Treatment Outcomes in Patients Receiving Regorafenib for Metastatic Colon Cancer
ORIGINAL ARTICLE CME
Treatment Outcomes in Patients Receiving Regorafenib for
Metastatic Colon Cancer
L Fok, KM Cheung, YL Kwok, KH Wong
Department of Clinical Oncology, Queen Elizabeth Hospital, Jordan, Hong Kong
Correspondence: Dr L Fok, Department of Clinical Oncology, Queen Elizabeth Hospital, Jordan, Hong Kong. Email: leslie.fok@link.cuhk.edu.hk
Submitted: 7 Jun 2019; Accepted: 3 Sep 2019.
Contributors: LF and KMC designed the study and acquired the data. All authors contributed to the analysis of data, drafted the manuscript,
and had critical revision of the manuscript for important intellectual content. All authors had full access to the data, contributed to the study,
approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: The authors have no conflicts of interest to disclose.
Funding/Support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics Approval: This study was approved by the Research Ethics Committee (Kowloon Central/Kowloon East) of the Hospital Authority,
Hong Kong (Ref KC/KE-19-0046-ER/4). The requirement for patient consent was waived. All patients were treated in compliance with the
Declaration of Helsinki.
Abstract
Introduction
To review the treatment outcomes of patients with chemorefractory metastatic colorectal cancer receiving the multikinase inhibitor regorafenib.
Methods
This was a retrospective cohort study including patients who received regorafenib after failure of standard
irinotecan- and oxaliplatin-based chemotherapy with or without biologics from 2016 to 2018 in a single centre in
Hong Kong.
Results
Fourteen patients met the inclusion criteria. All had good general condition (i.e., Eastern Cooperative
Oncology Group score 1). Seven patients had received bevacizumab previously. Median progression-free survival
(PFS) was 12.4 weeks and median overall survival (OS) was 26.5 weeks. Eight patients had grade ≥3 adverse events
and 10 (71.4%) required temporary treatment suspension. The commonest grade ≥3 adverse events were palmar-plantar
erythrodysaesthesia and fatigue (both 28.6%). Patients with a carcinoembryonic antigen drop of ≥50% from
baseline enjoyed longer PFS, though not to a significant extent. OS was longer for left-sided primary tumours (202
vs. 57 days, p = 0.001). Two patients with good performance after progression received trifluridine-tipiracil. Their
median OS was 400 days.
Conclusion
Our experience with regorafenib monotherapy for patients with chemorefractory metastatic colorectal
cancer was comparable to the landmark trials. The grade ≥3 adverse events were frequent, and dose reduction or
treatment delay was required. Potentially favourable prognostic factors included a left-sided primary tumour and
a carcinoembryonic antigen drop from baseline. Those who received further treatment after regorafenib enjoyed
reasonably long survival. Treatment after regorafenib with newer strategies should be considered in those who
remain functional.
