Diagnostic Accuracy of Unenhanced Abbreviated Diffusion-Weighted Magnetic Resonance Imaging Versus Postcontrast Abbreviated Breast Magnetic Resonance Imaging for Breast Cancer
ORIGINAL ARTICLE CME
Diagnostic Accuracy of Unenhanced Abbreviated
Diffusion-Weighted Magnetic Resonance Imaging Versus Postcontrast Abbreviated Breast Magnetic Resonance Imaging for Breast Cancer
YJ Kim
Department of Radiology, Konyang University Hospital, Republic of Korea
Correspondence: Dr YJ Kim, Department of Radiology, Konyang University Hospital, Republic of Korea. Email: jjoong0318@gmail.com
Submitted: 12 Mar 2020; Accepted: 18 May 2020.
Contributors: The author designed the study, acquired the data, analysed the data, drafted the manuscript, and critically revised the manuscript for important intellectual content. The author had full access to the data, contributed to the study, approved the final version for publication, and takes responsibility for its accuracy and integrity.
Conflicts of Interest: The author declared that no conflicts of interest.
Funding/Support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: This study was approved by the institutional review board of Konyang University Hospital (Ref: 2019-12-024).
Abstract
Objectives
The purpose of this study was to compare the detectability of breast cancer by unenhanced abbreviated
magnetic resonance imaging (MRI) based on diffusion-weighted imaging (DWI) with the detectability of breast
cancer by postcontrast abbreviated MRI.
Methods
Between January 2014 and December 2015, a total of 89 patients were enrolled. Bilateral breast cancers
were found in three patients, for a total of 92 breasts with breast cancer and 86 negative breasts. All breast MRIs were
performed using a 3T MRI scanner with a 7-channel radiofrequency coil. Postcontrast abbreviated MRI consisted
of T2-weighted images (T2WI), T1-weighted images (T1WI), and a postcontrast T1WI sequence. Unenhanced
abbreviated MRI included T2WI, T1WI, and DWI. Qualitative and quantitative analyses of the apparent diffusion
coefficient map were performed independently. The sensitivity and specificity were calculated. Receiver operating
characteristic analysis was performed and the areas under the curves (AUCs) were compared between these protocols.
We evaluated factors associated with false negativity.
Results
The sensitivity/specificity of postcontrast abbreviated MRI, qualitative and quantitative analyses of
abbreviated DWI MRI were 94.6%/94.2%, 84.8%/97.7%, and 87.0%/98.8%, respectively. The AUCs for the
postcontrast abbreviated MRI, and qualitative and quantitative analyses of DWI MRI were 0.944, 0.912, and 0.929,
respectively (p > 0.05). The false-negative rate of unenhanced abbreviated MRI was higher than that of postcontrast
abbreviated MRI without significant (p > 0.05). Smaller cancers (≤10 mm) was associated false negativity.
Conclusion
The diagnostic performance of abbreviated DWI breast MRI was comparable to that of postcontrast abbreviated MRI.
Key Words: Breast; Diagnosis; Magnetic resonance imaging; Neoplasms
中文摘要
彌散加權無顯影劑加強快速磁共振成像與顯影後快速磁共振成像對乳腺癌的診斷準確性比較
YJ Kim
目的
本研究旨在比較彌散加權(DWI)無顯影劑加強快速磁共振成像(MRI)與顯影後快速MRI對乳腺癌的檢測性能。
方法
2014年1月至2015年12月期間共納入89例患者,當中92個乳腺有乳腺癌(雙側乳腺癌3例),
86個乳腺為陰性。所有乳腺MRI均使用7通道射頻線圈的3T MRI掃描儀。顯影後快速MRI包括T2加
權圖像(T2WI)、T1加權圖像(T1WI)及顯影後T1WI序列。無顯影劑加強快速MRI包括 T2WI、
T1WI和DWI。獨立進行表觀擴散系數圖的定性和定量分析並計算敏感性和特異性。進行接受者操作
特徵分析並比較這些方案之間的曲線下面積(AUC)。評估與假陰性相關的因素。
結果
顯影後快速MRI、DWI MRI的定性及定量分析的敏感性與特異性分別為94.6%/94.2%、
84.8%/97.7%和87.0%/98.8%。顯影後快速MRI、DWI MRI的定性及定量分析的AUC分別為0.944、
0.912和 0.929(p > 0.05)。無顯影劑加強快速MRI的假陰性率高於顯影後快速MRI但統計不顯著
(p > 0.05)。腫瘤較細小(≤10毫米)與假陰性相關。
結論
乳腺DWI快速MRI與顯影後快速MRI的診斷表現相似。
INTRODUCTION
Breast magnetic resonance imaging (MRI) has a higher
sensitivity than digital mammography in women with a
familial or genetic predisposition.[1] [2] [3] Screening by breast
MRI is recommended for women with a personal history
or a high-risk lesion.[4] However, a full diagnostic protocol
(FDP) of breast MRI has drawbacks, including high
cost, lengthy acquisition time (mean time, 24 min; range,
17-40 min), and use of intravenous contrast.[5]
Abbreviated postcontrast breast MRI examinations have
been proposed as an alternative method; they typically
include only a precontrast sequence and an early-phase
postcontrast sequence with/without T2-weighted imaging
(T2WI).[5] [6] [7] [8] [9] [10] The mean acquisition time of these shortened
examinations is 9 min (range, 3-15 min).[11] Abbreviated
postcontrast MRI scans have been reported to provide
cancer detection rates and a diagnostic accuracy that are
equivalent to those provided by an FDP.[5] [6] [7] [8] [9] [10] These results
are promising for the development of screening MRI
protocols that are more efficient for women at high risk
of breast cancer.
