Mimics of Pituitary and Pineal Germ Cell Tumours on Imaging: A Pictorial Essay
PICTORIAL ESSAY
Hong Kong J Radiol 2023 Dec;26(4):283-92 | Epub 30 Nov 2023
Mimics of Pituitary and Pineal Germ Cell Tumours on Imaging: A Pictorial Essay
CC Huang1,2
1 Department of Radiology, MacKay Memorial Hospital, Taipei, Taiwan
2 Department of Medicine, MacKay Medical College, New Taipei City, Taiwan
Correspondence: Dr CC Huang, Department of Radiology, MacKay Memorial Hospital, Taipei, Taiwan. Email: hcc.5306@mmh.org.tw
Submitted: 15 Apr 2022; Accepted: 23 Oct 2022.
Contributors: The author designed the study, acquired the data, analysed the data, drafted the manuscript, and critically revised the manuscript for important intellectual content. The author had full access to the data, contributed to the study, approved the final version for publication, and takes responsibility for its accuracy and integrity.
Conflicts of Interest: The author has disclosed no conflicts of interest.
Funding/Support: This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: All procedures were conducted in accordance with the ethical standards of the institutional research committee and the Declaration of Helsinki. All patients were consented for the imaging examination in this study.
Acknowledgement: The author expresses thanks to the Division of Neuroradiology, Hospital of the University of Pennsylvania for their assistance during the study.
INTRODUCTION
Intracranial germ cell tumours (GCTs) comprise 0.4% to
9.4% of primary intracranial neoplasms and intracranial
germinoma accounts for 50% to 70% of all intracranial
GCTs.[1] [2] They are characteristically located in the
suprasellar and pineal regions. Apart from GCTs, other
diseases are also found in the suprasellar and pineal
regions.
In this pictorial essay, mimickers of intracranial GCTs are illustrated. These GCT mimics are based on a
retrospective analysis of 313 consecutive cases collected
over 29 years at a single hospital with a tentative or
initial diagnosis of GCT but proven to be otherwise on
histology.
GERM CELL TUMOURS
The most common locations of GCTs are the pineal gland and suprasellar regions. The levels of some
oncoproteins may elevate in the serum or cerebrospinal
fluid (CSF), including alpha-fetoprotein, beta-hCG, and
placental alkaline phosphatase, depending on the tumour
types.[3] Germinoma and teratoma are the most common
and the second most common types of intracranial
GCTs, respectively. Approximately 90% of intracranial
germinoma patients are diagnosed before the age of 20
years.[2] The two most common locations of intracranial
germinoma are the pineal region (37%-65%) and the
suprasellar region (25%-49%), with approximately
8% of cases showing bifocal involvement in these two
locations.[3] [4] The male-to-female ratio is 1.88:1 in the
suprasellar region; the ratio is even higher for pineal
germinomas.[1] Tumour location, size, and resultant
endocrine dysfunction are the main causes of clinical
symptoms and signs in germinoma. The prodrome in
suprasellar lesions can last from months to years, longer than that in pineal lesions. In suprasellar germinomas,