Key Words: Colorectal neoplasms; Protein kinase inhibitors
中文摘要
瑞戈非尼對大腸癌轉移患者的治療結果
霍善智、張嘉文、郭婉琳、黃錦洪
目的
探討多激酶抑制劑瑞戈非尼對化療難治性大腸癌轉移患者的治療結果。
方法
這項回顧性隊列研究納入2016年至2018年於香港單一中心進行標準伊立替康和奧沙利鉑化療無效後接受瑞戈非尼治療合用或未合用生物製劑的患者。
結果
14名患者符合納入標準。所有患者的身體狀況較好(ECOG 1分),當中7名患者曾接受貝伐單抗治療。無惡化存活期中位數為12.4週,總體存活期中位數為26.5週。8名患者出現≥3級不良事件,10名患者(71.4%)須暫停治療。最常見≥3級不良事件包括掌足紅腫綜合徵和疲勞(均為28.6%)。癌胚抗原從基線下降≥50%的患者有更長無惡化存活期,但只有邊緣顯著性。左側原發腫瘤患者的總體存活期較長(202天比57天,p = 0.001)。兩名患者在瑞戈非尼治療失敗後因體能狀態較佳,遂以三氟胸苷—替吡嘧啶作進一步治療。他們的總體存活期中位數為400天。
結論
本研究的瑞戈非尼單藥治療化療難治性大腸癌轉移患者的經驗與具有里程碑意義的試驗相若。≥3級不良事件很常見,須減少劑量或延遲治療。潛在的有利預後因素包括左側原發腫瘤和癌胚抗原從基線下降。瑞戈非尼後接受進一步治療患者有較長存活期。使用瑞戈非尼後仍保持功能的患者,可考慮以新策略作進一步治療。
INTRODUCTION
Colorectal cancer is the commonest malignancy in Hong
Kong, with an age-standardised incidence rate of 35.7
per 100 000 population in 2016.[1] Up to 23% of patients
have metastatic disease on presentation, and the 5-year
overall survival (OS) is 14%.[2]
Traditionally, chemotherapy and biologics using
fluoropyrimidine, irinotecan and oxaliplatin, with or
without anti–vascular endothelial growth factor (VEGF)
agents and anti–epithelial growth factor receptor
(EGFR) agents for RAS wild-type tumours, were the
main treatment strategies for inoperable or metastatic
colorectal cancer (mCRC). Despite the range of available
combination therapies, OS remained in the range of 20
to 30 months.[3] Options beyond these standard treatments
were limited, with regorafenib and trifluridine-tipiracil
being the only two Food and Drug Administration
(FDA)–approved treatments for this group of patients.[4]
Regorafenib is an oral multikinase inhibitor, which
is structurally similar to sorafenib. It blocks multiple
kinases involved in tumour angiogenesis (VEGFR 1-3,
Tie2), oncogenesis (KIT, RET, RAF1 and BRAF), and
tumour microenvironment (PDGFR and FGFR). In an
international multicentre phase III trial (CORRECT),
statistically significant, yet modest improvement in OS
was demonstrated compared with placebo in patients with colorectal cancer who had failed multiple lines
of chemotherapy (6.4 months vs. 5.0 months, hazard
ratio = 0.77, 95% confidence interval [CI] = 0.64-0.94,
p = 0.0052).[5] The results were similar in a subsequent
study targeting Asian populations.[6] However, grade 3
or 4 adverse events (AEs) were high in both trials and
in real-world settings,[7] affecting >50% of patients. The
modest magnitude of survival prolongation and its
significant toxicity suggests the importance of careful
patient selection and the urgency of identification of
additional treatment strategies. We aimed to review our
experience in using regorafenib monotherapy as a last-line
treatment, and to investigate predictive markers of
treatment response.
METHODS
Patients
This study was approved by the ethics committee of
Kowloon Central Cluster/Kowloon Eastern Cluster of the
Hospital Authority and conducted in compliance with the
Declaration of Helsinki. Records of patients with stage
IV colorectal adenocarcinoma who received regorafenib
from January 2016 to December 2018 in the Department
of Clinical Oncology of Queen Elizabeth Hospital
were retrieved and retrospectively analysed. Patients
were offered regorafenib after exhausting all available
treatments at that time, which included chemotherapy
fluoropyrimidine, irinotecan and oxaliplatin; and biologics with bevacizumab and cetuximab if clinically
suitable and affordable.
Treatment
Regorafenib was provided either on a compassionate
basis from the pharmaceutical company or as a selffinanced
item during the study period. Patients received
regorafenib 160 mg daily for the first 3 weeks of each
4-week cycle until disease progression, death, intolerable
AEs, or patients’ refusal to continue / inability to afford
treatment. Lower starting doses and dose reduction or
escalation during treatment were allowed per clinical
judgement of the prescribing physician.
Assessment
Patients were followed up fortnightly with routine
monitoring of complete blood counts, liver and renal
function tests, and carcinoembryonic antigen (CEA)
levels. Interval computed tomography scanning was
arranged every 10 to 12 weeks. The RECIST (Response
Evaluation Criteria in Solid Tumour) version 1.1 was
referred to in order to determine treatment response.
