Are We Adequately Communicating the Potential Radiation Risks to Patients Undergoing Nuclear Medicine Examinations? A Clinical Audit
ORIGINAL ARTICLE
Are We Adequately Communicating the Potential Radiation Risks to Patients Undergoing Nuclear Medicine Examinations?
A Clinical Audit
TK Chan, TK Au Yong, BT Kung, YH Hui
Nuclear Medicine Unit, Queen Elizabeth Hospital, Hong Kong
Correspondence: Dr TK Chan, Nuclear Medicine Unit, Queen Elizabeth Hospital, Hong Kong. Email: theo@hku.hk
Submitted: 18 Jun 2021; Accepted: 27 Sep 2021.
Contributors: All authors designed the study. TKC acquired the data. All authors analysed the data. TKC drafted the manuscript. TKAY, BTK
and YHH critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study,
approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: As an editor of the journal, TKAY was not involved in the peer review process. Other authors declare that they have no conflicts of interest.
Funding/Support: This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: All participants orally gave consent to take part in the clinical audit. All data collected are anonymised to safeguard confidentiality.
Abstract
Introduction
We assessed whether the communication of potential radiation risks from nuclear medicine examinations
to patients, which is required by law, is adequate.
Methods
We performed an audit to assess the adequacy of communication to patients, with two targets: (1) they
received sufficient information about the potential radiation risks; and (2) they understood the information before they
consented to the examination. We aimed at 100% of patients achieving both targets. If they did not, we planned to
implement changes to bring our practice in line with these standards. A total of 53 patients undergoing examinations
during a randomly selected week were recruited to fill out a questionnaire.
Results
The audit showed that the targets were not achieved, with only 45% of the participants (95% confidence
interval = 33-59%) reporting that they both received sufficient information and understood the potential risks. A
series of changes were implemented, including distribution of a newly designed one-page information pamphlet to
all participants, provision of a newly designed one-page reference sheet to the clinical team, and design of a new
workflow for radiographers. Another 53 patients were recruited for re-audit, and the effect of the changes was
assessed by comparing the results between the audit and re-audit, using the Chi squared test. These changes were
associated with statistically significant improvements in both targets from 45% to 100% (p < 0.0001).
Conclusion
When patients are provided with an easy-to-understand information pamphlet and the clinical team are
instructed to assist patients in understanding the information, the communication targets are achievable.
Key Words: Informed consent; Nuclear medicine; Clinical audit; Radiation; Communication
中文摘要
臨床審計:我們有否向接受核子醫學檢查的病人充分說明潛在的輻射風險?
陳德光、歐陽定勤、龔本霆、許殷豪
簡介
法律上我們須向接受核子醫學檢查的病人充分說明潛在的輻射風險。本臨床審計旨在評估相關溝通是否足夠。
方法
充分的溝通須達致兩個目標:(一)病人接收到有關潛在輻射風險的充分資訊;(二)他們在同意檢查前充分了解潛在的輻射風險。我們的目標是100%病人接收到並充分了解有關潛在輻射風險的資訊。如果未能實踐目標,我們將推行一系列改善措施並再評估成效。本審計在隨機選擇的一周內共招募了53名接受檢查的病人填寫問卷。
結果
審計結果顯示我們未能實踐目標,只有45%的審計參與者(95%置信區間 = 33-59%)表示他們收到並了解有關潛在輻射風險的資訊。因此,我們推行一系列改善措施,包括向所有檢查參與者派發新設計的一頁輻射風險資訊小冊子,向臨床團隊提供新設計的一頁參考資訊,以及為放射技師設計新的工作流程。其後,我們招募了另外53名接受檢查的病人填寫問卷,並透過卡方檢驗比較審計和改善後重新審計之間的差異。結果顯示我們的改善措施將審計達標率從45%提升至100%(統計學上顯著差異p < 0.0001)。
結論
向病人提供易於理解的資訊小冊子,並指導臨床團隊幫助病人理解資訊,審計目標將可得以實踐。
INTRODUCTION
Every nuclear medicine examination involves the
administration of a radioactive tracer. There exists
direct epidemiologic evidence of excess cancer risks
in a number of groups exposed to low-dose radiation.[1]
A risk of death of one in one million can be reasonably
considered negligible, since we are exposed to many risks
of such magnitude every day, such as from travelling by
car.[2] On the other hand, it may not be prudent to ignore
a risk of death of about one in one thousand, which may
correspond to potential additional life-long risk of cancer
