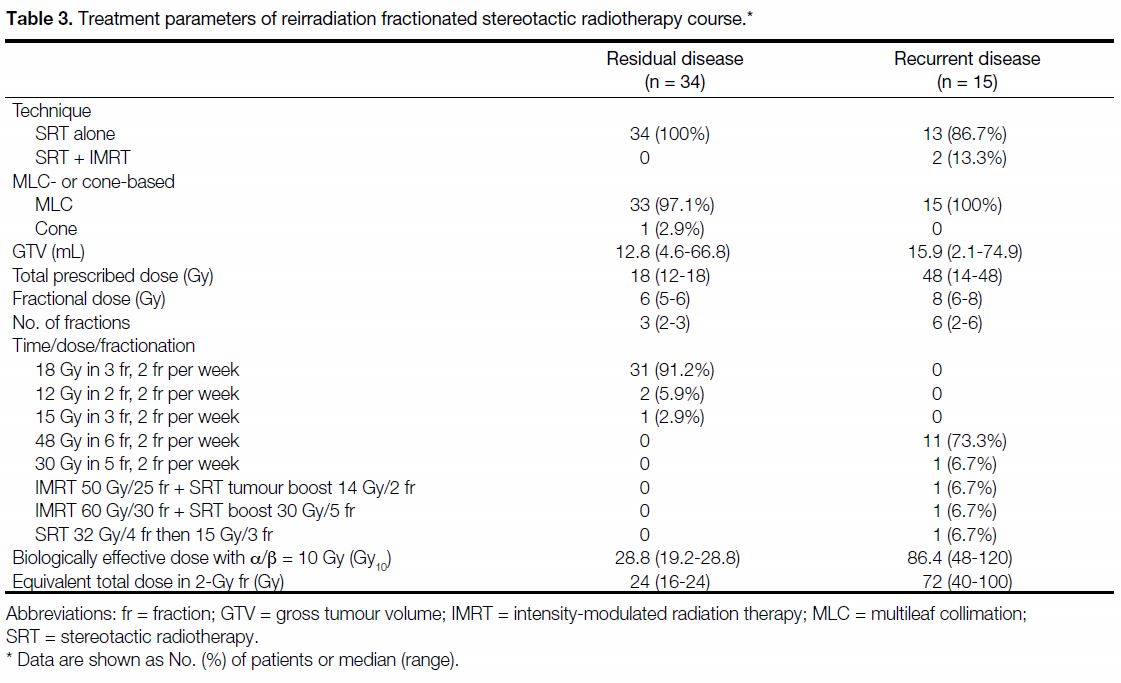
Table 3. Treatment parameters of reirradiation fractionated stereotactic radiotherapy course.
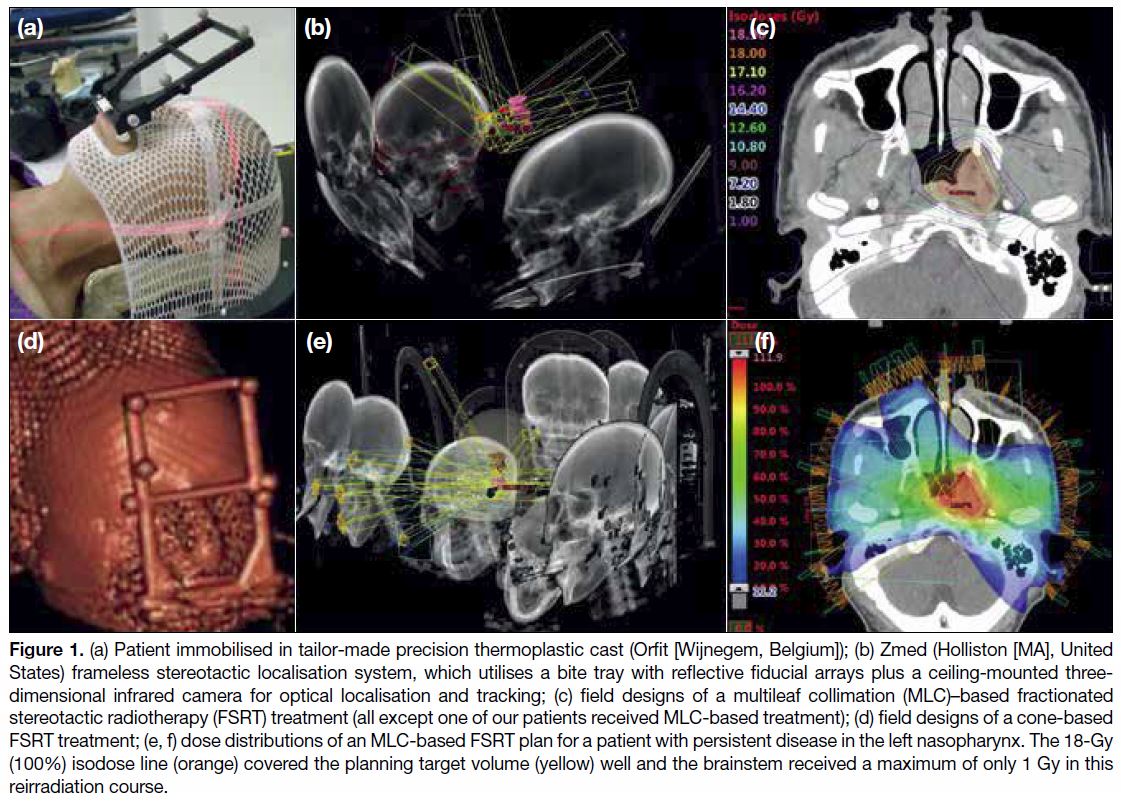
Figure 1. (a) Patient immobilised in tailor-made precision thermoplastic cast (Orfit [Wijnegem, Belgium]); (b) Zmed (Holliston [MA], United
States) frameless stereotactic localisation system, which utilises a bite tray with reflective fiducial arrays plus a ceiling-mounted three-dimensional
infrared camera for optical localisation and tracking; (c) field designs of a multileaf collimation (MLC)–based fractionated
stereotactic radiotherapy (FSRT) treatment (all except one of our patients received MLC-based treatment); (d) field designs of a cone-based
FSRT treatment; (e, f) dose distributions of an MLC-based FSRT plan for a patient with persistent disease in the left nasopharynx. The 18-Gy
(100%) isodose line (orange) covered the planning target volume (yellow) well and the brainstem received a maximum of only 1 Gy in this
reirradiation course.