AEs were defined and graded according to the CTCAE
(Common Terminology Criteria for Adverse Events)
version 4.0 by National Cancer Institute. A CEA response
was defined as a decrease of CEA from baseline after the
start of regorafenib.
Statistical Analyses
Statistical analyses were performed with SPSS
(Windows version 26.0; IBM Corp, Armonk [NY],
United States). Descriptive statistics on central tendency
(e.g., mean, median) and data dispersion (e.g., range,
standard deviation, 95% CI) were used. The Kaplan-
Meier method and log-rank test were used to depict
and analyse survival outcomes. A p value of <0.05 was
considered significant.
RESULTS
Patient Characteristics
From January 2016 to December 2018, a total of 14
mCRC patients received regorafenib. Patient baseline
characteristics are summarised in Table 1. The median
age was 61.3 years. All patients had an Eastern
Cooperative Oncology Group (ECOG) performance
score of 1. In all, 57.1% of them had primary colon
cancer while the rest had primary rectal cancer. In total,
85.7% had liver metastases when regorafenib treatment
was initiated, and approximately 60% of them had
three or more metastatic sites. Approximately 70% of
the patients had K- or N-RAS mutant tumours. Half of the patients had undergone anti-VEGF therapy prior to starting regorafenib. In the RAS wild-type subgroup,
three (75%) of the patients had received an anti-EGFR
agent. One patient (25%) chose not to receive anti-EGFR
therapy due to affordability. The median number of lines
of systemic therapy before regorafenib was two. Only
two patients continued onto a next line of treatment
after failure of regorafenib. The rest either had died by
the time of progression (n = 2), were unfit for further
oncological treatment due to disease progression (n = 4),
or were unable to afford any more treatment (n = 6).
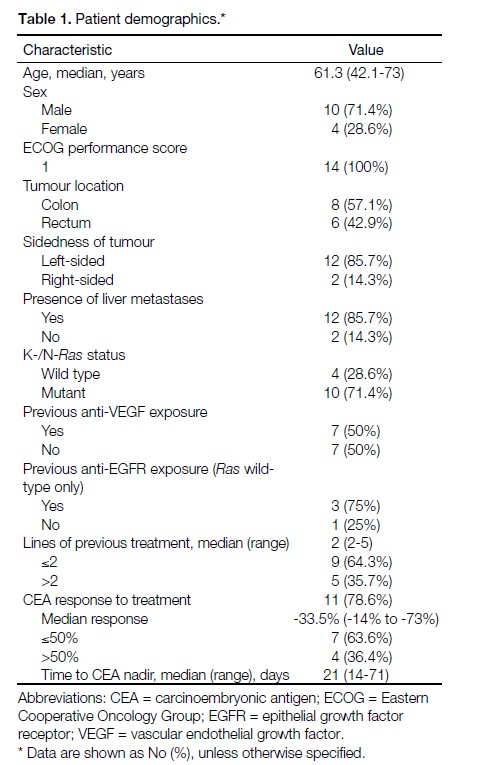
Table 1. Patient demographics.
Response
The median number of cycles of regorafenib received
was 2.67. The median dose intensity was 75% of the full
dose. Three patients achieved radiographically stable
disease, while the 11 others developed progressive
disease during regorafenib treatment. The overall
disease control rate was thus 21.4%. At the same time,
patients’ CEA response was analysed. All patients had
baseline CEA elevation (median CEA = 61; range, 8.3-1594). Eleven patients (78.6%) had a drop in CEA after
starting regorafenib. The median time to CEA nadir was
3 weeks and the median drop in CEA was 33.5% (range,
14%-73%). Using 50% as a cut-off, which was a value
also used in larger studies,13 seven (63.6%) of the CEA
responders had a drop in CEA ≤50%, while four (36.4%)
of them had a drop of >50%. No statistically significant
correlation was found between the degree of CEA drop
and the respective radiological response (Fisher’s exact
test, p = 1.00).