symptoms related to diabetes insipidus often occur first,
followed by other endocrine dysfunctions. As the tumour
grows, visual impairment may occur due to compression
of the optic chiasm; obstructive hydrocephalus is also
possible if the drainage of CSF is affected. In pineal
germinomas, the aqueduct and dorsal midbrain can
be affected, resulting in obstructive hydrocephalus,
diplopia, and Parinaud’s syndrome. Other symptoms
due to tumour dissemination or metastasis can develop.[4]
Computed tomography (CT) of the head seldom shows
calcification of the germinoma, but when located in
the pineal region, it can enlarge and engulf the pineal
calcification. In magnetic resonance imaging (MRI),
germinoma usually presents as iso- to hyperintense to
grey matter on T1-weighted and T2-weighted images,
with marked enhancement and cyst formation, and
hydrocephalus, as well as water restriction on diffusion-weighted imaging (DWI). Dissemination via the CSF and
invasion of adjacent brain parenchyma also commonly
occur.[3] [4] Because of the risk of CSF seeding, imaging
evaluation should include the entire neuroaxis; however,
even with extensive involvement, the prognosis of
germinomas is good because of the radiosensitive nature
of these tumours.[3] The prognosis of teratoma varies,
depending on the histological findings. Some clues to
the diagnosis of germinoma have been proposed, such
as engulfment of the pineal calcification and bifocal
involvement with normal alpha-fetoprotein level in the
serum and CSF. Nonetheless, surgical confirmation
is usually required.[3] [4] In our cases, there are typical
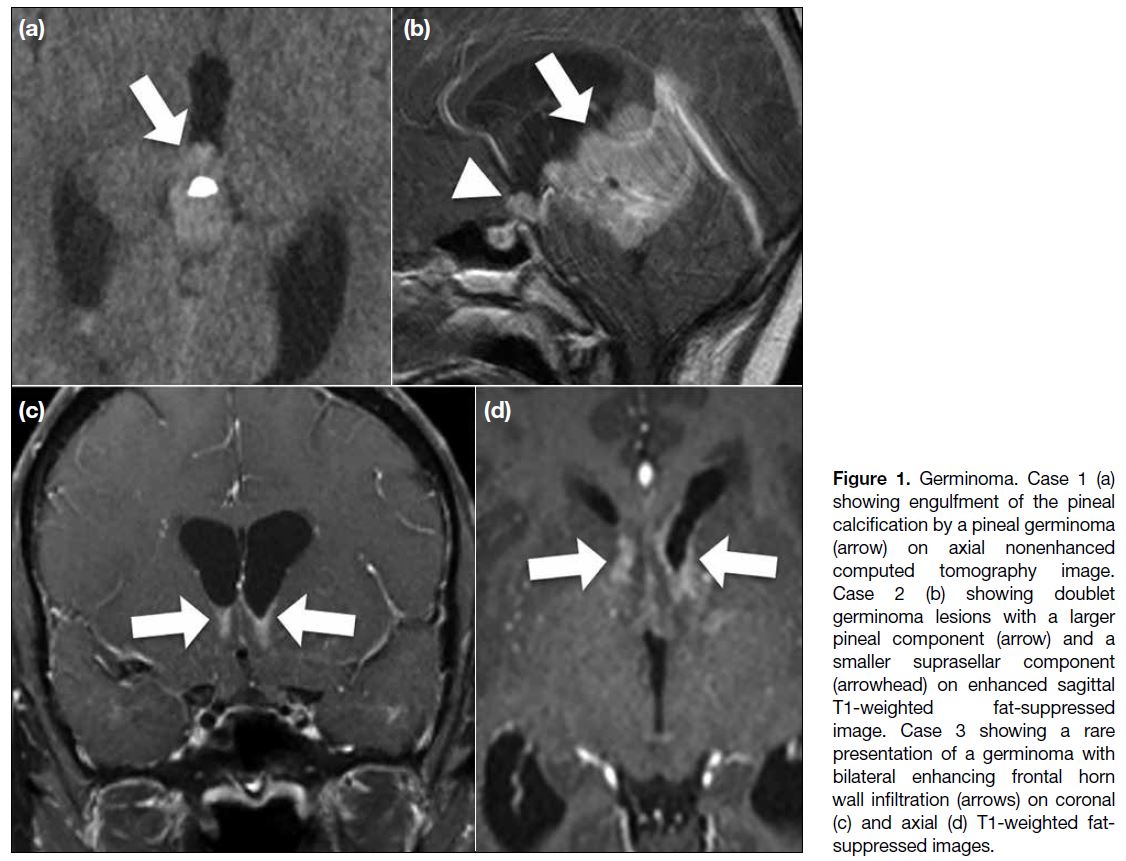
instances of engulfment of the pineal calcification,
doublet lesions, and also infiltrative lesions involving
both frontal lateral ventricular walls (Figure 1). However,
nongerminomatous GCTs can also demonstrate bifocal
involvement.[1] [2]
Figure 1. Germinoma. Case 1 (a)
showing engulfment of the pineal
calcification by a pineal germinoma
(arrow) on axial nonenhanced
computed tomography image.
Case 2 (b) showing doublet
germinoma lesions with a larger
pineal component (arrow) and a
smaller suprasellar component
(arrowhead) on enhanced sagittal
T1-weighted fat-suppressed
image. Case 3 showing a rare
presentation of a germinoma with
bilateral enhancing frontal horn
wall infiltration (arrows) on coronal
(c) and axial (d) T1-weighted fat-suppressed
images.
MIMICS OF SUPRASELLAR GERM CELL TUMOURS
Craniopharyngioma
Approximately 1.2% to 4.0% of paediatric brain
tumours are craniopharyngiomas, which are commonly
located in the sellar and suprasellar regions.[5] The adamantinomatous type is more common in childhood.
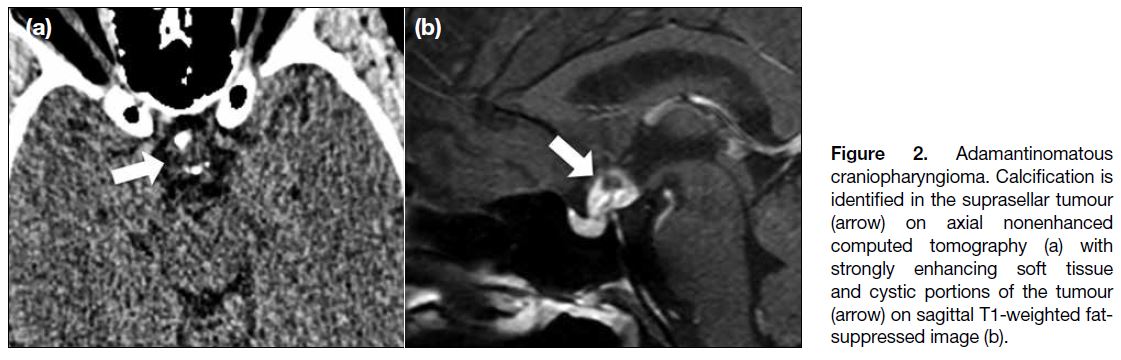
Imaging features include calcifications and cystic
components, which are found in our case (Figure 2).
Figure 2. Adamantinomatous
craniopharyngioma. Calcification is
identified in the suprasellar tumour
(arrow) on axial nonenhanced computed tomography (a) with strongly enhancing soft tissue and cystic portions of the tumour (arrow) on sagittal T1-weighted fat-suppressed image (b).