Current abbreviated breast MRI protocols still involve
administration of intravenous gadolinium contrast material. Intravenous contrast may increase the cost,
the examination time, or incidence of adverse effects.
In addition, gadolinium contrast is contraindicated in
patients with kidney disease or contrast allergy. These
are considerations for an asymptomatic young population
with indications for breast cancer screening.[11] [12]
Diffusion-weighted imaging (DWI), which measures
endogenous water movement within tissues, is a fast,
widely available MRI technique that requires no
gadolinium contrast material. The resulting apparent
diffusion coefficient (ADC) values are quantified by mean
diffusivity measurements in three orthogonal directions.
ADC values are influenced by tissue cellularity, fluid
viscosity, membrane permeability and blood flow, and
are known to be useful for discriminating between benign
and malignant lesions.[13] Several studies have reported
high yields from the combined use of DWI MRI added
to FDP MRI.[14] [15] [16] Recent studies distinguishing between
benign and malignant lesions have reported that DWI
MRI alone showed a diagnostic performance comparable
to that provided by FDP MRI.[14] [15] [16] Kang et al[17] compared
the performance of DWI MRI to that of abbreviated
postcontrast MRI. They reported that the performance of
DWI MRI as a screening examination in patients with a personal history of breast cancer was comparable to
that of abbreviated postcontrast MRI, with reduced times
for image acquisition and interpretation.[17] Few studies
have investigated the feasibility of unenhanced MRI
comprising T1-weighted images (T1WI), T2WI, and
DWI as a screening method in populations at risk.
The purpose of this study was to compare the
detectability of breast cancer by MRI based on DWI
with that by postcontrast abbreviated MRI. We also
compared the diagnostic performance of DWI MRI
using a quantitative analysis that required ADC values
and a qualitative analysis that was performed by visual
assessment without ADC mapping.
METHODS
Participants and Selection Process
This retrospective study was approved by our institutional
review board, and the requirement for informed consent
was waived. The initial study population consisted of
consecutive patients who underwent breast MRI with
FDP between January 2014 and December 2015 for
screening, difficult cases, evaluating the extension of
breast cancer, detection of additional lesions, or response
to neoadjuvant chemotherapy. Patients were excluded if
they had prior surgery, had been treated with neoadjuvant
chemotherapy, had MR findings that were difficult to
correlate with biopsy results, had implant or silicone
injection, or had previous mammotomy or stereotactic
biopsy.
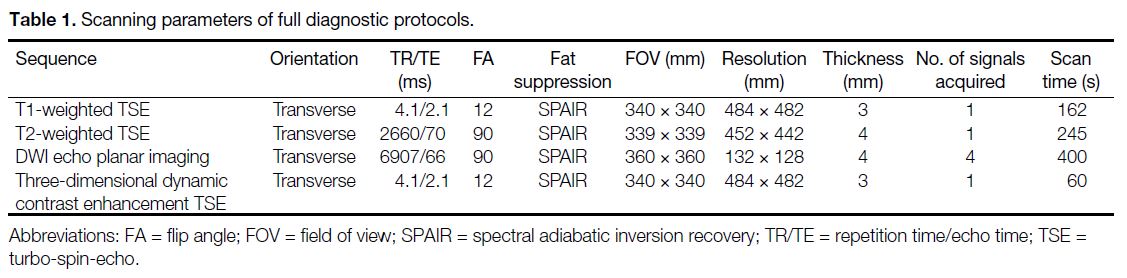
Magnetic Resonance Imaging Techniques
All breast MRIs were performed in a 3T MRI scanner
(Achieva; Philips Healthcare, Best, the Netherlands)
equipped with a 7-channel breast radiofrequency
coil, with the patient in the prone position. DWI was
examined by a spin-echo-type single-shot echo planar
imaging technique in the axial plane. Diffusion-sensitising
gradients were applied in three orthogonal
directions (lateral, sagittal, and craniocaudal) and images were obtained at b values of 0 and 1,000 s/mm2.[18] [19] The
scanning parameters are listed in Table 1. The contrast
agent (gadobutrol, 0.1 mmol/kg) was injected into an
antecubital vein using an automated injector at a rate of
1.2 mL/s, followed by a 20-mL saline flush. We acquired
subtraction images as follows: the baseline data, acquired
before infusion of contrast agent, were subtracted (slice
by slice, and for each slice, pixel by pixel).
Table 1. Scanning parameters of full diagnostic protocols.
Image Interpretation
All FDP MRIs were interpreted. The sequences making
up the postcontrast abbreviated MRI consisted of turbo-spin-echo (TSE) T2WI with fat suppression, TSE-T1WI,
a single intermediate (3 min after contrast injection)
postcontrast T1 sequence, and a subtraction image.
The sequences comprising unenhanced abbreviated
MRI, which was based on DWI, included TSE-T2WI
with fat suppression, TSE-T1WI, and DWI. All lesions
were evaluated according to a checklist, which included
the following items: location of the suspicious lesion,
size, shape, margin, enhancement pattern, T2WI signal
intensity (high, intermediate, or low), DWI detectability,
ADC value, ADC signal intensity, and Breast Imaging
Reporting and Data System (BI-RADS) assessment.