Response and Local Control Rates
The pathological complete response rate was 82.3%
for the residual disease group. For the recurrent disease
group, 93.3% achieved radiological shrinkage, with
pathological complete response achieved in 60%. The
actuarial 3-year local control rates were 78.9% and
68.2% for the residual and recurrent disease groups,
respectively. Overall, for the eight patients who failed
to achieve local control after FSRT, five had successful
salvage surgery, one developed bone metastases, one
had extensive inoperable neck failure, and one relapsed
locally again 1 year after reirradiation with disease too
advanced for further salvage.
Survival Outcomes
For the residual disease group, the median PFS was
66.1 months, median OS was 102.0 months, and median
DSS was not reached with our length of follow-up.
Similar to local control rates, the recurrent disease group
had inferior outcome, with median PFS of 29.3 months,
median OS of 33.2 months, and median DSS of
40.9 months.
The actuarial 3-year PFS, DSS, and OS for the
residual disease group were 66.2%, 82.2%, and 74.0%,
respectively. For the recurrent disease group, the
PFS, DSS, and OS were 40.0%, 58.7%, and 46.7%,
respectively. Upon log-rank testing, the residual disease
group had superior survival outcome after FSRT
compared with the recurrent disease group, and the
difference was statistically significant across all three
endpoints (Figure 2).
Figure 2. Kaplan-Meier curves and 3-year actuarial rates by type of local failure. Log-rank tests were performed to test for statistical
differences: (a) local control rate (LCR); (b) progression-free survival (PFS); (c) disease-specific survival (DSS); and (d) overall survival (OS).