Glial Cell Tumour
When located in the suprasellar region, the glial cell tumour is commonly referred to as an optic pathway
glioma. These cases are usually diagnosed at 4 to 5 years
of age.[6] Imaging findings include involvement along the
course of optic pathway with variable enhancement and
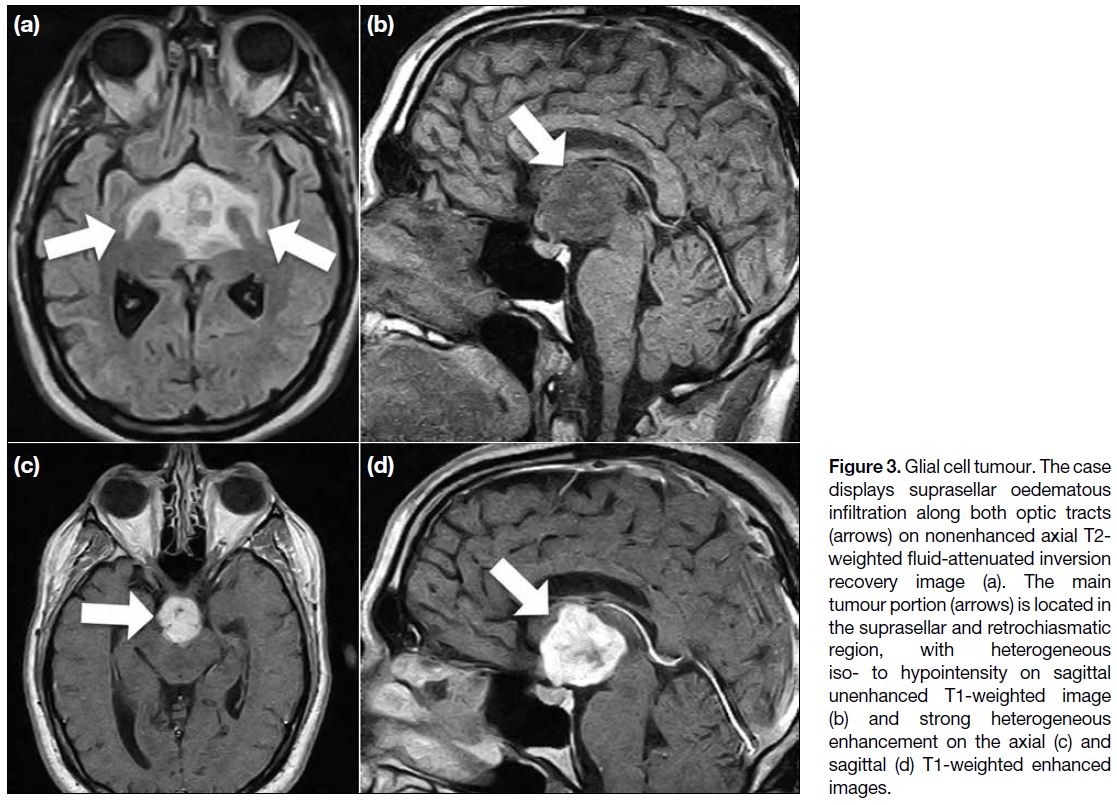
cystic components as well as calcifications. Sometimes,
oedema in the optic pathway near the tumour is a
diagnostic clue (Figure 3).[7]
Figure 3. Glial cell tumour. The case
displays suprasellar oedematous
infiltration along both optic tracts
(arrows) on nonenhanced axial T2-weighted fluid-attenuated inversion
recovery image (a). The main
tumour portion (arrows) is located in
the suprasellar and retrochiasmatic
region, with heterogeneous
iso- to hypointensity on sagittal
unenhanced T1-weighted image
(b) and strong heterogeneous
enhancement on the axial (c) and
sagittal (d) T1-weighted enhanced
images.
Lymphocytic Hypophysitis
Lymphocytic hypophysitis is an autoimmune
inflammatory disease with lymphocytic infiltration
involving the pituitary gland, stalk, and hypothalamus, which can affect adults and children of both sexes.[8]
Possible common symptoms include headache, hyper or
hypofunction of pituitary gland, and diabetes insipidus.[8]
Depending on disease involvement, typical imaging
findings are strong enhancement and enlargement of the
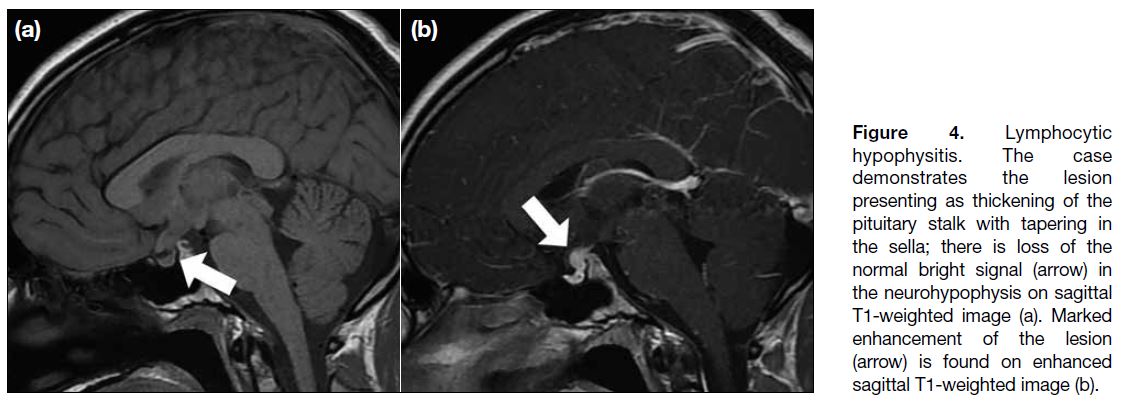
pituitary gland, stalk, and hypothalamus. Sometimes,
loss of the neurohypophyseal bright spot is noted (Figure 4).[8] Delayed enhancement of the whole pituitary gland
due to this disease is also described in the literature.[8]
Repeated imaging studies are sometimes necessary
because lesions might be found months after an initial
normal imaging study.[8]
Figure 4. Lymphocytic
hypophysitis. The case
demonstrates the lesion
presenting as thickening of the
pituitary stalk with tapering in
the sella; there is loss of the
normal bright signal (arrow) in
the neurohypophysis on sagittal
T1-weighted image (a). Marked
enhancement of the lesion
(arrow) is found on enhanced
sagittal T1-weighted image (b).