For postcontrast abbreviated MRI, the readers identified
any suspicious findings on the postcontrast sequence,
and then analysed the corresponding lesions on fat-suppressed
T2WI and T1WI. For abbreviated DWI MRI,
the readers detected breast lesions showing high signal
intensity on DWI with the high b value (b = 1,000 s/mm2),
and then reviewed the fat-suppressed T2WI and T1WI.
Qualitative evaluation was performed by a visual
assessment of the signal intensity on DWI acquired at
b=1000 s/mm2 and their corresponding ADC maps.
For visual evaluation, DWI detectability was classified
as detectable, equivocal, or undetectable. ADC signal
intensity was classified as high, low, or undetectable. A
solid tumour showing high signal intensity on the high-b-value
DWI and low signal intensity on the corresponding ADC map was considered to require histopathological
confirmation. We performed quantitative evaluations,
using the CAD system (CADstream version 6.0;
Confirma, Kirkland [WA], United States) to identify
ADC values corresponding to the lesions detected on
visual assessment. The ADC map of the largest diameter
of each tumour was selected, and a region of interest was
manually drawn to encompass the entire cross-section
of the lesion avoiding adjacent normal breast tissue or
fat. Cystic, necrotic, or haemorrhagic components of
the lesions that might affect the ADC values were also
avoided. The ADC cut-off was set as 1.25 × 10-3 mm2/s.[20] [21]
In this study, any lesion with an ADC value lower than the
cut-off value was considered to require histopathological
confirmation. Visual assessments and quantitative
analyses for ADC map were performed independently.
We assessed background intensity for DWI. There is no
definition of background intensity for DWI MRI in BI-RADS;
however, we applied a definition similar to that
of BI-RADS. The degree of background diffusion signals
on DWI was visually assessed and graded as minimal,
mild, moderate and severe. BI-RADS final assessment
categories 1, 2, and 3 were considered MRI-negative,
and categories 4 and 5 were considered MRI-positive.
Finally, the readers were instructed to record the length
of time needed to interpret each case. These image
evaluation sessions were assessed at 2-week intervals.
Histopathological Evaluation
The histopathological diagnoses were retrieved from
the electronic records of our institution. The final
histopathological diagnoses were made based on
evaluations of the surgical specimens of patients who
underwent surgery after the imaging studies. Evaluation
of core needle biopsy specimens was considered
representative for patients who refused surgery or who
were transferred to other hospitals.
The intrinsic lesion subtype was determined based on
the following markers: expression of oestrogen receptor,
progesterone receptor, and human epidermal growth
factor receptor 2 (HER2). Tumours with oestrogen
receptor and/or progesterone receptor positivity were
defined as having ≥10% of tumour cells with positive
nuclei. HER2 expression was evaluated by the
HercepTest (Dako, Glostrup, Denmark) and scored on
a scale from 0 to 3+. Tumours with scores ≥3 or with
a ≥2.2-fold increase in HER2 gene amplification, as
determined by fluorescence in situ hybridisation, were
considered positive for HER2 overexpression.
Statistical Analysis
Sensitivity and specificity were calculated. Receiver
operating characteristic curve analysis was performed,
and the areas under the curves were compared between
protocols. The Chi-square or Fisher’s exact test was used
to evaluate false negativity according to tumour size,
histopathological results, and background intensity on
DWI. The reading times for both protocols were assessed
by paired t tests. Statistical analysis was performed
by SPSS (Windows version 18.0; SPSS Inc., Chicago
[IL], United States). A p value of <0.05 was considered
significant.
RESULTS
Patients
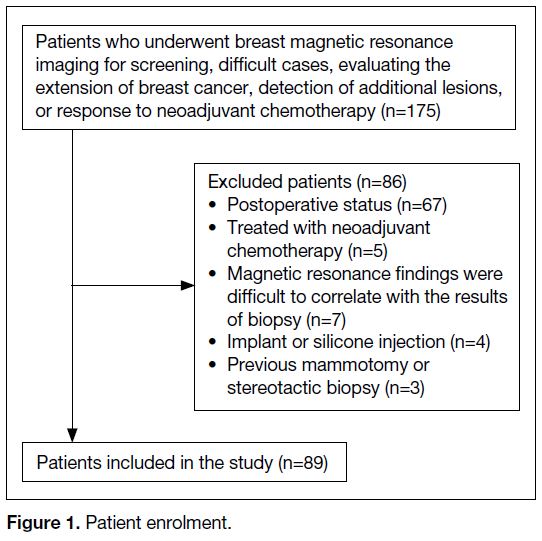
In total, 175 consecutive patients underwent breast MRI
with FDP between January 2014 and December 2015
for screening, difficult cases, evaluating the extension
of breast cancer, detection of additional lesions, or
response to neoadjuvant chemotherapy (Figure 1). A
total of 86 patients were excluded. For patients with
multicentric cancers, the largest tumour was considered
as the representative. Bilateral breast cancers were found
in three patients. Each lesion was counted as one lesion.
A total of 89 patients were enrolled, with 92 breasts with
breast cancer and 86 negative breasts.
