Treatment Outcomes and Toxicities of Stereotactic Body Radiotherapy for Oligoprogressive Metastatic Non–Small-Cell Lung Cancer
ORIGINAL ARTICLE CME
Hong Kong J Radiol 2023 Dec;26(4):248-54 | Epub 23 Nov 2023
Treatment Outcomes and Toxicities of Stereotactic Body Radiotherapy for Oligoprogressive Metastatic Non–Small-Cell Lung Cancer
KKS Wong, TY Kam, MW Yeung, SI Soong
Department of Clinical Oncology, Pamela Youde Nethersole Eastern Hospital, Hong Kong SAR, China
Correspondence: Dr KKS Wong, Department of Clinical Oncology, Pamela Youde Nethersole Eastern Hospital, Hong Kong SAR, China. Email: wks447@ha.org.hk
Submitted: 9 Oct 2022; Accepted: 20 Jan 2023.
Contributors: All authors designed the study. KKSW and TYK acquired and analysed the data. All authors drafted the manuscript and critically
revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the final
version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflict of interest.
Funding/Support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: This research was approved by the Hong Kong East Cluster Research Committee of Hospital Authority, Hong Kong (Ref No.:HKECREC2022032). Informed consent was waived by the Committee due to the retrospective nature of the study.
Abstract
Introduction
This study reviewed the toxicities and outcomes of stereotactic body radiotherapy (SBRT) for oligoprogressive metastatic non–small-cell lung cancer (NSCLC).
Methods
The cases of patients with oligoprogressive NSCLC receiving SBRT from 2015 to 2020 were reviewed retrospectively. Demographics were analysed by descriptive statistics. Important treatment outcomes including local control and survival were analysed by the Kaplan-Meier method. Simple and multivariable Cox regression analyses were carried out to investigate prognostic factors. Toxicities were reported using the Common Terminology Criteria for Adverse Event version 4.0.
Results
Forty-one cases with 51 oligoprogressive sites were included. The median age of the cohort was 65 years.
The most commonly ablated sites were the lung (68.6%) and bone metastasis (17.6%). The most common driver
mutation was the epithelial growth factor receptor mutation (82.9%). SBRT doses ranged from 30 to 60 Gy in 3 to
10 fractions. Median follow-up time was 64 weeks. SBRT achieved a 1-year local control rate of 85%. Median
progression-free survival (PFS) after SBRT was 8.8 months and median time from SBRT to the next line of systemic
treatment was 9 months. A robust response to pre-SBRT systemic treatment was significantly associated with longer
PFS after SBRT. Median overall survival was 58 months. There was one case of grade 3 pneumonitis (2%) and one
case of rib fracture (2%).
Conclusion
SBRT for oligoprogression in NSCLC is an effective strategy to prolong the time to the next systemic
treatment with minimal toxicities.
Key Words: Carcinoma, non-small-cell lung; Radiation oncology; Radiosurgery; Stereotactic body radiotherapy; Treatment outcome
中文摘要
寡進展轉移性非小細胞肺癌體部立體定向放射治療的療效及毒性
黃嘉誠、甘子揚、楊美雲、宋崧
簡介
本研究回顧寡進展轉移性非小細胞肺癌體部立體定向放射治療(SBRT)的毒性及結果。
方法
本研究回顧2015至2020年間接受SBRT的寡進展非小細胞肺癌患者個案。我們對患者的人口統計資料進行描述性統計,並使用Kaplan-Meier法分析重要的治療結果(包括局部控制及存活)以及簡單及多變量Cox迴歸分析研究預後因素。我們使用常見毒性標準(CTCAE)第4.0版本報告毒性。
結果
本研究包括了41例共51個寡進展部位。患者年齡中位數為65歲,最常見的消融部位是肺部(68.6%)及骨轉移(17.6%)。最常見的驅動基因突變是表皮生長因子受體突變(82.9%)。SBRT劑量介乎30至60 Gy,分3至10次。隨訪時間中位數為64星期。SBRT的一年局部控制率達85%。接受SBRT後的無惡化存活期中位數為8.8個月,而接受SBRT後至下次全身性治療的中位數時間則為9個月。接受SBRT前的全身性治療的顯著反應與較長的接受SBRT後的無惡化存活期顯著相關。整體存活期中位數為58個月。有一例3級肺炎(2%)及一例肋骨骨折(2%)。
結論
寡進展轉移性非小細胞肺癌SBRT在毒性減到最低的情況下能有效延長患者接受下次全身性治療前的存活期。
INTRODUCTION
Stereotactic body radiotherapy (SBRT) is a radiation
technique that delivers a high dose of radiation to a
small tumour target using highly conformal techniques.[1]
It is widely used to treat early-stage non–small-cell
lung cancer (NSCLC) with durable local control (LC)
and a high cure rate.[2] Oligoprogressive disease (OPD)
is defined as a clinical scenario in which there is
initial polymetastatic disease that responds to systemic
treatment until there is development of new subclones
with drug resistance.[3] It refers to a limited number of new
metastases with different authors quoting a range from a
maximum of three to five sites of progression.[4] [5] SBRT
can be used to ablate these resistant clones before they
proliferate and metastasise. Here we report the treatment
outcomes and toxicities of SBRT for oligoprogressive
metastatic NSCLC in our institution.