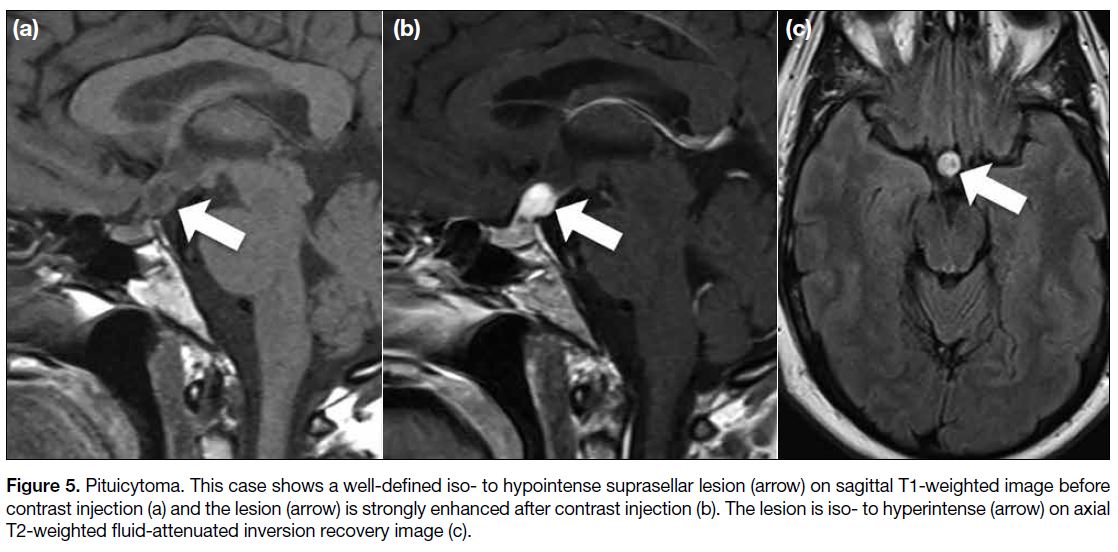
Pituicytoma
Pituicytomas are rare benign tumours originating from
a type of glial cell, the pituicyte, in the neurohypophysis
and pituitary stalk. They usually affect adults with a
slight male predominance.[9] The imaging presentation of
pituicytoma is nonspecific but it usually presents as a well-defined
homogeneously or heterogeneously enhancing
solid mass in the sellar or suprasellar region (Figure 5). Calcification, adjacent bony changes, or necrosis are
absent, but cystic portions sometimes can be identified.[9]
Figure 5. Pituicytoma. This case shows a well-defined iso- to hypointense suprasellar lesion (arrow) on sagittal T1-weighted image before contrast injection (a) and the lesion (arrow) is strongly enhanced after contrast injection (b). The lesion is iso- to hyperintense (arrow) on axial T2-weighted fluid-attenuated inversion recovery image (c).
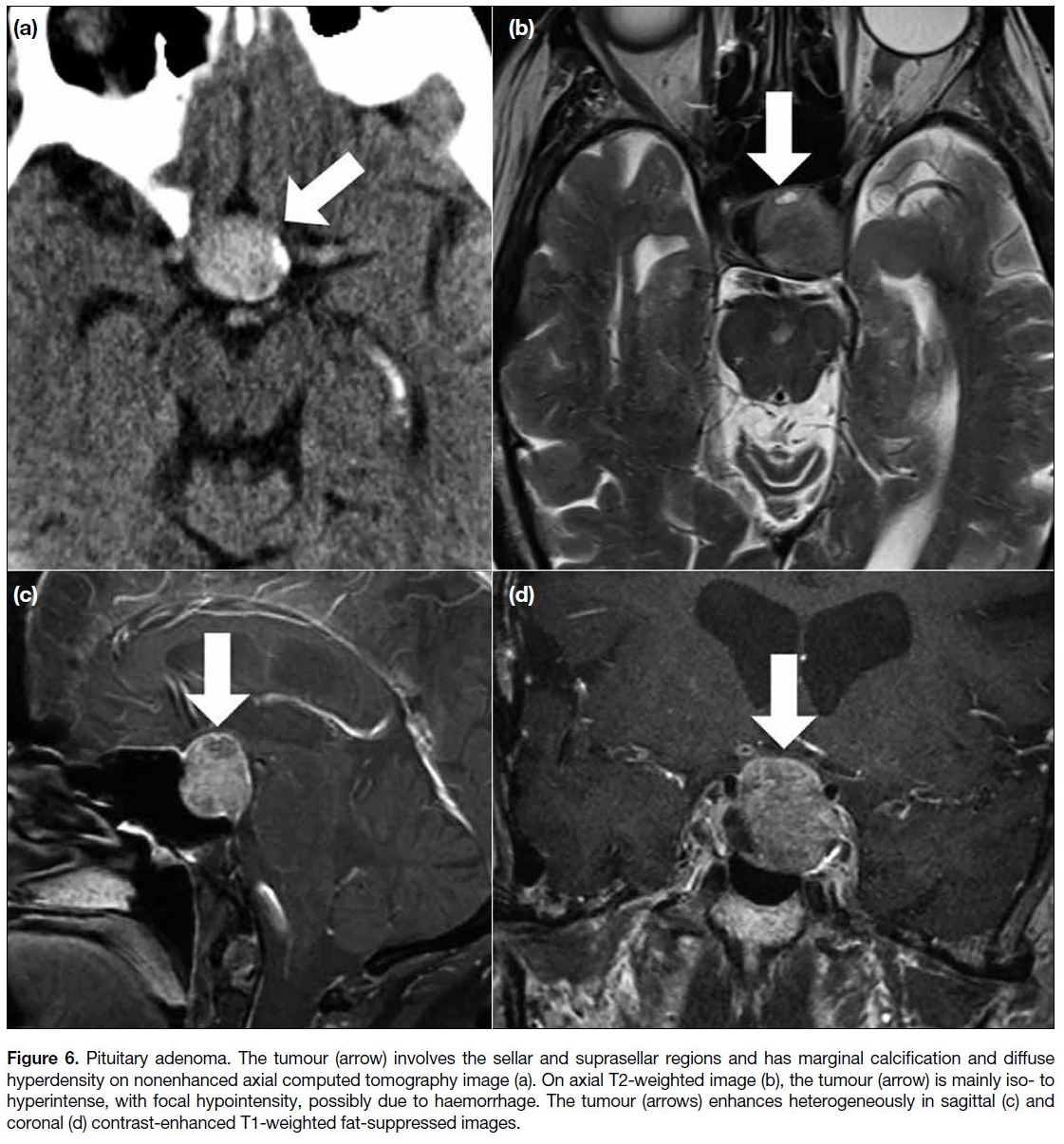
Pituitary adenoma
Pituitary adenoma is more common in adults but can
be found in children.[7] It may display signal intensities
similar to those of the adjacent normal pituitary gland
on pre-contrast MRI and relatively less enhancement on enhanced MRI. Cystic components, calcification, and
haemorrhage may be visible (Figure 6). The tumours
can display suprasellar or parasellar extension. Dynamic
contrast-enhanced MRI is useful to detect a relatively
hypointense microadenoma in its early enhancement
phase.[10]
Figure 6. Pituitary adenoma. The tumour (arrow) involves the sellar and suprasellar regions and has marginal calcification and diffuse
hyperdensity on nonenhanced axial computed tomography image (a). On axial T2-weighted image (b), the tumour (arrow) is mainly iso- to
hyperintense, with focal hypointensity, possibly due to haemorrhage. The tumour (arrows) enhances heterogeneously in sagittal (c) and
coronal (d) contrast-enhanced T1-weighted fat-suppressed images.