for an adult patient undergoing thallium scintigraphy.[3]
The Royal Society of the UK stated that a risk of
death of one in one thousand can be acceptable so
long as an individual patient knows the risk, receives
commensurable benefit, and understands that efforts are
made to minimise the risk.[4] In Montgomery v Lanarkshire
Health Board, the Supreme Court of the UK affirmed the
doctors’ duty to disclose material risks related to medical
treatments.[5] The common law duty for doctors to disclose
risk-related information was further affirmed in Hii Chii
Kok v Ooi Peng Jin London Lucien and National Cancer
Centre of Singapore Pte Ltd. by the Singaporean Court of Appeal.[6] Such legal duty is explicitly promulgated
in the Code of Professional Conduct published by the
Medical Council of Hong Kong (MCHK), which states
that informed consent must be obtained for all diagnostic
procedures: ‘The explanation should cover not only
significant risks, but also risks of serious consequence
even though the probability is low (i.e., low probability
serious consequence risks).’[7]
A literature review showed that 87% of patients were
of the view that potential radiation risks should be
discussed with them before imaging.[8] Only 29% of
patients in four different studies reported being informed
about the potential radiation risks of the computed
tomography (CT) they underwent.[8] Only 22% of
physicians reported providing this information to their
patients.[8] In a survey of radiology department chairs,
15% reported that their departments regularly informed
patients about potential radiation risks.[8] In fact, many
medical doctors requesting radiological examinations
do not have adequate understanding about the potential
radiation risks. A survey showed that only 22% of
emergency department physicians estimated the dose
from CT correctly.[9] Another study showed only 34% of non-radiologist medical doctors correctly estimated the
effective radiation dose from a thoracic CT.[10]
In our institute, we inform patients on a notice board
that in general the radiation dose of the radiotracer used
in our examinations is very low and will reduce to zero
over time very quickly. Patients are informed that the
radiation dose is so low that it does not cause harm to
them and is near that of a chest radiograph. The same
message is also delivered to patients through a one-page
pamphlet for patients undergoing bone scintigraphy.
This audit was a quality assurance project intended to
assess the adequacy of communication to patients in our
institute undergoing examinations (excluding positron
emission tomography) about the potential radiation risks
to comply with the legal and professional standards that
they should all (1) receive sufficient information about
the radiation risks, and (2) understand the information
before they consent to examinations. If necessary, we
aimed to take action to bring our practice in line with
these standards so as to safeguard patients’ right to
autonomy.
METHODS
All patients aged 18 to 70 years undergoing examinations in our institute during a randomly selected week were
consecutively recruited to fill out a questionnaire
(online supplementary Appendix 1). The data items to be collected
included patient demographics, type of nuclear medicine
examination, and whether they agreed that they had
(1) received and (2) understood the information about
potential radiation risks. We also enquired of the
participants whether they considered information about
the potential radiation risks of the examination important.
Standards
The targets were that 100% of participants agreed that
they had (1) received and (2) understood the information.
Indicators to be measured were the proportion of patients
who agreed that they received sufficient information
about the potential radiation risk of the examination
they were to undergo, and that they understood the
information before they consented to undergo the nuclear
medicine examination. If either one of the targets was
not achieved, a re-audit would be performed following
implementation of a series of changes.
