Metastatic Invasive Lobular Breast Carcinoma Mimicking Obstructive Primary Colonic Malignancy: A Case Report
CASE REPORT
Hong Kong J Radiol 2023 Sep;26(3):e19-23 | Epub 14 Aug 2023
Metastatic Invasive Lobular Breast Carcinoma Mimicking Obstructive Primary Colonic Malignancy: A Case Report
FFY Wan1, TWY Chin1, WK Ho2, YK So3
1 Department of Radiology and Imaging, Queen Elizabeth Hospital, Hong Kong SAR, China
2 Department of Clinical Oncology, Queen Elizabeth Hospital, Hong Kong SAR, China
3 Department of Pathology, Queen Elizabeth Hospital, Hong Kong SAR, China
Correspondence: Dr FFY Wan, Department of Radiology and Imaging, Queen Elizabeth Hospital, Hong Kong SAR, China. Email: wfy471@ha.org.hk
Submitted: 14 Jul 2022; Accepted: 15 Nov 2022.
Contributors: All authors designed the study and acquired and analysed the data. FFYW and YKS drafted the manuscript. FFYW, TWYC
and WKH critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study,
approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: This study was approved by the Kowloon Central Cluster / Kowloon East Cluster Research Ethics Committee of Hospital
Authority, Hong Kong (Ref No.: KC/KE-22-0098-ER-2). Patient consent for all treatments, procedures, and publication was obtained.
INTRODUCTION
Worldwide, breast cancer is the most common cancer
in women. Distant metastases primarily involve the
lungs, bones, liver and brain, while metastasis to the
gastrointestinal tract is extremely rare.[1] We present the radiological and pathological findings in a patient with metastatic invasive lobular breast carcinoma (ILC) and
initial presentation as an obstructing colonic tumour.
CASE REPORT
A 40-year-old woman with good past health presented
with a 2-week history of repeated vomiting. Abdominal
radiograph revealed multiple dilated large bowel loops.
Urgent computed tomography of the abdomen and pelvis
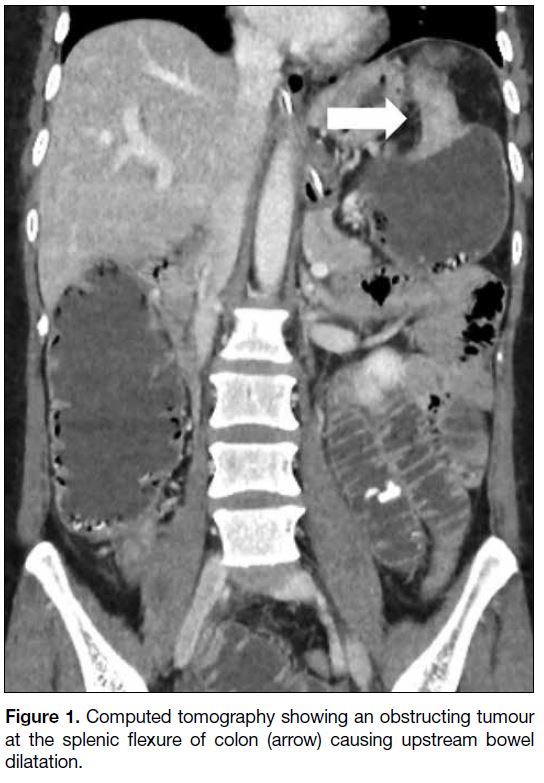
revealed a circumferential colonic tumour at the splenic
flexure causing upstream bowel dilatation (Figure 1)
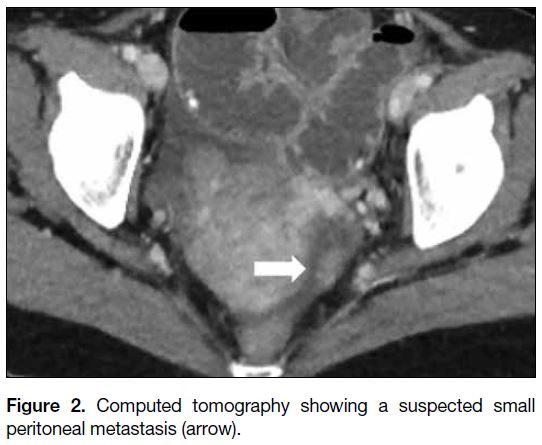
and a solid peritoneal nodule suspicious of metastasis
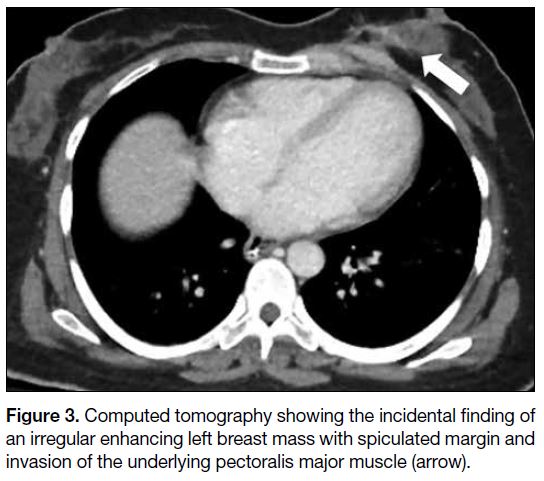
(Figure 2). An enhancing irregular left breast mass with
spiculated margin, invading the underlying pectoralis
major muscle, was noted incidentally on the computed
tomography scan (Figure 3). The patient subsequently underwent emergency surgery that revealed an
obstructing circumferential tumour at the splenic flexure
of the colon and multiple small peritoneal nodules.