MIMICS OF PINEAL GERM CELL TUMOURS
Primitive Neuroectodermal Tumour
The primitive neuroectodermal tumour of the pineal gland is also known as a pineoblastoma, which is a
highly malignant tumour and accounts for 40% of pineal
parenchymal tumours. The diagnosis of pineoblastoma
peaks before the age of 20 but can be at any age.[3] The
tumour is usually > 3 cm and the pineal calcification,
if present, is displaced from the midline by the tumour.
Because of its high cellularity, pineoblastomas are
hyperdense on CT and show water restriction on DWI
(Figure 7). Heterogeneous enhancement and obstructive
hydrocephalus are present and CSF dissemination is
common.[3]
Figure 7. Primitive neuroectodermal tumour. The tumour is hyperdense on nonenhanced axial computed tomography image with
displacement of the pineal calcification (arrow) from the midline by the tumour (a). Water restriction is noted on diffusion-weighted imaging
(b) and apparent diffusion coefficient (c) images. The tumour (arrows) is iso- to hyperintense on axial T2-weighted fluid-attenuated inversion
recovery image (d) and heterogeneously hypointense with heterogeneous enhancement on sagittal T1-weighted images (e and f).
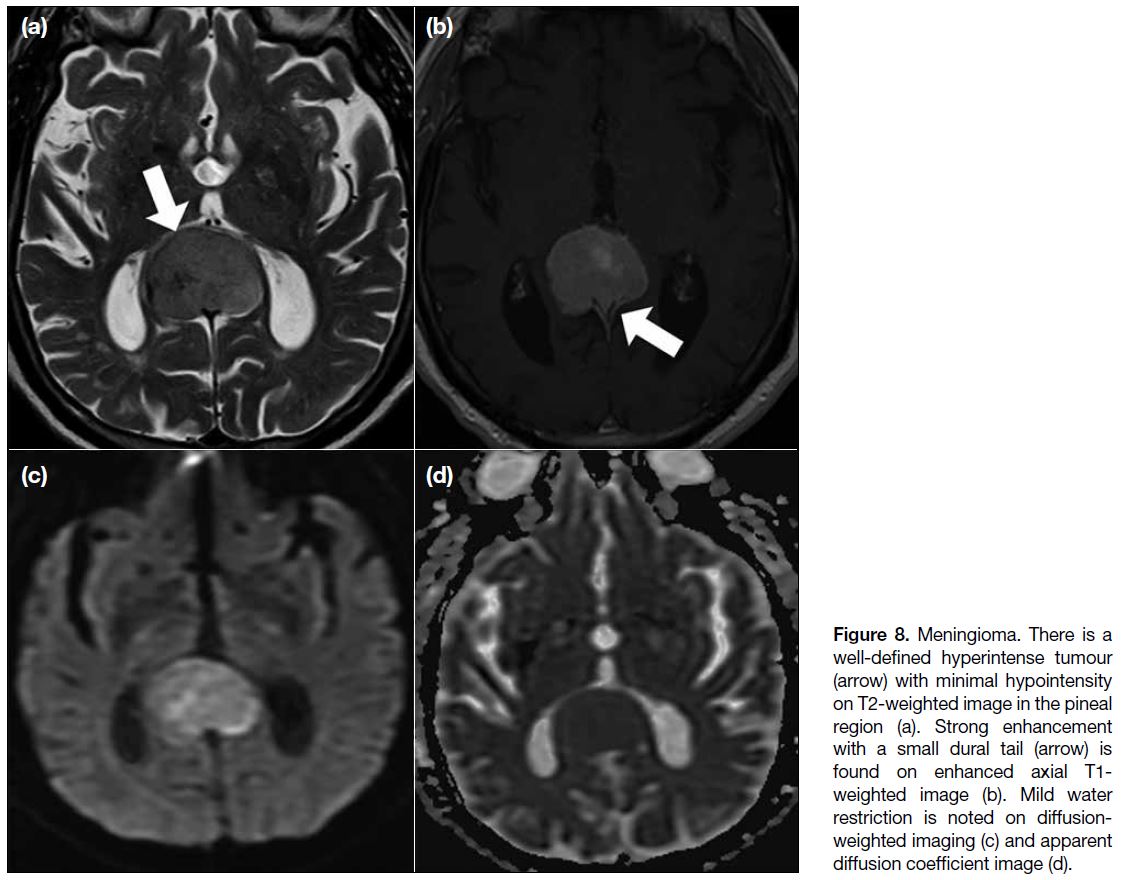
Meningioma
Pineal region meningiomas are uncommon, accounting
for 6.2% of all pineal tumours and 0.3% of all
intracranial meningiomas.[11] Because of the highly
cellular nature, meningioma shows hyperdensity on
CT and water restriction on DWI (Figure 8). Strong
enhancement is noted. Calcifications and a dural tail are
sometimes present.[3]
Figure 8. Meningioma. There is a
well-defined hyperintense tumour
(arrow) with minimal hypointensity
on T2-weighted image in the pineal
region (a). Strong enhancement
with a small dural tail (arrow) is
found on enhanced axial T1-weighted image (b). Mild water
restriction is noted on diffusion-weighted
imaging (c) and apparent
diffusion coefficient image (d).