Figure 1. Patient enrolment
Histopathological Evaluation
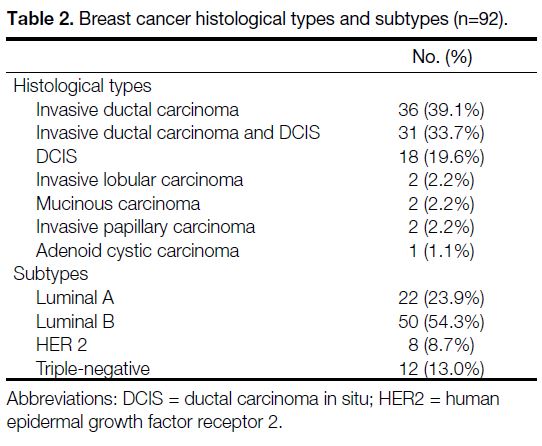
The histopathological diagnosis was obtained on a
surgical specimen in 65 patients and a core needle biopsy specimen in 24 patients. A total of 92 breast cancer lesions
were detected. The mean tumour size was 2.55 ± 1.59 cm
(range, 0.3-9.0). The histological types of the breast
cancers are shown in Table 2. Invasive ductal carcinoma
(IDC) and ductal carcinoma in situ (DCIS) accounted for
92.4% of the cancer lesions. Most of the lesions were
of the luminal B histological subtype, and 21.7% were
either HER2-overexpressing or triple-negative subtypes
(Table 2).
Table 2. Breast cancer histological types and subtypes (n=92).
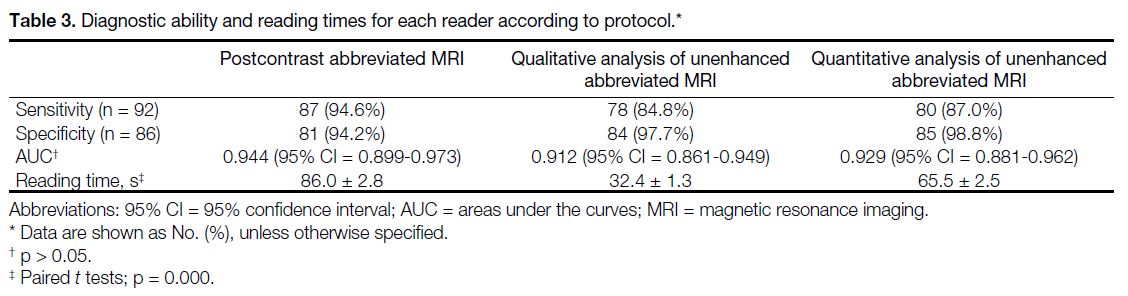
Performance of Abbreviated Protocols
The sensitivity and specificity of postcontrast
abbreviated MRI were 94.6% (87/92) and 94.2%
(81/86), respectively. For DWI MRI, the sensitivity
and specificity of qualitative and quantitative analysis
were as follows: qualitative, 84.8% (78/92), 97.7%
(84/86), respectively; and quantitative, 87.0% (80/92),
98.8% (85/86), respectively. The differences between
the diagnostic accuracy of these protocols were not
significant (Table 3).
Table 3. Diagnostic ability and reading times for each reader according to protocol.
Missed Tumours
Most of the lesions were detected with these protocols.
The tumour diameters ranged from 0.6 to 7.0 cm
(mean=2.25 ± 1.3 cm) on postcontrast abbreviated MRI
and 0.6 to 7.0 cm (mean=1.98 ± 1.1 cm) on unenhanced
abbreviated MRI. In addition, 10 lesions with diameters
≤1.0 cm were detected on unenhanced abbreviated MRI.
A total of 14 tumours were not detected by abbreviated
MRI. None of the tumours was missed by postcontrast
abbreviated MRI only. Of these tumours, five were
missed by both postcontrast and abbreviated DWI MRI
(mean diameter 1.0 ± 0.5 cm, 0.3-1.5 cm) [Figure 2].
Of the five undetected tumours, three had diameters
≤1 cm. The histopathological findings of these undetected
tumours were as follows: IDC (n=3), DCIS (n=1), and
IDC with DCIS (n=1). Three tumours were luminal A
and two were luminal B. Two IDC lesions were grade 1.
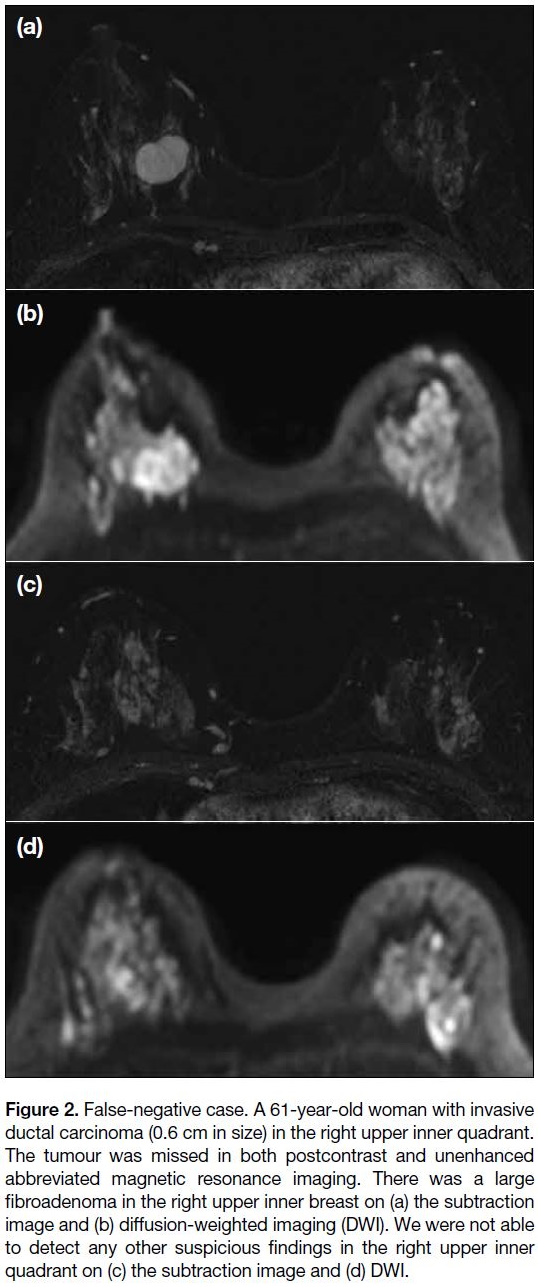
Figure 2. False-negative case. A 61-year-old woman with invasive
ductal carcinoma (0.6 cm in size) in the right upper inner quadrant.