Extended right hemicolectomy was performed.
Figure 1. Computed tomography showing an obstructing tumour at the splenic flexure of colon (arrow) causing upstream bowel dilatation.
Figure 2. Computed tomography showing a suspected small peritoneal metastasis (arrow).
Figure 3. Computed tomography showing the incidental finding of an irregular enhancing left breast mass with spiculated margin and invasion of the underlying pectoralis major muscle (arrow).
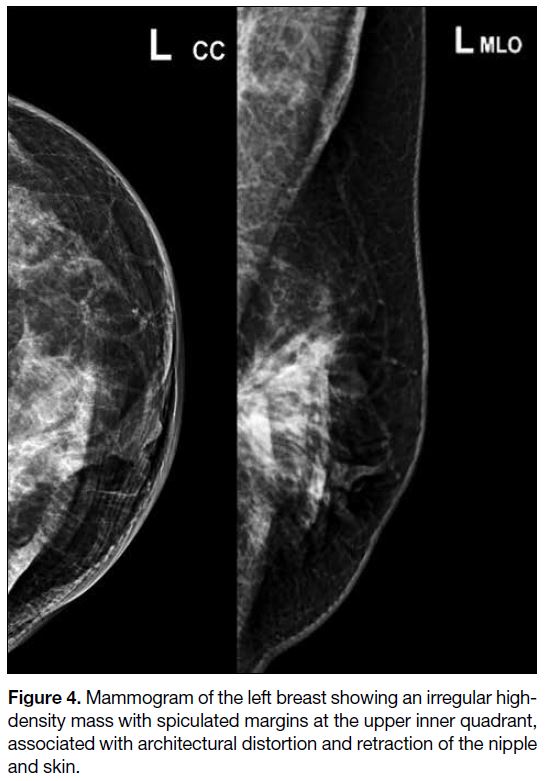
Mammography and breast ultrasonography were
performed 1 week postoperatively. The former showed
an irregular high-density mass with spiculated margins
at the upper inner quadrant of the left breast, associated
with architectural distortion and retraction of the nipple
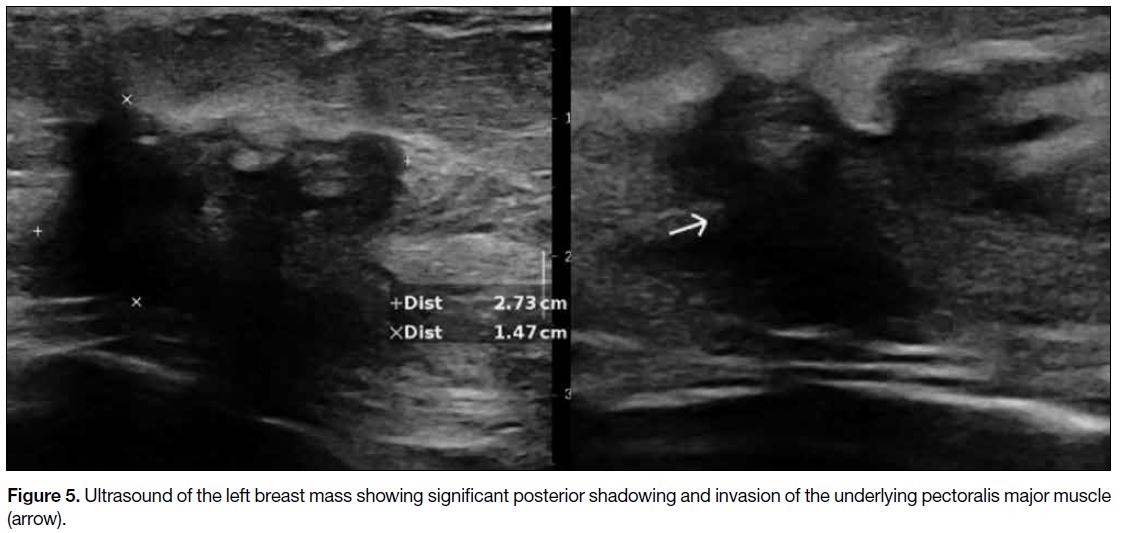
and skin (Figure 4). On ultrasound, the corresponding
left breast mass was hypoechoic with irregular shape,
spiculated margins, posterior shadowing, architectural
distortion, and invasion of the underlying pectoralis
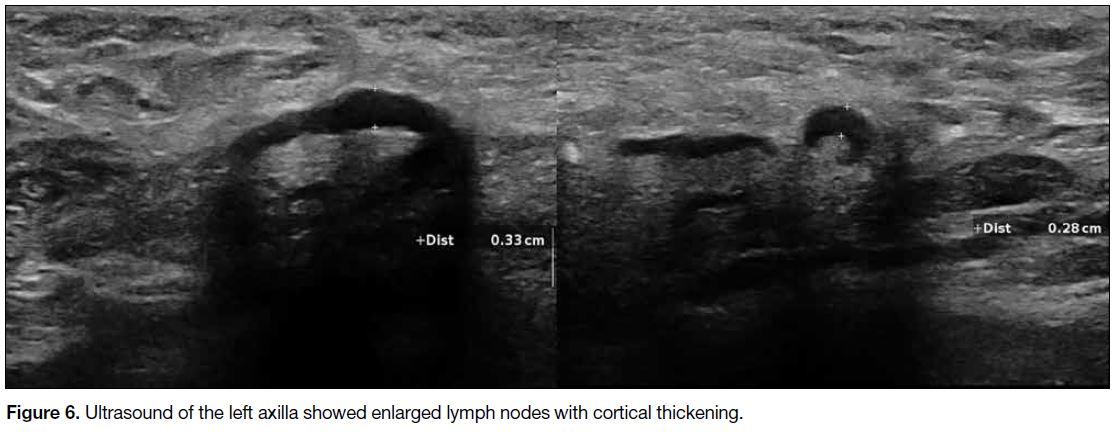
major muscle (Figure 5). Multiple enlarged left axillary
lymph nodes with cortical thickening were suspicious of
nodal involvement (Figure 6).
Figure 4. Mammogram of the left breast showing an irregular high-density mass with spiculated margins at the upper inner quadrant, associated with architectural distortion and retraction of the nipple and skin.
Figure 5. Ultrasound of the left breast mass showing significant posterior shadowing and invasion of the underlying pectoralis major muscle (arrow).
Figure 6. Ultrasound of the left axilla showed enlarged lymph nodes with cortical thickening.
The initial clinical diagnosis was synchronous colon and
breast cancer. Nonetheless pathological examination of