Prognostic Factors
The cut-off values of FSRT GTV as a predictor for
survival outcomes of the residual and recurrent disease
groups were found to be 16 mL and 10 mL, respectively.
FSRT GTVs of ≤16 mL for the residual disease group,
and that of ≤10 mL for the recurrent disease group
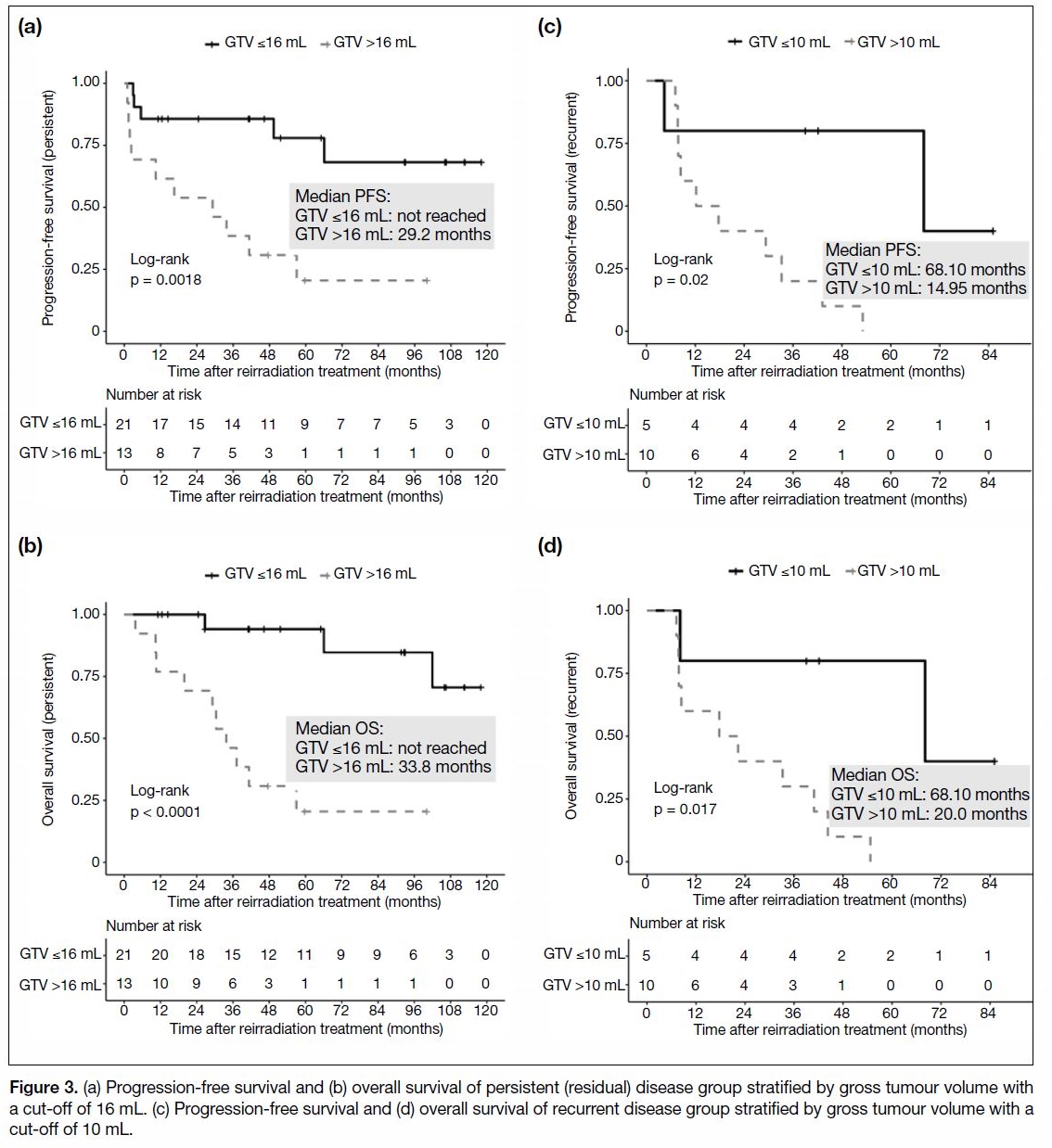
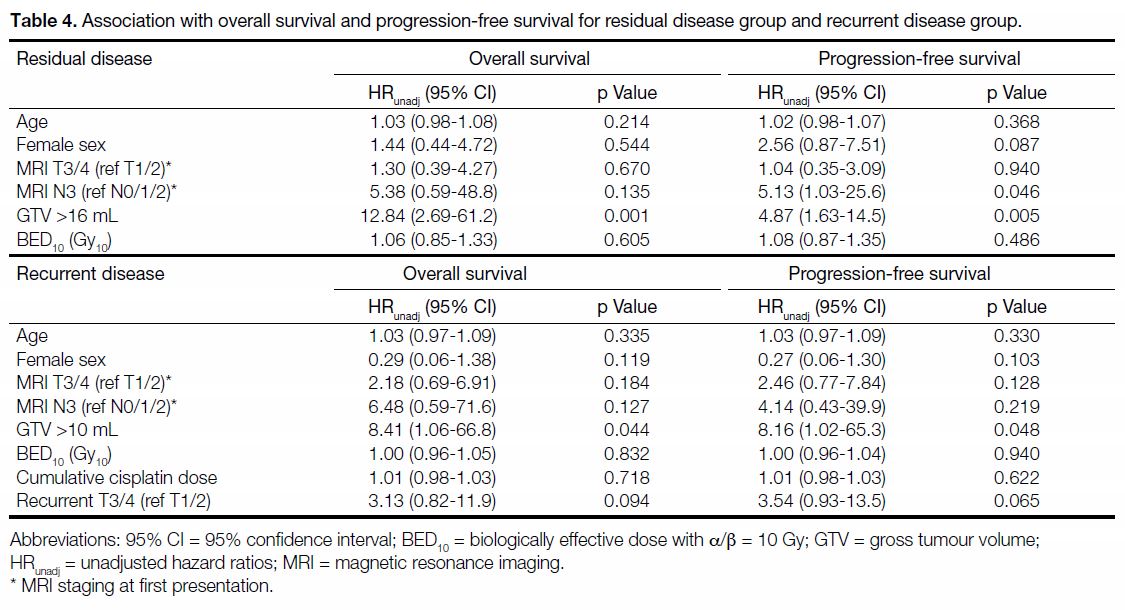
were associated with longer PFS and OS (Figure 3).
N3 nodal staging status was associated with poorer PFS
in the residual disease group. Other factors, including
advanced initial T stage (T3-4 vs. T1-2) in patients in the
residual disease group, and recurrent T stage (rT3-4 vs.
rT1-2) in patients in the recurrent disease group showed
only trends to inferior outcome in PFS or OS but were
not statistically significant prognostic factors. Table 4
summarises the results.
Figure 3. (a) Progression-free survival and (b) overall survival of persistent (residual) disease group stratified by gross tumour volume with
a cut-off of 16 mL. (c) Progression-free survival and (d) overall survival of recurrent disease group stratified by gross tumour volume with a
cut-off of 10 mL.
Table 4. Association with overall survival and progression-free survival for residual disease group and recurrent disease group.
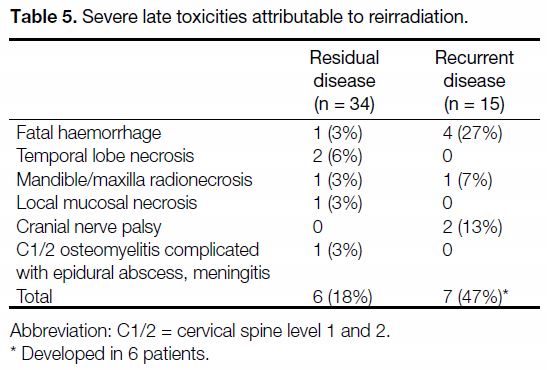
Complications
All patients were able to complete the scheduled FSRT.
No significant acute complications occurred. A total of 13
severe late complications occurred in 12 patients (24%)
after FSRT. The incidence of severe late complications
was higher in the recurrent disease group (in 6 patients,
40%) than in the residual disease group (18%).