The tumour was missed in both postcontrast and unenhanced
abbreviated magnetic resonance imaging. There was a large
fibroadenoma in the right upper inner breast on (a) the subtraction
image and (b) diffusion-weighted imaging (DWI). We were not able
to detect any other suspicious findings in the right upper inner
quadrant on (c) the subtraction image and (d) DWI.
The 14 tumours missed on abbreviated DWI MRI with
qualitative analysis ranged from 0.3 to 5.0 cm (mean 1.8 ± 1.3 cm) in diameter. Five of these tumours had diameters
≤1.0 cm. The 12 tumours missed on quantitative
analysis ranged from 0.3 to 5.0 cm (mean 1.86 ± 1.4 cm)
in diameter. Four of these tumours had diameters
≤1.0 cm. A significantly higher proportion of missed
breast cancers had diameters ≤1.0 cm (p = 0.006). The
histopathological findings of these tumours undetected
by abbreviated DWI MRI were as follows: mucinous
carcinoma (n=1), IDC (n=4), DCIS (n=5), and IDC with
DCIS (n=4) [Figure 3]. Seven tumours were luminal A
and seven were luminal B. In tumours missed on DWI
MRI, there was no significant difference according to
histopathological results and lesion subtype (p > 0.05).
The background intensities of the breasts with missed
tumours on DWI MRI were as follows: minimal (n=2),
mild (n=3), moderate (n=4), and severe (n=5). The
differences in tumour detection according to background
intensity on DWI MRI were not significant (p = 0.198).
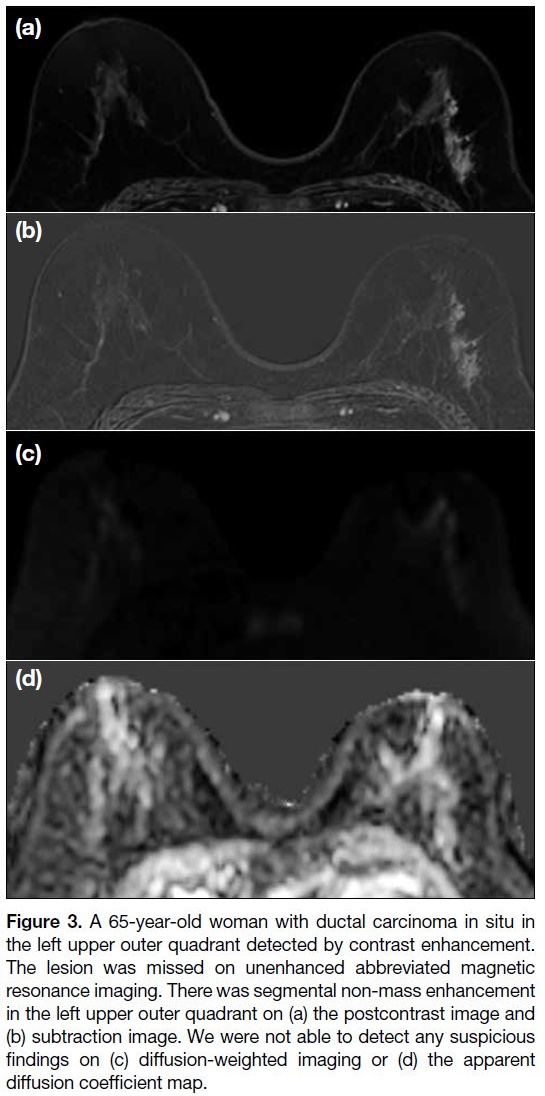
Figure 3. A 65-year-old woman with ductal carcinoma in situ in
the left upper outer quadrant detected by contrast enhancement.
The lesion was missed on unenhanced abbreviated magnetic
resonance imaging. There was segmental non-mass enhancement
in the left upper outer quadrant on (a) the postcontrast image and
(b) subtraction image. We were not able to detect any suspicious
findings on (c) diffusion-weighted imaging or (d) the apparent
diffusion coefficient map.
Reading Times
The postcontrast abbreviated MRI reading time was
significantly longer than that of unenhanced abbreviated
MRI (p = 0.000) [Table 3].
DISCUSSION
We compared the diagnostic performance of unenhanced
abbreviated breast MRI based on DWI with that of
postcontrast abbreviated MRI for breast cancer. We
found that the diagnostic performance of unenhanced
abbreviated breast MRI, including qualitative and
quantitative analysis, was comparable to that of
postcontrast abbreviated MRI.