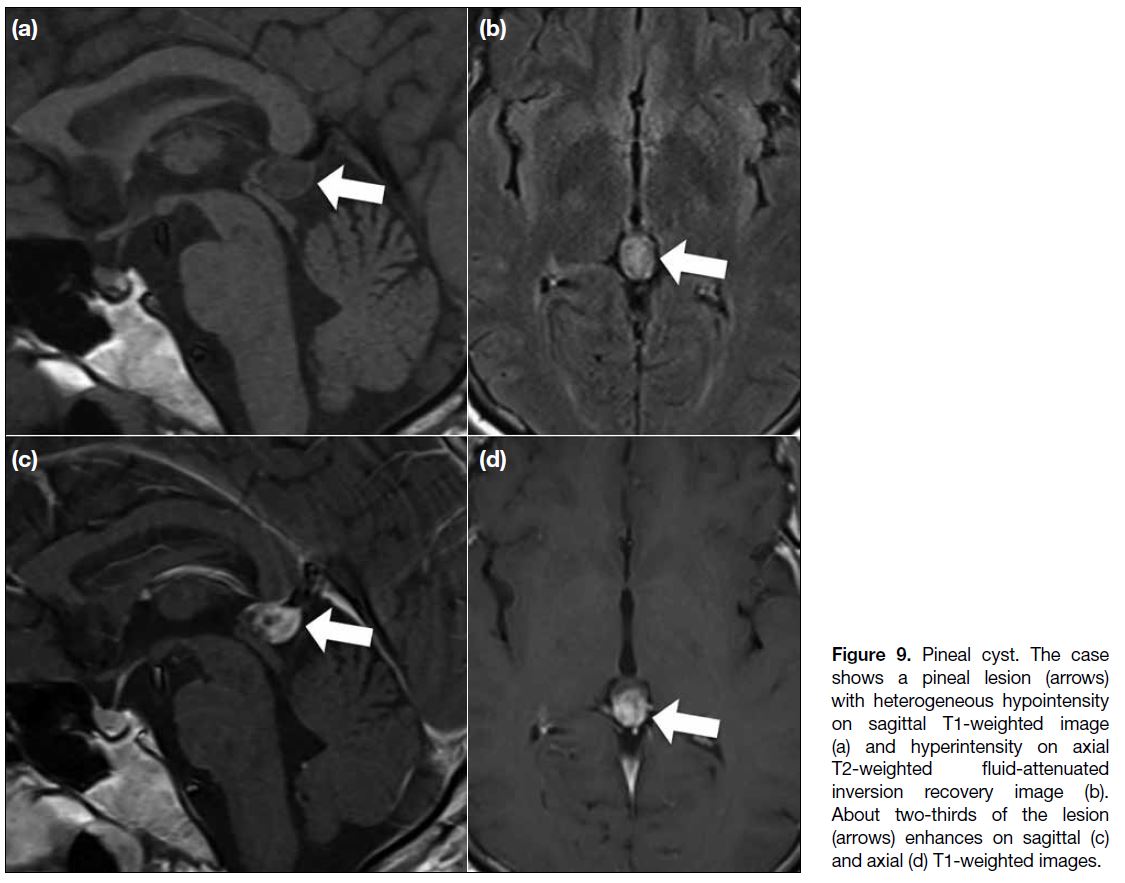
Pineal Cyst
Pineal cysts can be found radiographically in 23% of
patients, with a female predominance. Although they
can be found at all ages, they are most commonly
identified in adults. Typically, the lesion is < 15 mm;
larger lesions may show intracystic haemorrhage.[3] [12] The imaging findings are typically well-defined cystic
lesions with water signal intensity inside. Sometimes the
cystic portion shows hyperintensity on fluid-attenuated
inversion recovery images because of the proteinaceous
contents. Wall enhancement can be found and rarely,
and enhancement of the suspected cystic part has been
reported and was present in our case (Figure 9). The
likely mechanism is passive diffusion of the contrast into
the cyst.[3] [13]
Figure 9. Pineal cyst. The case
shows a pineal lesion (arrows)
with heterogeneous hypointensity
on sagittal T1-weighted image
(a) and hyperintensity on axial
T2-weighted fluid-attenuated
inversion recovery image (b).
About two-thirds of the lesion
(arrows) enhances on sagittal (c)
and axial (d) T1-weighted images.