Overall, five patients (10%) developed massive haemorrhage and all died of this event. Among them,
two had unsalvageable locoregional disease and the
haemorrhage could have been due to disease progression.
All of these haemorrhagic events happened within 5 years
of primary RT, and within 3 years of reirradiation FSRT.
Other known severe late complications associated with
reirradiation, including temporal lobe necrosis, cranial
nerve palsy, mandible/maxilla radionecrosis, and local
mucosal necrosis all occurred at a rate of <5%. One patient who had undergone a salvage nasopharyngectomy with
the maxillary swing approach after failing local control
with FSRT developed C1/2 osteomyelitis complicated
by an epidural abscess and meningitis 2 years later but
survived the event. Details of severe toxicities are shown
in Table 5.
Table 5. Severe late toxicities attributable to reirradiation.
DISCUSSION
Treatment options for NPC with local failure can be
operative or non-operative. Important distinctions exist
between residual and recurrent disease, with better
survival and disease control rates for those with residual
disease. This finding is consistent across multiple studies in the literature. It remains unclear whether
there is a relationship between tumour biology and
poorer reirradiation response of recurrent disease, i.e.,
revival of cancer cells, compared with residual disease,
which could be due to marginal miss of boost dose, or
prolonged/ incomplete regression. In our study, although
patients with residual and recurrent disease were treated
with a similar salvage RT technique, the radiation doses
they received were vastly different, and they were mostly
analysed as two distinctive groups.
For residual disease, in particular for early-stage disease
(rT1-2), treatment results and survival rates were highly
favourable and comparable to patients who had had a complete response after the first treatment course. Various
salvage options including surgery, further radiation boost
by external beam RT or brachytherapy, or photodynamic
therapy all result in good and comparable response
rates.[3] [14] [15] [16] [17] [18] [19] [20] [21] [22] [23] [24] Recurrent disease, however, is associated
with issues of radioresistance and higher morbidity with
irradiation, thus surgery is often preferred when it is
technically feasible. A matched cohort analysis showed
that salvage endoscopic nasopharyngectomy might be
superior to IMRT in terms of survival outcome, quality-of-life benefits, and complication rates for selected
rT1-T3 NPC.[25] Nonetheless, there has been no direct
prospective comparison between different techniques
of nasopharyngectomy with various non-operative
approaches, and such trials would not be feasible in
a randomised, well-stratified manner without large-scale
multi-centre cooperation and stringent quality
control. Besides, it is important to recognise that each
approach has its unique advantages and shortcomings
and the treatment decision has to take multiple factors
into account, such as the location and extent of
disease, availability of modality and expertise, patient
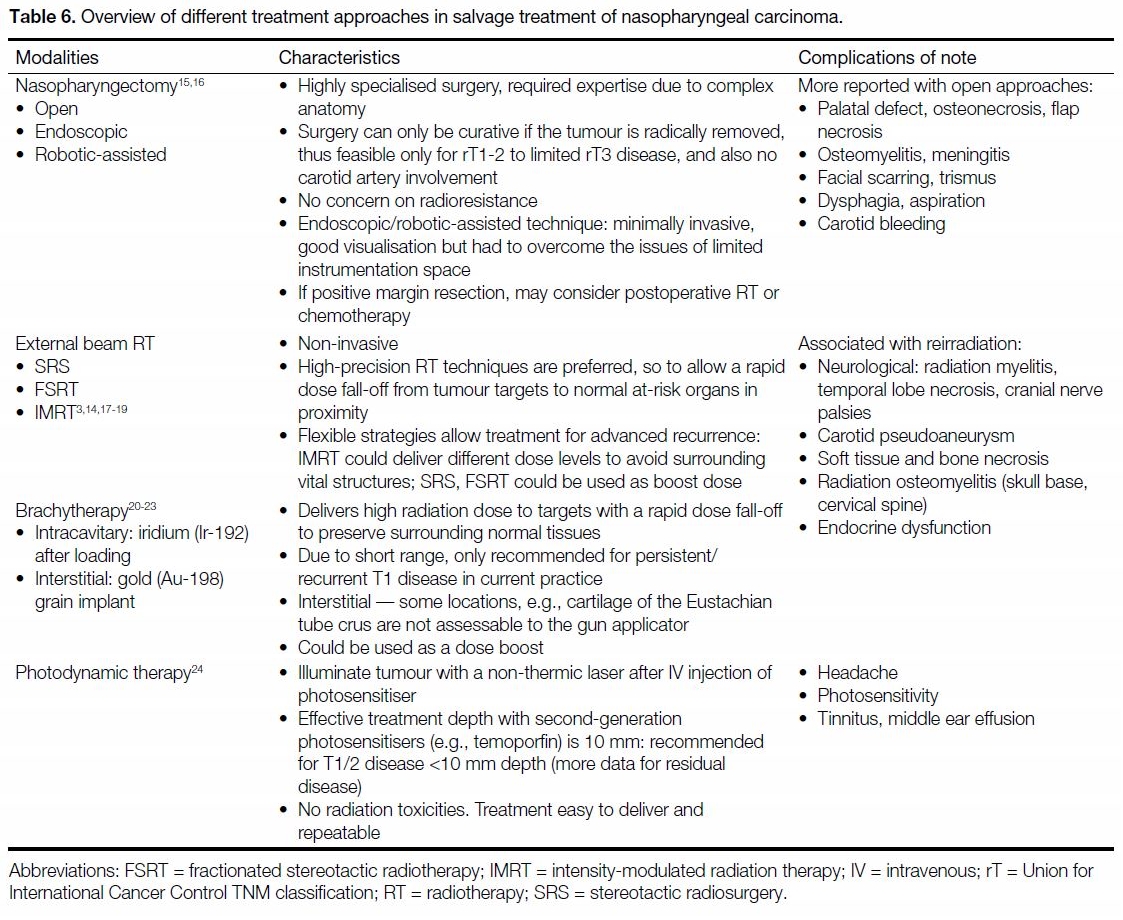
preference, and co-morbidities. Table 6 summarises
the characteristics of different treatment approaches in
salvage treatment of NPC.[3] [14] [15] [16] [17] [18] [19] [20] [21] [22] [23] [24]
Table 6. Overview of different treatment approaches in salvage treatment of nasopharyngeal carcinoma.