METHODS
We conducted a retrospective review of 41 patients
who received SBRT for oligoprogressive NSCLC from
1 January 2015 to 31 December 2020. Only patients
who had ≤ 3 foci of radiological progression during
systemic therapy (excluding central nervous system
progression) were included. Demographics were
analysed by descriptive statistics using SPSS (Window
version 23.0; IBM Corp, Armonk [NY], United States). Planning target volumes (PTVs) were generated by the
Eclipse Treatment Planning System (Varian Inc, Palo
Alto [CA], United States). Important treatment outcomes
including LC and survival were analysed by the Kaplan-Meier method. Univariate and multivariate analysis
were used to investigate prognostic factors. Toxicities
were reported using the Common Terminology Criteria
for Adverse Event version 4.0. Treatment response was
monitored by interval computed tomography (CT) or
positron emission tomography/computed tomography
(PET/CT) scan at intervals determined by the patients’
physicians and was reported by the RECIST (Response
Evaluation Criteria in Solid Tumours) version 1.1
criteria. Progression-free survival (PFS) was defined as
the time interval from date of initiation of SBRT to any
progression or death. PFS from the previous systemic
treatment (PFS1) was defined by the time from the start
of the previous systemic treatment to the initiation of
SBRT. Overall survival (OS) was defined as the time
interval from the start of systemic treatment to the date
of death from any cause. Complete follow-up data were
available at the time of analysis. The study adhered to the
STROBE (Strengthening the Reporting of Observational
Studies in Epidemiology) reporting guidelines.
Our radiotherapy treatment protocol followed the
Radiation Therapy Oncology Group trials protocol,[6] [7] the United Kingdom Stereotactic Ablative Radiotherapy
Consortium guidelines,[8] and the American Association
of Physicists in Medicine Task Group 101 report.[9] The
details of treatment simulation scan, image co-registration,
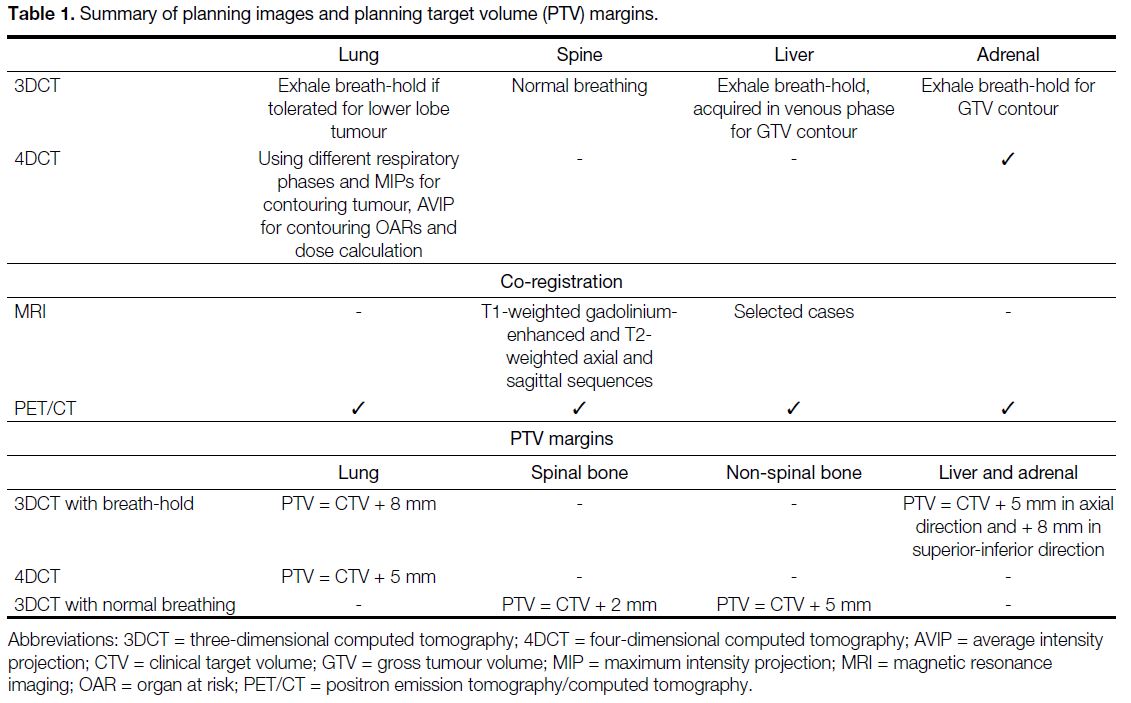
and PTV margins are described in Table 1. For lung
lesions, three-dimensional CT with breath-hold was used
for lower lobe tumours, while four-dimensional CT was
used for upper lobe tumours. Contouring was performed
on different respiratory phases and maximum intensity
projection. Regarding spinal metastases, planning CT