Tolerance
Average Time to Temporary Treatment Suspension
Ten patients (71.4%) required temporary treatment
suspension during their course of regorafenib due
to toxicities. The mean total time of suspension was
1.7 weeks, and the mean time of suspension per treatment
cycle was 4.9 days.
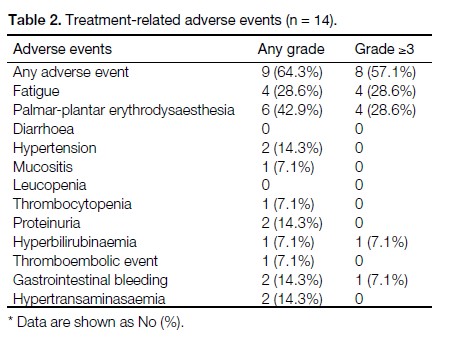
Adverse Events
Table 2 shows a summary of all the treatment-related
AEs. Nine patients (64.3%) experienced toxicity of
any kind and grade. Most AEs were severe, with eight
patients (57.1%) having grade ≥3 AEs. The most
common AEs were palmar-plantar erythrodysaesthesia
and fatigue (both 28.6%). Ten patients (71.4%) required
suspension at some point in their treatment.
Table 2. Treatment-related adverse events (n = 14).
Survival
Progression-free Survival
After a median follow-up of 194 days, disease progression
was noted in 13 patients. The median progression-free
survival (PFS) was 87 days (95% CI = 81.3-92.6 days).
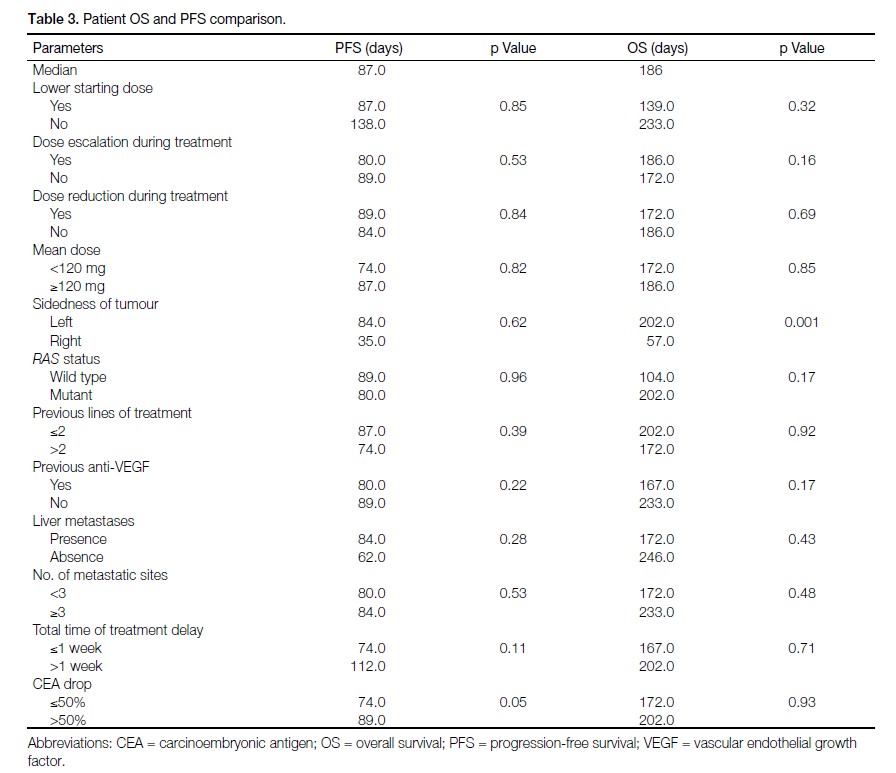
In univariate analysis, PFS was not significantly worse
for patients with a lower regorafenib starting dose or who
required dose reduction (n = 4), nor was it associated
with mean delay per cycle or the mean dose throughout
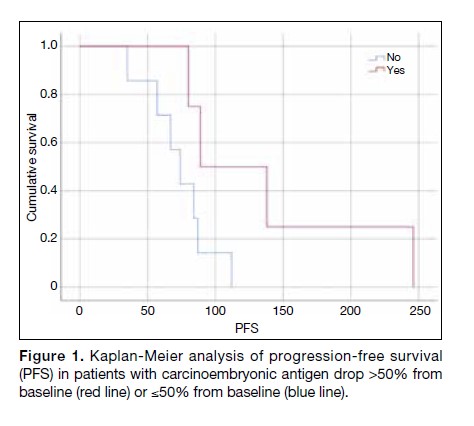
treatment (Table 3). The association between a >50%
decrease in CEA and better PFS was not significant
(89 vs. 74 days, p = 0.05) [Figure 1].