Our study provides updated data on the efficacy of
LINAC-based FSRT, using a frameless stereotactic
system and IMRT technique, in treating patients with NPC with local failure after high-dose primary IMRT.
The frameless system based on live tracking of fiducials
was non-invasive, required no neurosurgical expertise,
and improved patients’ comfort compared with the
conventional frame-based technique. Moreover, our
study findings are highly relevant to contemporary
practice as almost all of our patients received IMRT
(now the standard of care in many countries) as their
primary treatment. In fact, it has been proposed that with
the prevailing use of IMRT, the nature of recurrences is
likely to be different from those in patients in the older
era of two-dimensional or three-dimensional conformal
RT, when patients with NPC may have failed locally due
to marginal misses or underdosing to the clinical targets.
In the setting of modern imaging and RT techniques,
local recurrence after high-dose RT may instead
be accounted for by the presence of populations of
radioresistant cancer cells that survive the initial course of treatment and may pose a new set of challenges for
salvage. Therefore, previous results of salvage RT after
conventional techniques may not be as readily applicable
for patients treated in the IMRT era.[14] [26] From the overall
results of our study, in spite of these differences, our
clinical outcomes are comparable to previously reported
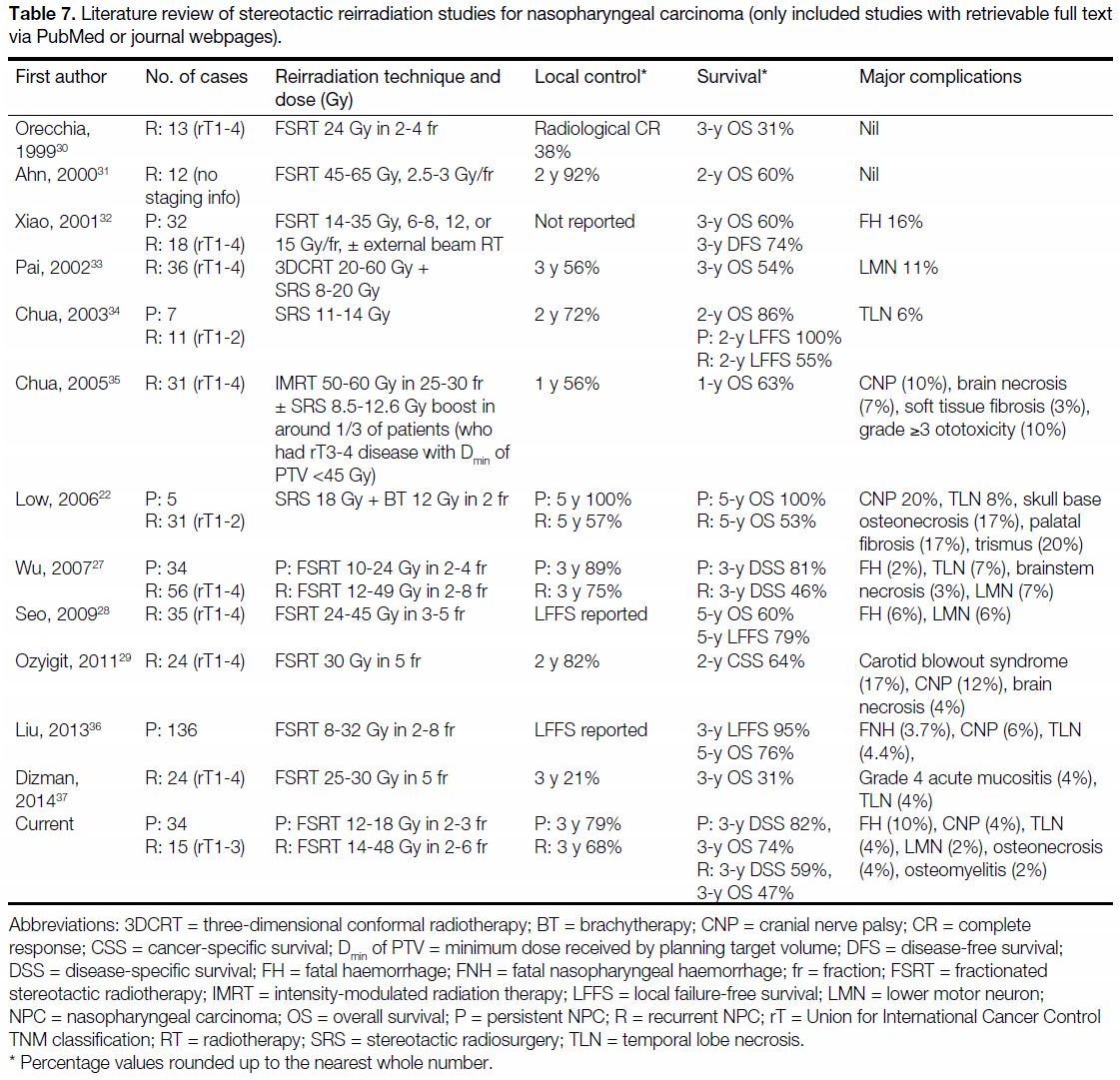
FSRT studies in the literature (Table 7[22] [27] [28] [29] [30] [31] [32] [33] [34] [35] [36] [37]).