images were co-registered with diagnostic MRI. For liver
and adrenal metastases, we used three-dimensional CT
with breath-hold technique if possible. When PET/CT
was available, it was registered to the planning CT to
assist in gross tumour volume contouring, which was
performed before treatment in 94.1% of our cases. For
spinal metastases, we followed the International Spine
Radiosurgery Consortium consensus guidelines[10] to
contour different parts of the vertebra as our clinical
target volume. For lung, liver, adrenal, and non-spinal
bone metastasis cases, there was no margin expansion
to form the clinical target volume. Treatment was
prescribed at the 60% to 90% isodose line. The treatment
aim was that 95% of the PTVs should receive at least the
prescribed dose and 99% of the PTVs should receive at least 90% of prescribed dose. Positioning was verified
with cone beam CT before each fraction. Treatment
was delivered using intensity-modulated radiotherapy
or volumetric modulated arc therapy, depending on the
radiotherapists’ preference.
Table 1. Summary of planning images and planning target volume (PTV) margins.
RESULTS
A total of 51 SBRT sites were included. All patients
had Eastern Cooperative Oncology Group performance
status ≤ 2. There were 33 cases with a single site of OPD
and 8 cases with > 1 site of OPD. Thirty-five cases had
developed OPD during targeted therapy, and 6 cases
had developed OPD during chemotherapy. The baseline
demographics and SBRT treatment sites are depicted in
Table 2.
Table 2. Patient demographics (n = 41), treatment sites, dose and fractionations, and planning target volume (PTV) in different sites of metastases.
The median age of the cohort was 65 years. Epithelial
growth factor receptor (EGFR) mutation was the most
common driver mutation (82.9%). The most commonly
ablated site was the lung (68.6%), followed by bone
metastasis (17.6%) as shown in Table 2. The SBRT
dose and fractionation ranged from 30 to 60 Gy in 3 to
10 fractions depended on the location of the metastasis.
Fractionation details are described in Table 2. Most
treatments were given every 2 days and completed within 2 weeks, except in peripheral lung lesions using 54 Gy
over 3 fractions, in which treatments were separated by
4 days and completed within 2 weeks. Volume details of
PTV in different SBRT sites are also reported in Table 2.