Postcontrast abbreviated MRI showed high sensitivity
(94.6%) in this study, which is consistent with previous
studies (sensitivity ranging from 86% to 92%).[6] [9] [22] Those studies demonstrated that the diagnostic accuracy
of postcontrast abbreviated breast MRI was similar to
that of a comprehensive diagnostic MRI protocol.[5] [7] [8] [9] [10] [22]
Based on these results, postcontrast abbreviated breast
MRI has been proposed as a promising screening tool for
women at high risk of breast cancer.[7] [10] [23]
Unenhanced DWI MRI was recently introduced as an
alternative methodology that avoids the risks and cost
of contrast material. Several studies have compared the
performance of abbreviated or standard DWI MRI to
that of postcontrast abbreviated MRI. The performance
of DWI MRI was reported to be equivalent to the
performance of postcontrast abbreviated MRI, with
reduction in image acquisition time and interpretation
time for each case.[17] [24] [25] In DWI MRI, qualitative
analysis is based on visual assessment and quantitative
analysis is based on ADC mapping. An ADC value
≤1.25 × 10-3 mm2/s was reported to be suitable for
differentiating between benign and malignant breast
lesions.[20] [21] Quantitative and qualitative analysis of
abbreviated unenhanced MRI has been shown to provide
higher specificity compared to that of contrast-enhanced
MRI.[15] [24]
Although unenhanced abbreviated MRI showed
comparable diagnostic accuracy, its false-negative rate
was higher than that of postcontrast abbreviated MRI. In
this study, smaller cancers (≤10 mm) were significantly
associated with false-negative findings. Several studies
have reported on the drawbacks of DWI MRI. DCIS
shows less diffusion impedance, as reflected by higher
ADC values, compared with invasive carcinomas,[28]
which might cause relatively low tumour conspicuity on
DWI MRI. Previous studies also reported that smaller
cancers, specifically ≤10 to 12 mm, were harder to
detect.[16] [17] [20] [21] The typical DWI MRI axial-in-plane
spatial resolution (2 × 2 mm2) and section thickness
(3-5 mm) are thought to lead to a marked partial volume
effect for small lesions, as well as possible concealment
by a susceptibility artefact such as adjacent biopsy
marker clips, which is more pronounced on DWI MRI
than on conventional T1WI and T2WI.[27]
Malignant lesions with high water content can also be
missed because of their high ADC values. Such lesions
include mucinous carcinoma and triple-negative cancer
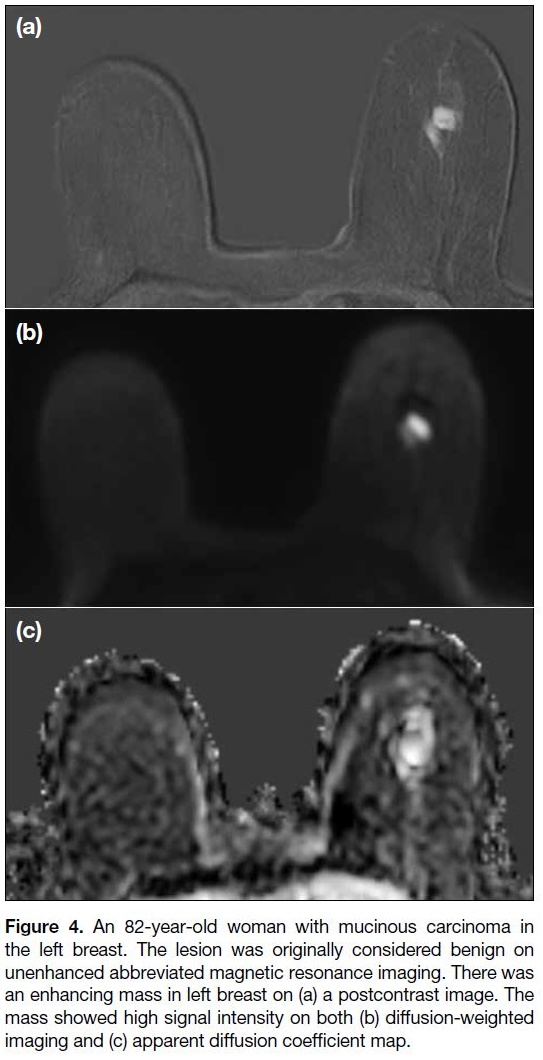
with extensive necrosis.[28] [29] One lesion, originally
considered to be benign, was mucinous cancer showing
a high ADC value and high signal intensity on T2WI
(Figure 4).
Figure 4. An 82-year-old woman with mucinous carcinoma in
the left breast. The lesion was originally considered benign on
unenhanced abbreviated magnetic resonance imaging. There was
an enhancing mass in left breast on (a) a postcontrast image. The
mass showed high signal intensity on both (b) diffusion-weighted
imaging and (c) apparent diffusion coefficient map.
DWI MRI shows high accuracy for detecting breast
cancer without contrast. In addition, DWI MRI is not
affected by breast density, menopausal status or timing
during the menstrual cycle, which impede the detection of
breast lesions on mammography or FDP MRI.[30] [31] [32] Most
of these studies were focused on quantitative DWI MRI,
which requires specific software for obtaining an ADC
value. However, some institutions do not implement
such a protocol. The qualitative analysis of DWI MRI
can be performed without a specific protocol by visual
assessment. This analysis could reduce reading times,
compared with quantitative DWI MRI. A previous study compared the detectability of postcontrast abbreviated
MRI and unenhanced abbreviated DWI MRI without
ADC mapping; they reported that the detectability
of DWI MRI was comparable to that of postcontrast
abbreviated MRI.[24] We compared the diagnostic
accuracies of quantitative and qualitative analyses of
DWI MRI. The sensitivity and specificity of quantitative
analysis were not significantly higher than those of
qualitative analysis. DWI MRI could be an alternative
screening tool for breast cancer.
This study has limitations. First, it was a retrospective
study. This design may lead to selection bias. Second,
all patients were already known to have breast cancer
and underwent breast MRI in a clinical setting. The
study population is not representative of patients with
breast cancer in the general population. Third, we did not
acquire DWI MRI with the use of advanced techniques.