Table 7. Literature review of stereotactic reirradiation studies for nasopharyngeal carcinoma (only included studies with retrievable full text
via PubMed or journal webpages).
On reviewing patients who suffered from fatal
nasopharyngeal haemorrhage as a complication, all
of these patients presented first with sentinel bleeds
up to 2 months before their fatal episode, suggesting
the possibility of earlier detection of carotid artery
pseudoaneurysms by raising physicians’ and patients’
awareness, especially in patients with known risk factors
such as reirradiation and skull base radionecrosis.[38]
Earlier detection may allow preventive endovascular
interventions to be performed.
As our study only included a limited number of patients
and survival events, conclusions regarding prognostic
factors should be drawn with caution, given the known
limitations of variable analyses of small cohorts.
Nonetheless, in the residual disease group, we found an
impact of FSRT GTV with a cut-off of 16 mL on OS
and PFS, similar to findings reported in many previous
studies.[27] [39] [40] [41] The cut-off values of this GTV in relation to
survival outcomes for the residual and recurrent disease
groups identified by receiver operating characteristic
analysis were different (16 mL and 10 mL, respectively).
We postulate that this difference could be due to the
inherently poorer radiosensitivity of recurrent disease. For the recurrent disease group, given the very limited
number of patients in the cohort, variable analysis
was more underpowered and might explain why some
commonly reported important prognostic factors such
as rT stage[28] [29] failed to reach statistical significance, and
the significance value of GTV was just below 0.05 (p =
0.044 for OS, p = 0.048 for PFS). However, on careful
assessment of all the regression analysis results, the
hazard ratios and p values of FSRT GTV far outweigh
those of either initial or recurrent T stage in both groups.
Over the years, much effort has been made to formulate
prognostic algorithms to predict radioresistance in
recurrence, so that low-risk patients could be confidently treated with reirradiation, whereas the unfavourable
high-risk group may be managed more aggressively or
recruited into clinical trials.[42] [43] With the changing patient
population and treatment advancements, the prognostic
effects of different factors could be dynamic and should
be continuously examined.
Looking to the future, the use of charged-particle RT,
including intensity-modulated ion therapy (IMIT) and
intensity-modulated proton therapy (IMPT), potentially
offers physical and biologic advantages over photon-based
IMRT. The multicentric in silico ROCOCO trial
has demonstrated further reduced doses to OARs using
IMIT or IMPT compared with photon therapy.[44] Very
recently, various group had been studying intensitymodulated
carbon ion RT in clinical practice for salvage
reirradiation of NPC, and has published promising early
results.[45] [46] However, long-term follow-up is needed to
assess the long-term outcome and late toxicities, and also
the optimal dose and fractionation.
CONCLUSION
In summary, we have presented our findings on the
efficacy and safety of LINAC-based FSRT, using a
frameless stereotactic system and IMRT technique, in
treating patients with NPC with local failure (including
residual and recurrent disease) after high-dose primary
IMRT. Using this less-invasive and resource-friendly
technique, the clinical outcomes were comparable to
those in the literature. FSRT GTV was identified as a
predictor of PFS and OS in patients irradiated for residual
disease. We look forward to more studies reporting
outcomes of reirradiation for patients treated in the
IMRT era, and also eagerly await long-term results from
IMIT and IMPT, which may further raise the therapeutic
ratio and overcome the existing barriers to reirradiation.
REFERENCES
1. Hong Kong Cancer Registry, Hospital Authority, Hong Kong
SAR Government. 2018. Available from: https://www3.ha.org.
hk/cancereg/. Accessed 10 Jan 2018.
2. Zhang B, Mo Z, Du W, Wong Y, Liu L, Wei Y. Intensity-modulated
radiation therapy versus 2D-RT or 3D-CRT for the treatment of
nasopharyngeal carcinoma: A systematic review and meta-analysis.
Oral Oncol. 2015;51:1041-6. Crossref
3. Chan OS, Sze HC, Lee MC, Chan LL, Chang AT, Lee SW.
Reirradiation with intensity-modulated radiotherapy for locally
recurrent T3 to T4 nasopharyngeal carcinoma. Head Neck.
2017;39:533-40. Crossref
4. Wu LR, Liu YT, Jiang N, Fan YX, Wen J, Huang SF, et al. Ten-year survival outcomes for patients with nasopharyngeal carcinoma receiving intensity-modulated radiotherapy: an analysis of 614
patients from a single center. Oral Oncol. 2017;69:26-32. Crossref
5. Au KH, Ngan RK, Ng AW, Poon DM, Ng WT, Yuen KT, et al. Treatment outcomes of nasopharyngeal carcinoma in modern era
after intensity modulated radiotherapy (IMRT) in Hong Kong:
A report of 3328 patients (HKNPCSG 1301 study). Oral Oncol.