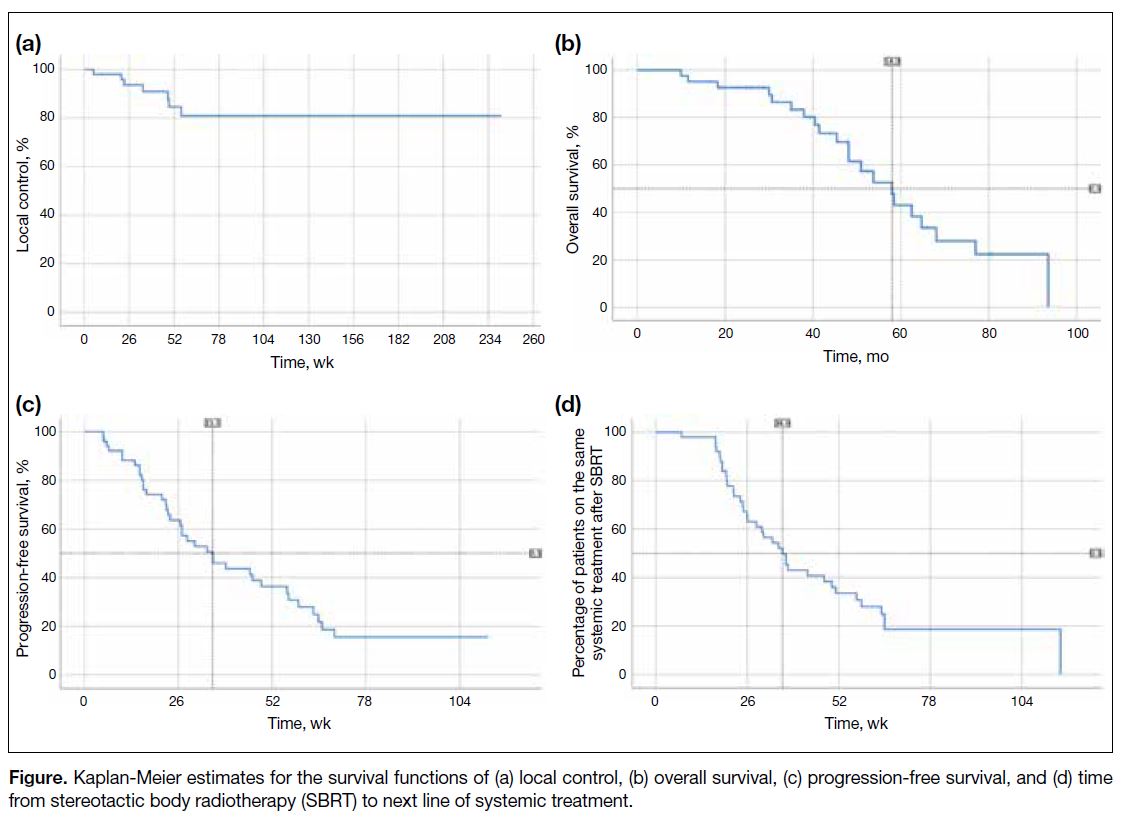
For the treatment outcome, 20 out of 41 patients (48.8%) were alive at last follow-up. With a median follow-up
time of 64 weeks, a total of 7 out of 51 sites (13.7%)
developed local failure. The 1-year LC rate was 85%.
The median PFS after SBRT, which was defined by
the time interval from date of initiation of SBRT to any
progression or death, was 8.8 months. The median time
from SBRT to the next line of systemic treatment was 9
months. The median OS was 58 months (Figure).
Figure. Kaplan-Meier estimates for the survival functions of (a) local control, (b) overall survival, (c) progression-free survival, and (d) time from stereotactic body radiotherapy (SBRT) to next line of systemic treatment.