We used classic diffusion-weighted 3.0-T MRI with a
conventional single-shot echo planar imaging–based
sequence.[19] At 3.0 T, visibility of the lesion on DWI
MRI is substantially improved compared with 1.5 T,[33]
but image artefacts are twice as strong. Clinical DWI
is based on single-shot echo planar imaging, which is
prone to image artefacts.[34] DWI MRI based on readout-segmented
echo planar imaging was introduced to reduce
geometric distortions, image blurring, and ghosting
artefacts at 3.0 T.[35] [36]
CONCLUSION
In conclusion, the diagnostic performance of an
abbreviated DWI MRI for detecting breast cancers
was comparable to that of a postcontrast abbreviated
MRI protocol. The DWI protocol might be useful for
screening breast MRI in high-risk populations; however,
further validation is needed to demonstrate the feasibility
of DWI as a breast screening tool.
REFERENCES
1. Oeffinger KC, Fontham ET, Etzioni R, Herzig A, Michaelson JS,
Shih YC, et al. Breast cancer screening for women at average risk:
2015 guideline update from the American Cancer Society. JAMA
2015;314:1599-614. Crossref
2. Kriege M, Brekelmans CT, Boetes C, Besnard PE, Zonderland HM,
Obdeijn IM, et al. Efficacy of MRI and mammography for breast-cancer
screening in women with a familial or genetic predisposition.
N Engl J Med. 2004;351:427-37. Crossref
3. Kuhl C, Weigel S, Schrading S, Arand B, Bieling H, König R,
et al. Prospective multicenter cohort study to refine management
recommendations for women at elevated familial risk of breast
cancer: the EVA trial. J Clin Oncol. 2010;28:1450-7. Crossref
4. Sippo DA, Burk KS, Mercaldo SF, Rutledge GM, Edmonds C, Guan Z, et al. Performance of screening breast MRI across women with different elevated breast cancer risk indications. Radiology.
2019;292:51-9. Crossref
5. Kuhl CK, Schrading S, Strobel K, Schild HH, Hilgers RD,
Bieling HB. Abbreviated breast magnetic resonance imaging
(MRI): first postcontrast subtracted images and maximum-intensity
projection — a novel approach to breast cancer screening with MRI.
J Clin Oncol. 2014;32:2304-10. Crossref
6. Grimm LJ, Soo MS, Yoon S, Kim C, Ghate SV, Johnson KS. Abbreviated screening protocol for breast MRI: a feasibility study. Acad Radiol. 2015;22:1157-62. Crossref
7. Harvey SC, Di Carlo PA, Lee B, Obadina E, Sippo D, Mullen L.
An abbreviated protocol for high-risk screening breast MRI saves
time and resources. J Am Coll Radiol. 2016;13:R74-80. Crossref
8. Moschetta M, Telegrafo M, Rella L, Stabile Ianora AA, Angelelli G.
Abbreviated combined MR protocol: a new faster strategy for
characterizing breast lesions. Clin Breast Cancer. 2016;16:207-11. Crossref
9. Machida Y, Shimauchi A, Kanemaki Y, Igarashi T, Harada M,
Fukuma E. Feasibility and potential limitations of abbreviated
breast MRI: an observer study using an enriched cohort. Breast
Cancer. 2017;24:411-9. Crossref
10. Panigrahi B, Mullen L, Falomo E, Panigrahi B, Harvey S. An
abbreviated protocol for high-risk screening breast magnetic
resonance imaging: impact on performance metrics and BI-RADS
assessment. Acad Radiol. 2017;24:1132-8. Crossref
11. Chhor CM, Mercado CL. Abbreviated MRI protocols: wave of
the future for breast cancer screening. AJR Am J Roentgenol.
2017;208:284-9. Crossref
12. Gulani V, Calamante F, Shellock FG, Kanal E, Reeder SB,
International Society for Magnetic Resonance in Medicine.
Gadolinium deposition in the brain: summary of evidence and
recommendations. Lancet Neurol. 2017;16:564-70. Crossref
13. Partridge SC, Mullins CD, Kurland BF, Allain MD, DeMartini WB,
Eby PR, et al. Apparent diffusion coefficient values for
discriminating benign and malignant breast MRI lesions: effects
of lesion type and size. AJR Am J Roentgenol. 2010;194:1664-73. Crossref
14. Zhang L, Tang M, Min Z, Lu J, Lei X, Zhang X. Accuracy of
combined dynamic contrast-enhanced magnetic resonance imaging
and diffusion-weighted imaging for breast cancer detection: a meta-analysis.