the colonic tumour showed histological features and an immunohistochemical profile (GATA binding protein
3 and oestrogen and progesterone receptor positive;
E-cadherin negative) suggestive of a primary breast
ILC (Figure 7). The full-thickness circumferential
involvement of the colonic wall from the mucosa to serosa and the absence of adjacent peritoneal nodule
were consistent with haematogenous metastasis to the
colon. A section of an intraoperative peritoneal nodule
revealed fibroadipose tissue infiltrated with metastatic
carcinoma and morphology similar to that of the colonic tumour. Subsequent ultrasound-guided core biopsy of
the left breast mass using a 14-G Tru-Cut biopsy needle
(Achieve Automatic Biopsy Device; Merit Medical,
South Jordan [UT], United States) and fine-needle
aspiration of the left axillary lymph node using a 21-G
needle confirmed ILC with ipsilateral axillary lymph
node metastasis. Stage IV breast cancer with colonic
metastasis was confirmed and the patient was referred
for consideration of systemic treatment.
Figure 7. Histological examination of the colon specimen. Haematoxylin and eosin staining showed neoplastic infiltration with mucosal [(a), ×10] and muscularis propria [(b), ×4] involvement. Immunohistochemical staining (×20) showed positive results for GATA binding protein 3 (c), oestrogen receptor (d), progesterone receptor (e), and negative result for E-cadherin (f).
DISCUSSION
ILC is the second most common histological type
of invasive breast carcinoma after invasive ductal carcinoma (IDC), accounting for 5% to 15% of all
breast cancers.[1] It is known to have an atypical pattern of
metastatic spread and diffuse growth. Positron emission
tomography–computed tomography (PET-CT) is useful
for systemic staging of breast cancer. Nonetheless not all
ILCs are hypermetabolic on 18F-flurodeoxyglucose PET-CT.