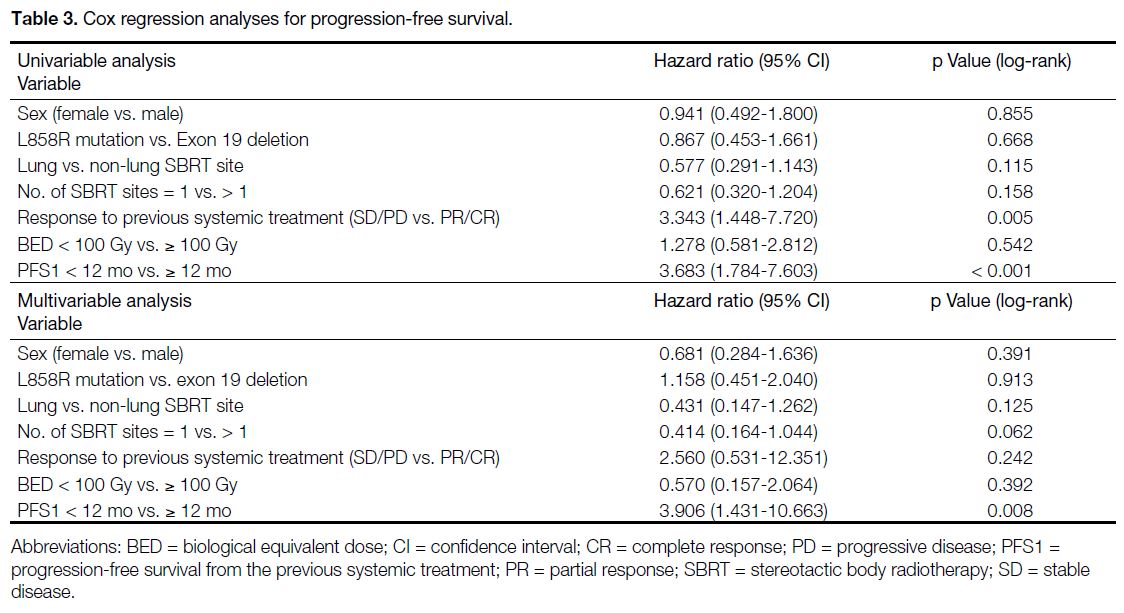
Possible prognosticators affecting PFS are assessed
in Table 3. In simple Cox regression analysis, deeper
response to previous systemic treatment and longer
PFS1 (≥ 12 months) were significantly associated with
longer PFS. In multivariable analysis, only PFS1 ≥ 12
months remained statistically significant. Meanwhile,
sex, driver mutation type, lung or non-lung metastases,
number of SBRT sites, degree of response to previous
systemic treatment, and biological equivalent dose > 100
Gy did not significantly affect PFS.
Table 3. Cox regression analyses for progression-free survival.
We demonstrated a significant association between a
better response to pre-SBRT systemic treatment (either
partial response or complete response) and a longer PFS
following SBRT. A longer PFS1 was also significantly
associated with longer PFS after SBRT (Table 3).
For treatment-related toxicities, only one patient (2.4%) developed grade 3 pneumonitis, and one patient (2.4%)
developed a rib fracture. There were no grade 4 to 5
toxicities. The patient who developed symptomatic
pneumonitis had radiographic features of pneumonitis
on CT scan. Pneumonitis was treated with a course of
empirical antibiotics and a tapering course of steroids.
EGFR tyrosine kinase inhibitor was temporarily
suspended during management of pneumonitis. The
patient was still alive at last follow-up and required 2
L/min of long-term oxygen therapy. For the rib fracture
in our study, it was detected by follow-up PET/CT scan
and the patient was asymptomatic without the need of
analgesic.
DISCUSSION
Targeted therapy in NSCLC significantly changes the
treatment landscape of metastatic NSCLC. However,
disease progression is inevitable when a drug-resistant clone develops and proliferates. Oligoprogression is a
distinct clinical entity which specifies a state where the
number of progression sites is limited to ≤ 5.[4] [5] A strategy
for OPD is not yet well defined. Theoretically, eradicating
the resistant subclone by SBRT will potentially prolong
the use of tyrosine kinase inhibitors upon progression.
The benefits of SBRT in OPD have yet to be explored in
prospective studies and current data mostly came from
phase II studies. Most of the studies were retrospective
in nature and they included a heterogeneous group of
patients with different molecular profiles. Different local
ablative therapies other than SBRT were included in
some studies. Several retrospective studies of patients
with EGFR or anaplastic lymphoma kinase–mutated
NSCLC treated with local ablative therapy and continued
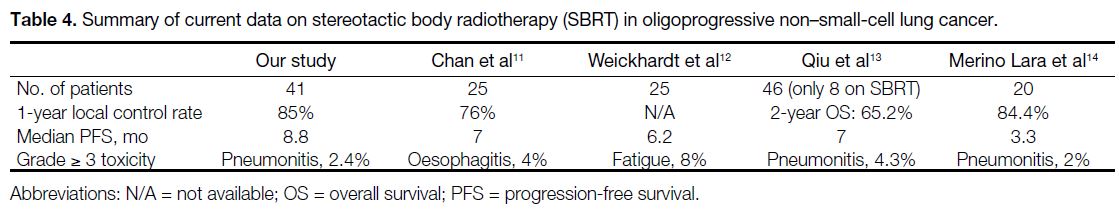
treatment with EGFR- or anaplastic lymphoma kinase–targeted therapy resulted in improved PFS (Table 4),[11] [12] [13] [14]
with reported magnitudes of PFS ranging from 3.3 to 7
months.