Statistical Analyses
The sample size was calculated using Power Analysis
and Sample Size 2021 (PASS 21.0.3, LLC, Kaysville [UT], US). A total of 82 subjects were needed, using
the Chi squared test for independence, with one degree
of freedom, type 1 error = 0.05, power = 0.95, for
detection of 20% of difference after implementation of
changes (assuming a proportion of 50% under the null
hypothesis). The Chi squared test was performed using
Number Cruncher Statistical Systems (NCSS 21.0.3,
LLC, Kaysville [UT], US).
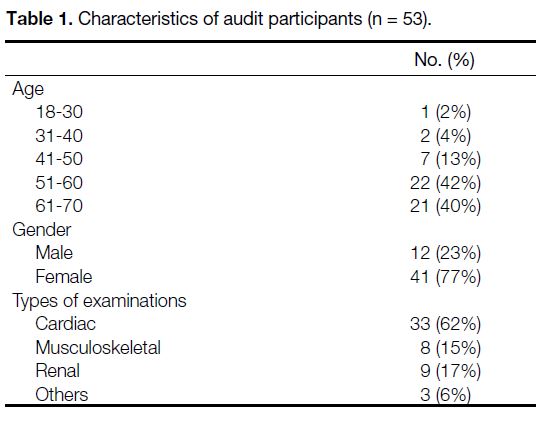
RESULTS
The audit was conducted from 15 February 2021 to 19
February 2021. A total of 54 participants fulfilled the
inclusion criteria. One participant, who had impaired
cognitive function, was excluded. Fifty-three of them
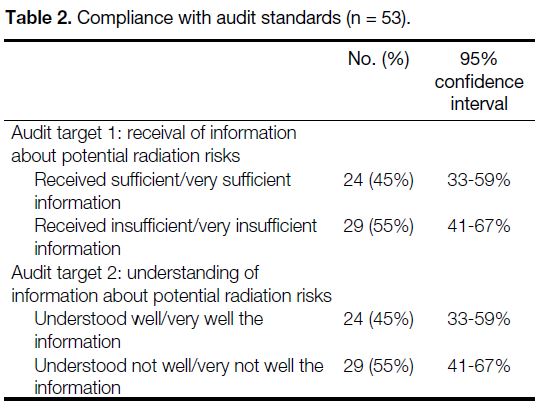
completed the survey (Table 1). Only 45% of the
participants (95% confidence interval = 33-59%)
considered that they received sufficient/very sufficient
information regarding the potential radiation risks