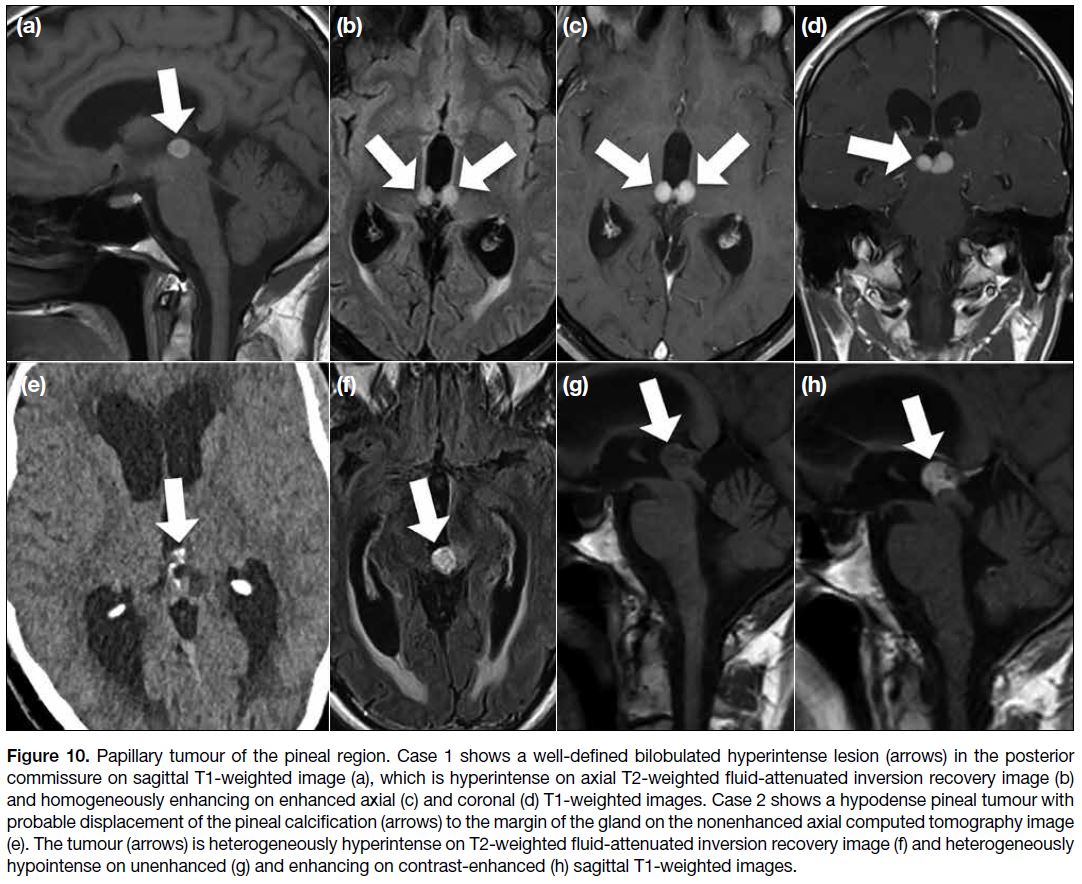
Papillary Tumour of the Pineal Region
Papillary tumour of the pineal region is a rare
neuroepithelial tumour located in the pineal region. It
can affect children and adults without sex difference.[3]
On imaging studies, this tumour is described as a well-defined enhancing lesion with possible cystic portions.
Usually, there is absence of fat, haemorrhage, melanin
or calcification. Sometimes, there is T1 signal
hyperintensity in the lesion (Figure 10), which is
considered to be due to proteinaceous content.[3] One of
our cases shows calcification at the lesion site (Figure 10), but it could be just due to normal pineal calcification
considering the old age of the patient.
Figure 10. Papillary tumour of the pineal region. Case 1 shows a well-defined bilobulated hyperintense lesion (arrows) in the posterior
commissure on sagittal T1-weighted image (a), which is hyperintense on axial T2-weighted fluid-attenuated inversion recovery image (b)
and homogeneously enhancing on enhanced axial (c) and coronal (d) T1-weighted images. Case 2 shows a hypodense pineal tumour with
probable displacement of the pineal calcification (arrows) to the margin of the gland on the nonenhanced axial computed tomography image
(e). The tumour (arrows) is heterogeneously hyperintense on T2-weighted fluid-attenuated inversion recovery image (f) and heterogeneously
hypointense on unenhanced (g) and enhancing on contrast-enhanced (h) sagittal T1-weighted images.
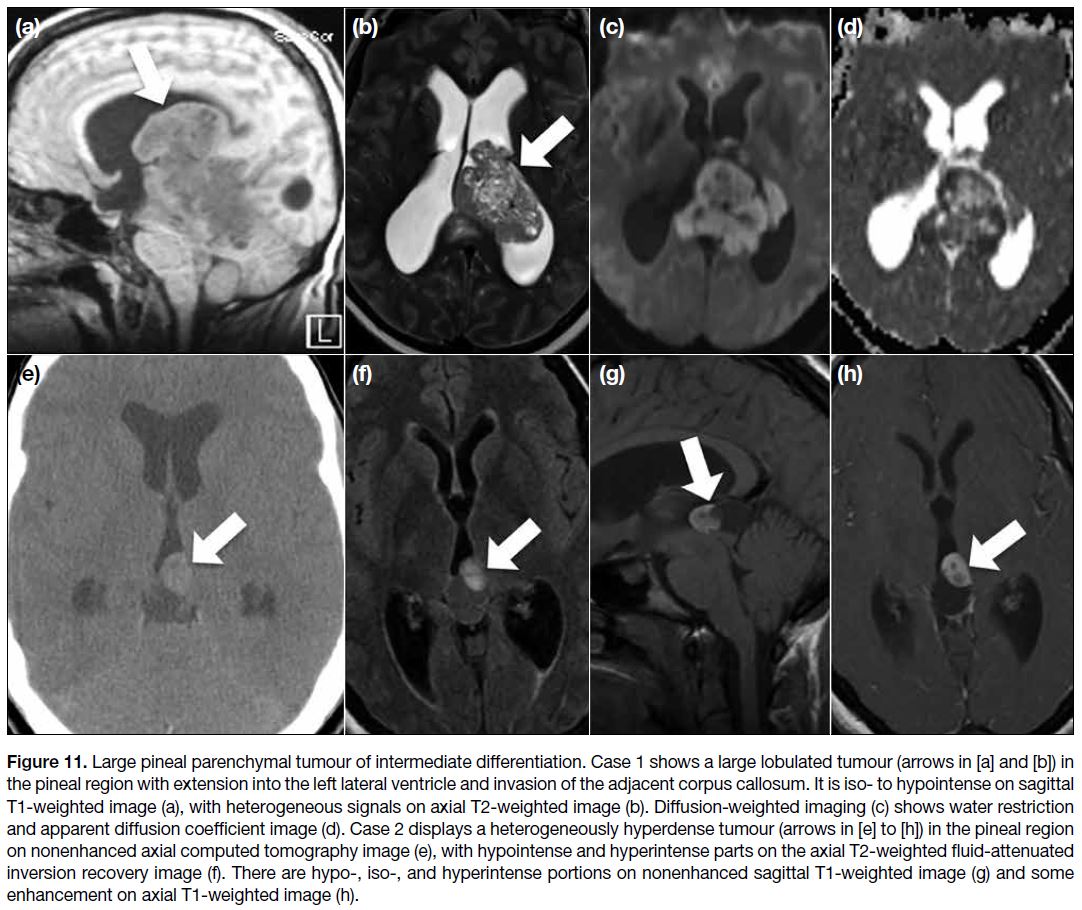
Pineal Parenchymal Tumours
Pineal parenchymal tumour of intermediate
differentiation accounts for about 20% of all pineal
parenchymal tumours and can be found at any age with
a slight female predominance.[3] Imaging findings are
variable and may be indistinguishable from pineocytoma or pineoblastoma. Usually, this tumour presents as a
lobulated heterogeneously enhancing lesion, sometimes
with a cystic component. Pineal calcification can be
displaced to the periphery by the tumour. Because of
its high cellularity, it can show hyperdensity on CT and
water restriction on DWI (Figure 11). Local invasion is
reported in approximately 80% of cases.[3] [14]
Figure 11. Large pineal parenchymal tumour of intermediate differentiation. Case 1 shows a large lobulated tumour (arrows in [a] and [b]) in
the pineal region with extension into the left lateral ventricle and invasion of the adjacent corpus callosum. It is iso- to hypointense on sagittal
T1-weighted image (a), with heterogeneous signals on axial T2-weighted image (b). Diffusion-weighted imaging (c) shows water restriction
and apparent diffusion coefficient image (d). Case 2 displays a heterogeneously hyperdense tumour (arrows in [e] to [h]) in the pineal region
on nonenhanced axial computed tomography image (e), with hypointense and hyperintense parts on the axial T2-weighted fluid-attenuated
inversion recovery image (f). There are hypo-, iso-, and hyperintense portions on nonenhanced sagittal T1-weighted image (g) and some
enhancement on axial T1-weighted image (h).
CONCLUSION