Table 4. Summary of current data on stereotactic body radiotherapy (SBRT) in oligoprogressive non–small-cell lung cancer.
Our data showed that SBRT delivers reasonably good
LC at the metastatic sites, and our LC rate of 85% is on
par with other different case series.[11] [12] [13] [14] Also, our findings
revealed that SBRT to OPD brings a benefit of PFS of 8.8
months, which is in line with the existing literature. This
benefit is not only demonstrated on follow-up imaging,
but it is also clinically meaningful in a sense that it can
be translated into a delay in the use of the next systemic
treatment by 9 months. With these data, the magnitude of
benefit from SBRT in OPD can be quantified. Therefore,
opening up the option of SBRT at the first appearance
of OPD could potentially forestall the use of cytotoxic
chemotherapy, and hence preserve the quality of life of
patients for a longer period.
To maximise the benefit of SBRT, selecting the correct
patients is crucial. Significant prognostic factors
associated with longer PFS after SBRT include longer
PFS1 and better radiological response to previous
systemic treatment. These two factors constitute a favourable profile of tumours which are likely to derive
sustained systemic response after SBRT for OPD. Hence,
they can potentially serve as criteria when selecting
suitable patients to receive SBRT and hence maximise
the survival benefit.
Oligoprogression should also be well defined by
sensitive imaging such as PET/CT before delivering
SBRT. Treatment should be limited to a maximum of
three to five sites of disease progression according to
the literature.[4] [5] However, we could not demonstrate a
significantly shorter PFS for > 1 SBRT site in our study,
probably due to the limitation of the small number of
cases.
Merino Lara et al[14] reported 108 patients with metastatic NSCLC treated with extracranial SBRT, and revealed
an incidence of grade ≥ 3 pneumonitis within 1 year of
treatment of approximately 2%; SBRT-induced bone
fracture was reported in 3% of the patients, and there
were no grade 4 or 5 toxicities. Similar to their findings,
the toxicities observed in our retrospective cohort aligned
with these reported ranges.
Limitations
Our study has several limitations. First, the retrospective
nature and small sample size (41 patients with 51
treatment sites) may lead to underreporting of toxicities
and inadequate statistical power to detect significant
differences. Also, retrospective studies are prone
to selection and sampling bias. Second, our cohort
predominantly consisted of patients who developed OPD
during targeted therapy treatment. Therefore, we need
to be cautious about the limitation when generalising
the data on other patients who have OPD during non–targeted therapy treatment. As interval imaging post-SBRT is based on the clinician’s discretion, the regular
imaging to document local failure or progression is not as
strict as in randomised trials. There is no randomisation
and no control arm comparing the benefits of SBRT and
changing systemic treatment at the first appearance of OPD. Hence, the clinical question whether SBRT is
better than changing systemic treatment at the discovery
of OPD remains unanswered. Moreover, measuring
the time from SBRT to the next systemic treatment as
a surrogate of clinical benefit may be affected by the
patient’s decision and choice of treatment, instead of
objective assessment using radiographic progression.
Lastly, patients with limited metastases may have
intrinsic biology that allows them to have a longer
survival independent of the success of local or systemic
therapies.
CONCLUSION
Our data concur with existing literature that SBRT
to OPD in NSCLC is an effective and safe strategy
to prolong the time to next systemic treatment with
minimal toxicities. Further studies including the HALT
study (Stereotactic Body Radiotherapy for the Treatment
of OPD)[15] and the STOP trial [Randomized Study of
Stereotactic Body Radiation Therapy (SBRT) in Patients
With Oligoprogressive Metastatic Cancers of the Breast
and Lung][16] will provide prospective data on PFS and
OS for SBRT in the setting of OPD.