of the examination they underwent. Also, only 45% of participants (95% confidence interval = 33-59%)
considered that they understood well/very well the
radiation risk of the examination (Table 2). Both fell
short of the audit targets. It was therefore necessary to
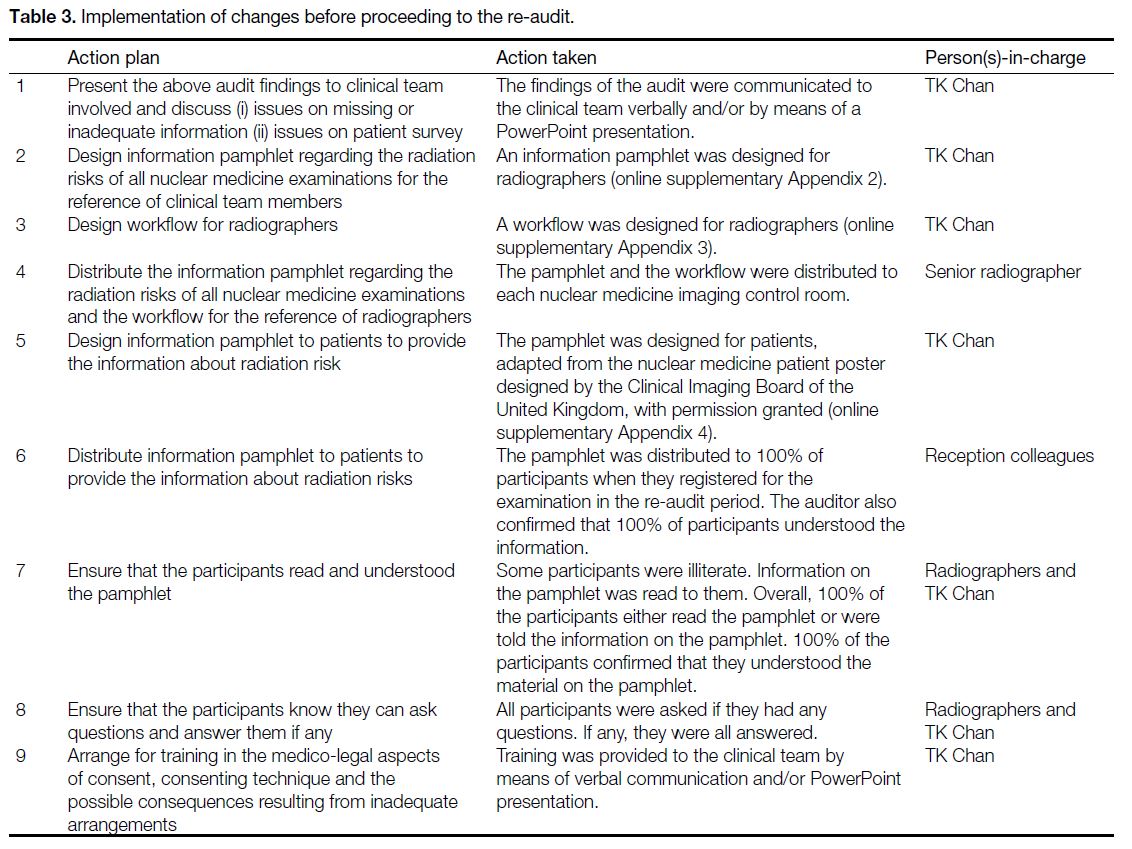
implement changes (Table 3) and proceed to re-audit.
Table 1. Characteristics of audit participants (n = 53).
Table 2. Compliance with audit standards (n = 53).
Table 3. Implementation of changes before proceeding to the re-audit.
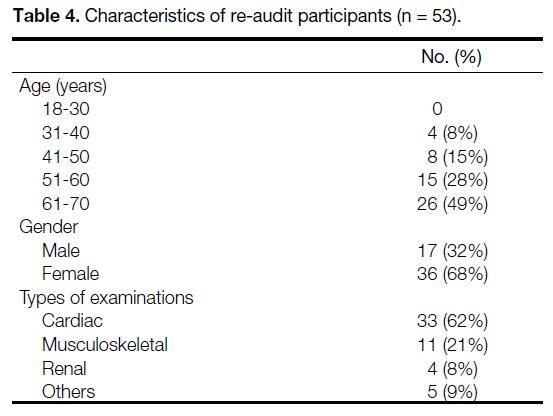
The re-audit was conducted from 22 to 26 February 2021.
A total of 54 participants fulfilled the inclusion criteria.
One participant, who had impaired cognitive function,
was excluded. Fifty-three of them completed the survey,
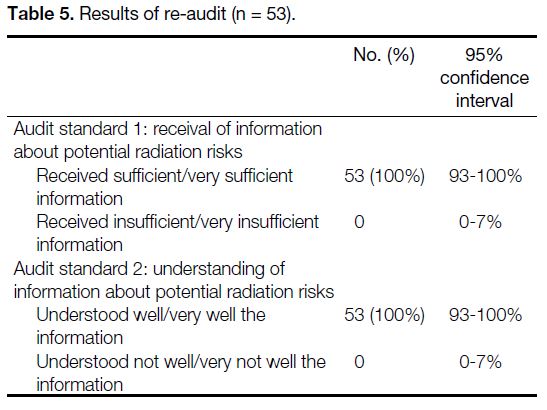
response rate being 100% (Table 4). In the re-audit,
subsequent to implementation of the changes described
in Table 3, there were significant improvements in the
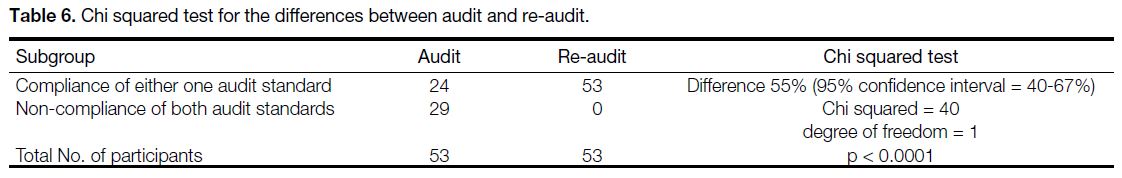
compliance with the audit targets (Tables 5and 6).