Acta Radiol. 2016;57:651-60. Crossref
15. Chen X, Li WL, Zhang YL, Wu Q, Guo YM, Bai ZI. Meta-analysis
of quantitative diffusion-weighted MR imaging in the differential
diagnosis of breast lesions. BMC Cancer. 2010;10:693. Crossref
16. Kazama T, Kuroki Y, Kikuchi M, Sato Y, Nagashima T, Miyazawa Y,
et al. Diffusion-weighted MRI as an adjunct to mammography
in women under 50 years of age: an initial study. J Magn Reson
Imaging. 2012;36:139-44 Crossref
17. Kang JW, Shin HJ, Shin KC, Chae EY, Choi WJ, Cha JH, et al.
Unenhanced magnetic resonance screening using fused diffusion-weighted
imaging and maximum-intensity projection in patients
with a personal history of breast cancer: role of fused DWI for
postoperative screening. Breast Cancer Res Treat. 2017;165:119-28. Crossref
18. Kim JY, Kim JJ, Lee H, Lee JW, Lee NK, Nam KJ, et al. Diffusion-weighted
MRI of estrogen receptor-positive, HER2-negative,
node-negative breast cancer: association between intratumoral
heterogeneity and recurrence risk. Eur Radiol. 2020;30:66-76. Crossref
19. Kim JY, Kim JJ, Lee H, Kang T, Park H. Diffusion-weighted
imaging of invasive breast cancer: relationship to distant
metastasis–free survival. Radiology. 2019;291:300-7. Crossref
20. Baltzer PA, Bickel H, Spick C, Wengert G, Woitek R, Kapetas P,
et al. Potential of noncontrast magnetic resonance imaging with
diffusion-weighted imaging in characterization of breast lesions:
intraindividual comparison with dynamic contrast-enhanced magnetic resonance imaging. Invest Radiol. 2018;53:229-35. Crossref
21. Pinker K, Moy L, Sutton EJ, Mann RM, Weber M, Thakur SB, et al.
Diffusion-weighted imaging with apparent diffusion coefficient
mapping for breast cancer detection as a stand-alone parameter:
comparison with dynamic contrast-enhanced and multiparametric
magnetic resonance imaging. Invest Radiol. 2018;53:587-95. Crossref
22. Chen SQ, Huang M, Shen YY, Liu CL, Xu CX. Application of
abbreviated protocol of magnetic resonance imaging for breast
cancer screening in dense breast tissue. Acad Radiol. 2017;24:316-20. Crossref
23. Choi BH, Choi N, Kim MY, Yang JH, Yoo YB, Jung HK.
Usefulness of abbreviated breast MRI screening for women
with a history of breast cancer surgery. Breast Cancer Res Treat.
2018;167:495-502. Crossref
24. Yamada T, Kanemaki Y, Okamoto S, Nakajima Y. Comparison
of detectability of breast cancer by abbreviated breast MRI based
on diffusion-weighted images and postcontrast MRI. Jpn J Radiol.
2018;36:331-9. Crossref
25. Shin HJ, Chae EY, Choi WJ, Ha SM, Park JY, Shin KC, et al.
Diagnostic performance of fused diffusion-weighted imaging using
unenhanced or postcontrast T1-weighted MR imaging in patients
with breast cancer. Medicine (Baltimore). 2016;95:e3502. Crossref
26. Choi SY, Chang YW, Park HJ, Kim HJ, Hong SS, Seo DY.
Correlation of the apparent diffusion coefficiency values on
diffusion-weighted imaging with prognostic factors for breast
cancer. Br J Radiol. 2012;85:e474-9. Crossref
27. Le Bihan D, Poupon C, Amadon A, Lethimonnier F. Artifacts and
pitfalls in diffusion MRI. J Magn Reson Imaging. 2006;24:478-88. Crossref
28. Woodhams R, Matsunaga K, Kan S, Hata H, Ozaki M, Iwabuchi K,
et al. ADC mapping of benign and malignant breast tumors. Magn
Reson Med Sci. 2005;4:35-42. Crossref
29. Youk JH, Son EJ, Chung J, Kim JA, Kim EK. Triple-negative invasive breast cancer on dynamic contrast-enhanced and diffusionweighted
MR imaging: comparison with other breast cancer
subtypes. Eur Radiol. 2012;22:1724-34. Crossref
30. Hahn SY, Ko ES, Han BK, Lim Y, Gu S, Ko EY. Analysis of factors
influencing the degree of detectability on diffusion-weighted MRI
and diffusion background signals in patients with invasive breast
cancer. Medicine (Baltimore). 2016;95:e4086. Crossref
31. Horvat JV, Durando M, Milans S, Patil S, Massler J, Gibbons G, et al.
Apparent diffusion coefficient mapping using diffusion-weighted
MRI: impact of background parenchymal enhancement, amount
of fibroglandular tissue and menopausal status on breast cancer
diagnosis. Eur Radiol. 2018;28:2516-24. Crossref
32. Iacconi C, Thakur SB, Dershaw DD, Brooks J, Fry CW, Morris EA.
Impact of fibroglandular tissue and background parenchymal
enhancement on diffusion weighted imaging of breast lesions. Eur
J Radiol. 2014;83:2137-43. Crossref
33. Matsuoka A, Minato M, Harada M, Kubo H, Bandou Y, Tangoku A,
et al. Comparison of 3.0-and 1.5-tesla diffusion-weighted imaging
in the visibility of breast cancer. Radiat Med. 2008;26:15-20. Crossref
34. Yeom KW, Holdsworth SJ, Van AT, Iv M, Skare S, Lober RM,
et al. Comparison of readout-segmented echo-planar imaging
(EPI) and single-shot EPI in clinical application of diffusion-weighted
imaging of the pediatric brain. AJR Am J Roentgenol.
2013;200:W437-43. Crossref
35. Bogner W, Pinker-Domenig K, Bickel H, Chmelik M, Weber M,
Helbich TH, et al. Readout-segmented echo-planar imaging
improves the diagnostic performance of diffusion-weighted MR
breast examinations at 3.0 T. Radiology. 2012;263:64-76. Crossref
36. Bogner W, Gruber S, Pinker K, Grabner G, Stadlbauer A, Weber M, et al. Diffusion-weighted MR for differentiation of breast lesions at 3.0 T: how does selection of diffusion protocols affect diagnosis? Radiology. 2009;253:341-51. Crossref