Since nearly 95% of ILCs are oestrogen receptor–positive, a novel PET tracer targeting the oestrogen
receptor (18F-fluoroestradiol) has shown promising
results in detecting metastases in ILC.[2]
Compared with IDC, ILC has a higher propensity to
metastasise to unusual sites such as the gastrointestinal tract, peritoneum, and ovaries.[3] Despite the higher
prevalence of IDC (90%) among patients with breast
cancer, most gastrointestinal metastases are from ILC.[3]
There is currently no clear explanation for the difference
in metastatic patterns of ILC and IDC. It has been
postulated that the loss of E-cadherin in ILC, which is
responsible for cell-to-cell adhesion, accounts for its
more invasive behaviour.[4]
Among the cases of ILC with gastrointestinal
metastases, stomach and small intestine are the more
frequent sites of metastases compared with the colon.
Gastrointestinal metastases are usually metachronous
and develop after diagnosis of a breast primary. In a case
series of gastrointestinal metastases from ILC, the mean
interval between diagnosis of primary breast cancer and
gastrointestinal metastasis was 4 years.[5] Our patient with ILC and initial presentation as large bowel obstruction
due to colonic metastasis is extremely rare. It should
be noted that endoscopic biopsy of gastrointestinal
metastases has a higher false negative rate compared
with primary colonic cancer because of the relatively
late involvement of the avascular mucosal layer by the
metastatic tumour. Once a diagnosis of gastrointestinal
metastasis has been made, the 5-year survival rate is
reported to be 29%.[5] This is similar to the rate of 25%
reported by the National Cancer Institute for metastatic
breast cancer.[6]
Since both colonic metastases from breast cancer
and primary colonic carcinoma can have a similar
macroscopic appearance, the presence of a suspicious
breast lesion or a history of breast ILC should raise
the suspicion of gastrointestinal metastasis. Further
pathological examination is warranted with appropriate
immunohistochemical staining. In our case, positivity of
GATA binding protein 3,[7] oestrogen and progesterone
receptors suggested a breast primary, while negative
staining for E-cadherin was suggestive of ILC.[8]
CONCLUSION
To the best of our knowledge, this is the first report in the literature of ILC with initial presentation as obstructing
colonic metastasis. It highlights the unique feature of
ILC of its potential for gastrointestinal tract involvement.
Knowledge of the pattern of spread of ILC raises clinical suspicion and can guide subsequent investigation and management.
REFERENCES
1. Chow CE, Cendan JC, Herrmann G, Richardson L, Benda RK. Metastatic lobular breast cancer presenting with malignant ascites: case report and review of literature. Breast J. 2003;9:414-6. Crossref
2. Ulaner GA, Jhaveri K, Chandarlapaty S, Hatzoglou V, Riedl CC, Lewis JS, et al. Head-to-head evaluation of 18F-FES and 18F-FDG PET/CT in metastatic invasive lobular breast cancer. J Nucl Med. 2021;62:326-31. Crossref
3. Mathew A, Rajagopal PS, Villgran V, Sandhu GS, Jankowitz RC, Jacob M, et al. Distinct pattern of metastases in patients with invasive lobular carcinoma of the breast. Geburtshilfe Frauenheilkd.
2017;77:660-6. Crossref
4. Arpino G, Bardou VJ, Clark GM, Elledge RM. Infiltrating lobular carcinoma of the breast: tumor characteristics and clinical outcome. Breast Cancer Res. 2004;6:R149-56. Crossref
5. Switzer N, Lim A, Du L, Al-Sairafi R, Tonkin K, Schiller D. Case series of 21 patients with extrahepatic metastatic lobular breast carcinoma to the gastrointestinal tract. Cancer Treat Commun. 2015;3:37-43. Crossref
6. National Cancer Institute, the United States Government. SEER Cancer Statistics Review, 1975-2011. 2014. Available from: https://seer.cancer.gov/archive/csr/1975_2011/results_single/sect_28_table.03.pdf. Accessed 1 Jul 2022.
7. Miettinen M, McCue PA, Sarlomo-Rikala M, Rys J, Czapiewski P, Wazny K, et al. GATA3: a multispecific but potentially useful marker in surgical pathology: a systematic analysis of 2500
epithelial and nonepithelial tumors. Am J Surg Pathol. 2014;38:13-22. Crossref
8. Siitonen SM, Kononen JT, Helin HJ, Rantala IS, Holli KA, Isola JJ. Reduced E-cadherin expression is associated with
invasiveness and unfavorable prognosis in breast cancer. Am J
Clin Pathol. 1996;105:394-402. Crossref