The change was found to be statistically significant
(p < 0.0001). Four of the participants (8%) needed
further explanation of the information after reading
the information pamphlet. All participants considered
that they were provided sufficient or very sufficient
information about the radiation risks of the examination they underwent. Also, 100% of participants considered
that they understood well or very well about the radiation
risks of the examination they underwent. This was in
full compliance with the audit standards that 100% of participants should receive sufficient information and
understand it.
Table 4. Characteristics of re-audit participants (n = 53).
Table 5. Results of re-audit (n = 53).
Table 6. Chi squared test for the differences between audit and re-audit.
Out of a total of 106 participants from the audit and re-audit, 105 participants (99%) considered it important
or very important for them to know and understand the
radiation risks of their examination.
DISCUSSION
Approaches to Radiation Risks Disclosure
There are three current strategies of risk disclosure for examinations: no mention, understatement, and full
disclosure. One philosophy is not to mention potential
radiation risks. The basic argument is that radiologists are
too busy to lose time in obtaining informed consent and
too wise to undertake inappropriate examinations. The
long-term nature of the risk and/or its minimal to mild
magnitude appear to provide an excuse for overlooking
the issue of informed consent.[1]
Another approach is to understate the potential risks. We commonly read statements such as ‘a nuclear medicine
examination is safe, with an irradiation corresponding
to a simple radiograph’ or ‘almost always less than a
common radiological examination’. Both patients and
clinicians might believe that a simple radiograph would
be a chest radiograph. In reality, however, the radiation dose ranges from 50 chest radiographs for thyroid
scintigraphy to 4000 chest radiographs for a cortical
adrenal gland scintigraphy.[1]
In the alternative, the potential radiation risks can be fully
disclosed. This approach was more strictly required in the research setting.[11]
This is the approach we used in our implementation of
change. We mentioned in our newly designed patient
pamphlet that ‘The amount of radiation in a nuclear
medicine examination varies, but is less than 5 years’
duration of your natural exposure. The radiation risks
are negligible to low.’ We made it clear in a remark that
‘There is a 20% life time chance of developing a fatal
cancer in the general population. A nuclear medicine
examination may expose an adult patient to less than or
much less than 0.1% extra chance’ (online supplementary Appendix 4).