2018;77:16-21. Crossref
6. Suárez C, Rodrigo JP, Rinaldo A, Langendijk JA, Shaha AR,
Ferlito A. Current treatment options for recurrent nasopharyngeal
cancer. Eur Arch Otorhinolaryngol. 2010;267:1811-24. Crossref
7. Xu T, Tang J, Gu M, Liu L, Wei W, Yang H. Recurrent
nasopharyngeal carcinoma: a clinical dilemma and challenge. Curr
Oncol. 2013;20:e406-19. Crossref
8. Stoker SD, van Diessen JN, de Boer JP, Karakullukcu B,
Leemans CR, Tan IB. Current treatment options for local
residual nasopharyngeal carcinoma. Curr Treat Options Oncol.
2013;14:475-91. Crossref
9. Chua DT, Wu SX, Lee V, Tsang J. Comparison of single versus
fractionated dose of stereotactic radiotherapy for salvaging local
failures of nasopharyngeal carcinoma: a matched-cohort analysis.
Head Neck Oncol. 2009;1:13. Crossref
10. Kwong DL, Nicholls J, Wei WI, Chua DT, Sham JS, Yuen PW,
et al. The time course of histologic remission after treatment of
patients with nasopharyngeal carcinoma. Cancer. 1999;85:1446-53. Crossref
11. Nieder C, Langendijk J, editors. Re-irradiation: New Frontiers.
Switzerland: Springer; 2017. Crossref
12. Wang HZ, Luo JW, Yi JL, Huang XD, Zhang SP, Wang K, et al.
The tolerance of brainstem in reirradiation with intensity modulated
radiation therapy in recurrent nasopharyngeal carcinoma. Int J
Radiat Oncol Biol Phys. 2016;96:E340. Crossref
13. Schultheiss TE. The radiation dose-response of the human spinal
cord. Int J Radiat Oncol Biol Phys. 2008;71:1455-9. Crossref
14. Kong L, Wang L, Shen C, Hu C, Wang L, Lu JJ. Salvage
intensity-modulated radiation therapy (IMRT) for locally recurrent
nasopharyngeal cancer after definitive IMRT: a novel scenario of
the modern era. Sci Rep. 2016;6:32883. Crossref
15. Tsang RK, Wei WI. Salvage surgery for nasopharyngeal cancer.
World J Otorhinolaryngol Head Neck Surg. 2015;1:34-43. Crossref
16. Liu J, Yu H, Sun X, Wang D, Gu Y, Liu Q, et al. Salvage endoscopic
nasopharyngectomy for local recurrent or residual nasopharyngeal
carcinoma: a 10-year experience. Int J Clin Oncol. 2017;22:834-
42. Crossref
17. Lu TX, Mai WY, The BS, Zhao C, Han F, Huang Y, et al. Initial
experience using intensity-modulated radiotherapy for recurrent
nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys.
2004;58:682-7. Crossref
18. Roeder F, Zwicker F, Saleh-Ebrahimi L, Timke C, Thieke C,
Bischof M, et al. Intensity modulated or fractionated stereotactic
reirradiation in patients with recurrent nasopharyngeal cancer.
Radiat Oncol. 2011;6:22. Crossref
19. Qiu S, Lin S, Tham IW, Pan J, Lu J, Lu JJ. Intensity-modulated
radiation therapy in the salvage of locally recurrent nasopharyngeal
carcinoma. Int J Radiat Oncol Biol Phys. 2012;83:676-83. Crossref
20. Leung TW, Tung SY, Sze WK, Sze WM, Wong VY, O SK. Salvage
brachytherapy for patients with locally persistent nasopharyngeal
carcinoma. Int J Radiat Oncol Biol Phys. 2000;47:405-12. Crossref
21. Leung TW, Tung SY, Sze WK, Sze WM, Wong VY, Wong CS, et al.
Salvage radiation therapy for locally recurrent nasopharyngeal
carcinoma. Int J Radiat Oncol Biol Phys. 2000;48:1331-8. Crossref
22. Low JS, Chua ET, Gao F, Wee JT. Stereotactic radiosurgery
plus intracavitary irradiation in the salvage of nasopharyngeal
carcinoma. Head Neck. 2006;28:321-9. Crossref
23. Kwong DL, Wei WI, Cheng AC, Choy DT, Lo AT, Wu PM, et al.
Long term results of radioactive gold grain implantation for the
treatment of persistent and recurrent nasopharyngeal carcinoma.
Cancer. 2001;91:1105-13. Crossref
24. Wildeman MA, Nyst HJ, Karakullukcu B, Tan BI. Photodynamic
therapy in the therapy for recurrent/persistent nasopharyngeal
cancer. Head Neck Oncol. 2009;1:40. Crossref
25. You R, Zou X, Hua YJ, Han F, Li L, Zhao C, et al. Salvage
endoscopic nasopharyngectomy is superior to intensity-modulated
radiation therapy for local recurrence of selected
T1-T3 nasopharyngeal carcinoma — a case-matched comparison.
Radiother Oncol. 2015;115:399-406. Crossref
26. Kong L, Lu JJ. Reirradiation of locally recurrent nasopharyngeal
cancer: history, advances, and promises for the future. Chin Clin
Oncol. 2016;5:26. Crossref
27. Wu SX, Chua DT, Deng ML, Zhao C, Li FY, Sham JS, et al.
Outcome of fractionated stereotactic radiotherapy for 90 patients
with locally persistent and recurrent nasopharyngeal carcinoma.