This pictorial essay suggests that the presence of doublet
lesions in both the suprasellar and pineal regions, although
less common, might be a useful clue for intracranial
germinomas. However, imaging diagnosis for single
germinomas in either the suprasellar or pineal region
remains challenging because of the overlapping imaging
presentations of intracranial germinomas and their
mimics. When the mimics are also frequently observed in children or young adults, such as craniopharyngioma
and glial cell tumour in the suprasellar region and
primitive neuroectodermal tumour in the pineal region,
the diagnosis becomes even more difficult.
Imaging diagnosis of intracranial GCTs is challenging
due to their diverse presentation and overlapping
appearance with other diseases. The phenomenon of
doublet lesions in the suprasellar and pineal regions may
be a clue to diagnose germinoma but is uncommon and might also happen in other tumours.
REFERENCES
1. Jennings MT, Gelman R, Hochberg F. Intracranial germ-cell tumors: natural history and pathogenesis. J Neurosurg. 1985;63:155-67. Crossref
2. Echevarría ME, Fangusaro J, Goldman S. Pediatric central nervous system germ cell tumors: a review. Oncologist. 2008;13:690-9. Crossref
3. Smith AB, Rushing EJ, Smirniotopoulos JG. From the archives of the AFIP: lesions of the pineal region: radiologic-pathologic correlation. Radiographics. 2010;30:2001-20. Crossref
4. Osorio DS, Allen JC. Management of CNS germinoma. CNS Oncol. 2015;4:273-9. Crossref
5. Müller HL. Childhood craniopharyngioma — current concepts in diagnosis, therapy and follow-up. Nat Rev Endocrinol. 2010;6:609-18. Crossref
6. Aihara Y, Chiba K, Eguchi S, Amano K, Kawamata T. Pediatric optic pathway/hypothalamic glioma. Neurol Med Chir (Tokyo). 2018;58:1-9. Crossref
7. McCrea HJ, George E, Settler A, Schwartz TH, Greenfield JP. Pediatric suprasellar tumors. J Child Neurol. 2016;31:1367-76. Crossref
8. Rivera JA. Lymphocytic hypophysitis: disease spectrum and approach to diagnosis and therapy. Pituitary. 2006;9:35-45. Crossref
9. Yang X, Liu X, Li W, Chen D. Pituicytoma: a report of three cases and literature review. Oncol Lett. 2016;12:3417-22. Crossref
10. Kucharczyk W, Bishop JE, Plewes DB, Keller MA, George S. Detection of pituitary microadenomas: comparison of dynamic keyhole fast spin-echo, unenhanced, and conventional contrast-enhanced MR imaging. AJR Am J Roentgenol. 1994;163:671-9. Crossref
11. Konovalov AN, Spallone A, Pitzkhelauri DI. Meningioma of the pineal region: a surgical series of 10 cases. J Neurosurg. 1996;85:586-90. Crossref
12. Richardson JK, Hirsch CS. Sudden, unexpected death due to “pineal apoplexy”. Am J Forensic Med Pathol. 1986;7:64-8. Crossref
13. Pu Y, Mahankali S, Hou J, Li J, Lancaster JL, Gao JH, et al. High prevalence of pineal cysts in healthy adults demonstrated by high-resolution, noncontrast brain MR imaging. AJNR Am J Neuroradiol. 2007;28:1706-9. Crossref
14. Yoon DJ, Park J, Lezama LM, Heller GD. Pineal parenchymal tumour of intermediate differentiation: a rare differential diagnosis of pineal region tumours. BJR Case Rep. 2016;2:20150371. Crossref