International Ethical Principles
The World Medical Association’s Medical Ethics
Manual provides that informed consent is one of the
central concepts of present-day medical ethics: ‘The
patient has the right to … make free decisions regarding
himself/herself. The physician will inform the patient of
the consequences of his/her decisions.’[12]
Prevailing Legal Paradigm
In law, a medical professional must serve a patient’s best
interests. It was established in the UK case Airedale NHS
Trust v Bland that ‘the best interests of the patient are
served by respecting the patient’s wishes.’[13] In order for
patients to make informed decisions, the Montgomery
case affirmed doctors’ duty to disclose material risks
related to medical treatments,[5] citing Lord Woolf MR
as saying in another UK case Pearce v United Bristol
Healthcare NHS Trust: ‘[I]f there is a significant risk
which would affect the judgment of a reasonable patient,
it is the responsibility of a doctor to inform the patient
of that significant risk, if the information is needed so
that the patient can determine for him or herself as to
what course he or she should adopt.’[14] Insofar as what
amounts to a significant risk, it was said in the UK
case Wyatt v Curtis that ‘a risk which … doubles, or at least enhances, the background risk of a potentially
catastrophic abnormality may well be both substantial
and grave, or at least sufficiently real’ for a patient to be
told about.[15]
It was further established in the Hii Chii Kok case that ‘material information should not be limited to risk-related
information’. This should include all information
that ‘may be needed to enable patients to make informed
decision about their health ... [A]s to what exactly it is
about the various types of information that would be
considered relevant or material, in our judgment, this is
largely a matter of common sense.’[6]
The International Covenant on Economic, Social and
Cultural Rights of the United Nations provides that the
right to health is an inclusive right, in which the entitlement
includes provision of health-related information.[16] Such
right and entitlement is also protected in Article 39 of the
Basic Law of Hong Kong.[17]
Local Professional and Corporate Guidelines
Locally, the Code of Professional Conduct published
by the MCHK provides that informed consent must be
obtained for all diagnostic procedures.[7] Consent may
be either implied or expressed, and is valid only if the
doctor has provided proper explanation of the nature,
effects, and risks of the proposed procedure and/or
treatment.[7] The explanation has to cover ‘risks of serious
consequence even though the probability is low (i.e.,
low probability serious consequence risks)’.[7] The doctor
must ensure that the patient understands the explanation.[7]
The local Hospital Authority Head Office Operations
Circular No. 19/2015 ‘Update on HA Informed Consent
for Operation/Procedure/Treatment’ has largely adopted
the relevant provisions in the Code of Professional
Conduct published by the MCHK.[18]
Is Potential Radiation Risk Material?
Since the 1970s, the potential risk of radiation has been
estimated using the linear non-threshold (LNT) model,
which assumes a linear relationship between radiation
dose and cancer risk. Much of the evidence on radiation-induced
cancer risks came from a Japanese atomic
bomb survivors’ lifespan study and studies of medically
exposed populations.[19] As the LNT model implies that
even the smallest dose may trigger carcinogenesis, there
have been many challenges to its scientific basis in the
last two decades.[20] [21] [22] Some studies even demonstrated
health benefits of low-dose radiation exposure, including reduced cancer incidence and increased longevity.[23] The
prudent use of radiation by means of ALARA (as low
as reasonably achievable) approach is further criticised
as being radiophobic.[21] A model suggesting protective
effects at imaging radiation levels (radiation hormesis)
was proposed to counter the LNT model (harm at any
dose).[21]
This said, there exists direct epidemiologic evidence
of excess cancer risks in a number of groups exposed
to low radiation doses.[1] [19] [24] The controversy remains
unresolved. The French Academy of Sciences report
concluded that the use of LNT model is not based on
scientific evidence.[25] In contrast, the Biological Effects of
Ionizing Radiation VII report and that of the International
Commission on Radiological Protection endorsed the use
of the LNT model.[26] [27] After all, prevailing radiology and
nuclear medicine professional guidelines still embrace
the principle of ALARA inherited from the LNT model.
While some criticised the use of LNT model as ignoring
the huge benefits of imaging, promoting fear and imaging
avoidance,[21] patients are entitled to be informed of the
potential radiation risks estimated by the LNT model.
A medical doctor cannot be faulted for overcaution so
far as patients are told of the potential nature of the risk
and also the substantial benefits of the examination. The
law has made it clear that the best interests of a patient
are served by respecting his or her wishes.[13] In law, a
doctor is allowed to withhold from a patient a material
risk ‘only if he reasonably considers that its disclosure
would be seriously detrimental to the patient’s health...’.