Int J Radiat Oncol Biol Phys. 2007;69:761-9. Crossref
28. Seo Y, Yoo H, Yoo S, Cho C, Yang K, Kim MS, et al.
Robotic system-based fractionated stereotactic radiotherapy in
locally recurrent nasopharyngeal carcinoma. Radiother Oncol.
2009;93:570-4. Crossref
29. Ozyigit G, Cengiz M, Yazici G, Yildiz F, Gurkaynak M, Zorlu F,
et al. A retrospective comparison of robotic stereotactic body
radiotherapy and three-dimensional conformal radiotherapy for
the reirradiation of locally recurrent nasopharyngeal carcinoma.
Int J Radiat Oncol Biol Phys. 2011;81:e263-8. Crossref
30. Orecchia R, Redda MG, Ragona R, Nassisi D, Jereczek-Fossa B,
Zurrida S, et al. Results of hypofractionated stereotactic re-irradiation
on 13 locally recurrent nasopharyngeal carcinomas.
Radiother Oncol. 1999;53:23-8. Crossref
31. Ahn YC, Lee KC, Kim DY, Huh SJ, Yeo IH, Lim DH, et al.
Fractionated stereotactic radiation therapy for extracranial head
and neck tumors. Int J Radiat Oncol Biol Phys. 2000;48:501-5. Crossref
32. Xiao J, Xu G, Miao Y. Fractionated stereotactic radiosurgery for
50 patients with recurrent or residual nasopharyngeal carcinoma.
Int J Radiat Oncol Biol Phys 2001;51:164-70. Crossref
33. Pai PC, Chuang CC, Wei KC, Tsang NM, Tseng CK, Chang CN.
Stereotactic radiosurgery for locally recurrent nasopharyngeal
carcinoma. Head Neck. 2002;24:748-53. Crossref
34. Chua DT, Sham JS, Kwong PW, Hung KN, Leung LH. Linear
accelerator-based stereotactic radiosurgery for limited, locally
persistent, and recurrent nasopharyngeal carcinoma: efficacy and
complications. Int J Radiat Oncol Biol Phys. 2003;56:177-83. Crossref
35. Chua DT, Sham JS, Leung LH, Au GK. Re-irradiation of
nasopharyngeal carcinoma with intensity-modulated radiotherapy. Radiother Oncol. 2005;77:290-4. Crossref
36. Liu F, Xiao JP, Xu GZ, Gao L, Xu YJ, Zhang Y, et al. Fractionated
stereotactic radiotherapy for 136 patients with locally residual
nasopharyngeal carcinoma. Radiat Oncol. 2013;8:157. Crossref
37. Dizman A, Coskun-Breuneval M, Altinisik-Inan G, Olcay GK,
Cetindag MF, Guney Y. Reirradiation with robotic stereotactic
body radiotherapy for recurrent nasopharyngeal carcinoma. Asian
Pac J Cancer Prev. 2014;15:3561-6. Crossref
38. Chen KC, Yen TT, Hsieh YL, Chen HC, Jiang RS, Chen WH,
et al. Postirradiated carotid blowout syndrome in patients with
nasopharyngeal carcinoma: a case-control study. Head Neck.
2015;37:794-9. Crossref
39. Lee AW, Foo W, Law SC, Poon YF, Sze WM, O SK, et al.
Reirradiation for recurrent nasopharyngeal carcinoma: factors
affecting the therapeutic ratio and ways for improvement. Int J
Radiat Oncol Biol Phys. 1997;38:43-52. Crossref
40. Chua DT, Sham JS, Hung KN, Leung LH, Au GK. Predictive
factors of tumor control and survival after radiosurgery for local
failures of nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys.
2006;66:1415-21. Crossref
41. Xiao W, Liu S, Tian Y, Guan Y, Huang S, Lin C, et al. Prognostic
significance of tumor volume in locally recurrent nasopharyngeal
carcinoma treated with salvage intensity-modulated radiotherapy.
PLoS One. 2015;10:e0125351. Crossref
42. Chua DT, Hung KN, Lee V, Ng SC, Tsang J. Validation of a
prognostic scoring system for locally recurrent nasopharyngeal
carcinoma treated by stereotactic radiosurgery. BMC Cancer.
2009;9:131. Crossref
43. Li YQ, Tian YM, Tan SH, Liu MZ, Kusumawidjaja G, Ong EH,
et al. Prognostic model for stratification of radioresistant
nasopharynx carcinoma to curative salvage radiotherapy. J Clin
Oncol. 2018;36:891-9. Crossref
44. Eekers DB, Roelofs E, Jelen U, Kirk M, Granzier M, Ammazzalorso F,
et al. Benefit of particle therapy in re-irradiation of head and
neck patients. Results of a multicentric in silico ROCOCO trial.
Radiother Oncol. 2016;121:387-94. Crossref
45. Hu J, Bao C, Gao J, Guan X, Hu W, Yang J, et al. Salvage treatment
using carbon ion radiation in patients with locoregionally recurrent
nasopharyngeal carcinoma: Initial results. Cancer. 2018;124:2427-37. Crossref
46. Gao J, Hu J, Guan X, Yang J, Hu W, Kong L, et al. Salvage carbon-ion
radiation therapy for locoregionally recurrent head and neck
malignancies. Sci Rep. 2019;9:4259. Crossref