REFERENCES
1. Timmerman RD, Herman J, Cho LC. Emergence of stereotactic body radiation therapy and its impact on current and future clinical practice. J Clin Oncol. 2014;32:2847-54. Crossref
2. Ball D, Mai GT, Vinod S, Babington S, Ruben J, Kron T, et al. Stereotactic ablative radiotherapy versus standard radiotherapy in stage 1 non–small-cell lung cancer (TROG 09.02 CHISEL): a phase 3, open-label, randomised controlled trial. Lancet Oncol. 2019;20:494-503. Crossref
3. Guckenberger M, Lievens Y, Bouma AB, Collette L, Dekker A, deSouza NM, et al. Characterisation and classification of oligometastatic disease: a European Society for Radiotherapy and Oncology and European Organisation for Research and Treatment of Cancer consensus recommendation. Lancet Oncol. 2020;21:e18-28. Crossref
4. Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol. 1995;13:8-10. Crossref
5. Patel PH, Palma D, McDonald F, Tree AC. The dandelion dilemma revisited for oligoprogression: treat the whole lawn or weed selectively? Clin Oncol (R Coll Radiol). 2019;31:824-33. Crossref
6. Timmerman R, Paulus R, Galvin J, Michalski J, Straube W, Bradley J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303:1070-6. Crossref
7. Bezjak A, Paulus R, Gaspar LE, Timmerman RD, Straube WL, Ryan WF, et al. Safety and efficacy of a five-fraction stereotactic body radiotherapy schedule for centrally located non–small-cell lung cancer: NRG Oncology/RTOG 0813 Trial. J Clin Oncol. 2019;37:1316-25. Crossref
8. Faculty of Clinical Oncology, The Royal College of Radiologists. Stereotactic ablative body radiation therapy (SABR): a resource. Version 6.1. Available from: https://www.sabr.org.uk/wp-content/uploads/2019/04/SABRconsortium-guidelines-2019-v6.1.0.pdf. Accessed 13 Nov 2023.
9. American Association of Physicists in Medicine. Report No. 101–Stereotactic body radiation therapy: the report of AAPM Task Group 101 (2010). Available from: https://www.aapm.org/pubs/reports/detail.asp?docid=102. Accessed 13 Nov 2023.
10. Cox BW, Spratt DE, Lovelock M, Bilsky MH, Lis E, Ryu S, et al. International Spine Radiosurgery Consortium consensus guidelines for target volume definition in spinal stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2012;83:e597-605. Crossref
11. Chan OS, Lee VH, Mok TS, Mo F, Chang AT, Yeung RM. The role of radiotherapy in epidermal growth factor receptor mutation–positive patients with oligoprogression: a matched-cohort analysis. Clin Oncol (R Coll Radiol). 2017;29:568-75. Crossref
12. Weickhardt AJ, Scheier B, Burke JM, Gan G, Lu X, Bunn PA Jr, et al. Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene-addicted non–small-cell lung cancer. J Thorac Oncol. 2012;7:1807-14. Crossref
13. Qiu B, Liang Y, Li Q, Liu G, Wang F, Chen Z, et al. Local therapy for oligoprogressive disease in patients with advanced stage non–small-cell lung cancer harboring epidermal growth factor receptor mutation. Clin Lung Cancer. 2017;18:e369-73. Crossref
14. Merino Lara T, Helou J, Poon I, Sahgal A, Chung HT, Chu W, et al. Multisite stereotactic body radiotherapy for metastatic non–small-cell lung cancer: delaying the need to start or change systemic therapy? Lung Cancer. 2018;124:219-26. Crossref
15. ClinicalTrials.gov, US National Library of Medicine. Stereotactic Body Radiotherapy for the Treatment of OPD (HALT). Available from: https://clinicaltrials.gov/study/NCT03256981. Accessed 31 Oct 2023.
16. ClinicalTrials.gov, US National Library of Medicine. Randomized Study of Stereotactic Body Radiation Therapy (SBRT) in Patients With Oligoprogressive Metastatic Cancers of the Breast and Lung. Available from: https://www.clinicaltrials.gov/study/NCT03808662. Accessed 31 Oct 2023.