However, this does not enable a ‘doctor to prevent the
patient from making an informed choice where [he/] she
is liable to make a choice which the doctor considers to
be contrary to [his/] her best interests.’[5]
All of our participants except one considered information
about potential radiation risks of examinations important
or very important. We fully concur with the Court of
Appeal in Singapore that the judgement is ‘a matter of
common sense’.[6] Any possible increased risk of getting a
fatal cancer, the controversy granted, must still be ‘both
substantial and grave’ for a reasonable patient.[15] In
short, the ethical and legal authorities, local professional
code and corporate guideline unequivocally require that
nuclear medicine physicians should adopt the approach
of full disclosure regarding potential radiation risks. Our
results indicate that such standard is achievable through
changes.
Should Patients be Asked to Sign a Consent
Form?
The law does not require that consent has to be in
writing. A valid consent can even be implied. In many
minor examinations or procedures patients give implied
consent. For example, the patient lies on the examination
couch when the doctor proceeds to conduct a physical
examination; or the patient may roll up a sleeve and offer
an arm when the doctor proceeds to take a blood sample.
Mandatory written informed consent may result in undue
anxiety and occasional refusal of examinations that may
be lifesaving when in fact the radiation risk is minimal.
So far as a patient understands the information about
the radiation risk, given the large volume of workload
in the nuclear medicine centre, it is reasonable and fair
to proceed with the examination on the basis of implied
consent. It is relevant to note that the MCHK’s Code
of Professional Conduct also stipulates that for non-invasive
treatments, consent can usually be implied.[7]
Limitations
The clinical team was not blinded to the participant
group. Reporting bias may have been introduced when
the informed consent process took place during the audit
period and when some less educated patients needed
prompts in answering the questions.
Also, the audit was conducted based on subjective
answers from the participants. What the participants
subjectively consider sufficient may not be sufficient
from legal and professional perspectives. What the
participants thought they understood also may not
represent actual and complete understanding of the
information.
CONCLUSIONS
Despite the target that patients know and understand
the potential radiation risks of their examination, our
previous practice fell short of it frequently. Our audit
results demonstrate that once a patient is provided
sufficient information in the form of a pamphlet and
the clinical team is given instructions to assist patients
in understanding the information, the standard is
achievable.
Future research can be conducted amongst the
stakeholders to investigate how much information to
give, how best to provide it, when to provide it, whom
to provide it to, with a view to perfecting the informed
consent process.
REFERENCES
1. Berrington de González A, Darby S. Risk of cancer from diagnostic
X-rays: estimates for the UK and 14 other countries. Lancet.
2004;363:345-51. Crossref
2. Picano E. Informed consent and communication of risk from
radiological and nuclear medicine examinations: how to escape
from a communication inferno. BMJ. 2004;329:849-51. Crossref
3. Overbeek FJ, Pauwels EK, Bloem JL, Camps JA, Geleijns J,
Broerse JJ. Somatic effects in nuclear medicine and radiology.
Appl Radiat Isot. 1999;50:63-72. Crossref
4. Lawton MP. Legal aspects of iatrogenic disorders: discussion paper.
J R Soc Med. 1983;76:289-91. Crossref
5. Montgomery v Lanarkshire Health Board. UKSC 11; 2015.
6. Hii Chii Kok v (1) Ooi Peng Jin London Lucien; (2) National Cancer Centre of Singapore Pte Ltd. SGCA 38; 2017.
7. Medical Council of Hong Kong. Code of Professional Conduct.
Available from: https://www.mchk.org.hk/english/code/files/Code_of_Professional_Conduct_.... Accessed 15 Jun 2021.
8. Lam DL, Larson DB, Eisenberg JD, Forman HP, Lee CI.
Communicating potential radiation-induced cancer risks from
medical imaging directly to patients. AJR Am J Roentgenol.
2015;205:962-70. Crossref
9. Lee CI, Haims AH, Monico EP, Brink JA, Forman HP. Diagnostic
CT scans: assessment of patient, physician, and radiologist
awareness of radiation dose and possible risks. Radiology.
2004;231:393-8. Crossref
10. Heyer CM, Peters S, Lemburg S, Nicolas V. Awareness of radiation exposure of thoracic CT scans and conventional radiographs: what do non-radiologists know? [in German]. Rofo. 2007;179:261-7. Crossref
11. Office for Protection from Research Risks, Department of Health
and Human Services, US Government. Code of federal regulations.
Title 45: public welfare. Department of Health and Human Services.
Part 46 protection of human subjects. Bethesda, MD: National
Institutes of Health; 2009. Available from: https://www.hhs.gov/ohrp/sites/default/files/ohrp/policy/ohrpregulations.... Accessed 10 Jan 2021.
12. World Medical Association. Medical Ethics Manual. Chapter two—Physicians and Patients. Available from: https://www.wma.net/wp-content/uploads/2016/11/Ethics_manual_3rd_Nov2015.... Accessed 13 Jan 2021.
13. Airedale NHS Trust v Bland. AC 789; 1993.
14. Pearce and Pearce v United Bristol Healthcare NHS Trust. PIQR P 53; 1999.
15. Sarah Wyatt v Dr Anne Curtis. EWCA Civ 1779; 2003.
16. Office of the United Nations High Commissioner for Human Rights and World Health Organization. The right to health. Available from:
https://www.ohchr.org/sites/default/files/Documents/Publications/Factshe.... Accessed 13 Jan 2021.
17. Hong Kong SAR Government. Basic Law. Available from: https://www.basiclaw.gov.hk/en/basiclaw/index.html. Accessed 15 Jun 2021.
18. Hospital Authority. Head Office Operations Circular No. 19/2015:
Update on HA informed consent for operation/procedure/treatment. Hong Kong: Hospital Authority; 2015.
19. Little MP, Wakeford R, Tawn EJ, Bouffler SD, Berrington de
Gonzalez A. Risks associated with low doses and low dose rates
of ionizing radiation: why linearity may be (almost) the best we
can do. Radiology. 2009;251:6-12. Crossref
20. Tubiana M, Feinendegen LE, Yang C, Kaminski JM. The linear
no-threshold relationship is inconsistent with radiation biologic
and experimental data. Radiology. 2009;251:13-22. Crossref
21. Siegel JA, Pennington CW, Sacks B. Subjecting radiologic imaging to the linear no-threshold hypothesis: a non sequitur of non-trivial
proportion. J Nucl Med. 2017;58:1-6. Crossref
22. Chen WL, Luan YC, Shieh MC, Chen ST, Kung HT, Soong KL,
et al. Effects of cobalt-60 exposure on health of Taiwan residents
suggest new approach needed in radiation protection. Dose
Response. 2007;5:63-75. Crossref
23. Sacks B, Meyerson G, Siegel JA. Epidemiology without biology:
false paradigms, unfounded assumptions, and specious statistics in
radiation science (with commentaries by Inge Schmitz-Feuerhake
and Christopher Busby and a reply by the authors). Biol Theory.
2016;11:69-101. Crossref
24. Valentin J. Low-dose extrapolation of radiation-related cancer risk. Ann ICRP. 2005;35:1-140. Crossref
25. Tubiana M. Dose-effect relationship and estimation of the
carcinogenic effects of low doses of ionizing radiation: the joint
report of the Académie des Sciences (Paris) and of the Académie
Nationale de Médecine. Int J Radiat Oncol Biol Phys. 2005;63:317-9. Crossref
26. National Research Council, Committee to Assess Health Risks from
Exposure to Low Levels of Ionizing Radiation. Health risks from
low levels of ionizing radiation: BEIR VII, Phase 2. Washington,
DC: The National Academies Press; 2006.
27. International Commission on Radiological Protection. ICRP
Publication 99. Low-dose extrapolation of radiation-related cancer
risk. Amsterdam, the Netherlands: Elsevier; 2006.