Table 3. Patient OS and PFS comparison.
Figure 1. Kaplan-Meier analysis of progression-free survival (PFS) in patients with carcinoembryonic antigen drop >50% from
baseline (red line) or ≤50% from baseline (blue line).
Overall Survival
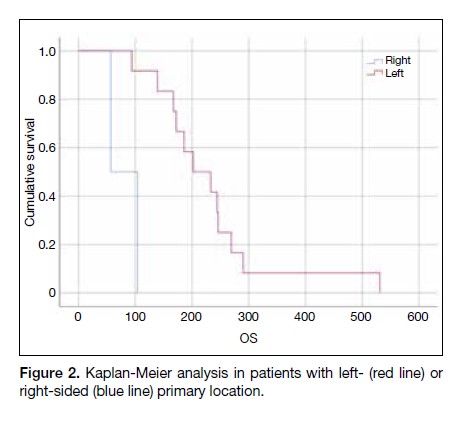
The median OS was 186 days (95% CI = 131-241 days).
Patients with left-sided tumours had longer OS when
compared with those with right-sided tumours (202 vs.
57 days, p = 0.001) [Figure 2]. No other factors were
significantly associated with OS (Table 3).
Figure 2. Kaplan-Meier analysis in patients with left- (red line) or
right-sided (blue line) primary location.
Subsequent Treatment
Only two (14%) of the patients had satisfactory World
Health Organization performance status (i.e., ECOG
score 1) and were able to afford and undergo further
treatment after disease progression. All of them received
trifluridine-tipiracil (Lonsurf; Taiho Oncology, Japan)
after regorafenib. Their survivals after regorafenib were
207 and 464 days, respectively.
DISCUSSION
Regorafenib is one of the few treatment options available
for mCRC that has failed oxaliplatin- and irinotecan-based
chemotherapy. In two large international
randomised controlled phase III studies, CORRECT5
and CONCUR,6 the median PFS was approximately 60.9
to 91.3 days, and the OS was approximately 197.8 to 273.9 days. In the current study, the median PFS was
87 days (95% CI = 81.3-92.6 days) and the median
OS was 186 days (95% CI = 131-241 days), which are
comparable to these two phase III studies.
AEs remain a concern in treatment with regorafenib. In
both the CORRECT and CONCUR trials, 54% of the
patients experienced grade ≥3 AEs,5,6 which are similar
to our study (57.1%). The most common AE in our study
was palmar-plantar erythrodysaesthesia, with >40% of
patients affected. Approximately two-thirds of these
patients had grade ≥3 palmar-plantar erythrodysaesthesia,
which commonly led to treatment suspension. Although
only 28.6% of the patients experienced fatigue, all of
them reported grade 3 fatigue (fatigue limiting self-care)
during the course of treatment. The occurrence of fatigue in trials varies considerably. In the CONCUR trial, only
17% of patients reported fatigue of any grade and only
2.9% had fatigue of grade ≥3.6 In contrast, 48% of
patients experienced fatigue in the CORRECT trial, with
9.6% of them having fatigue grade ≥3.5 In a systematic
review, the incidence of fatigue ranges from 2% to 73%
in different studies.[8]
As regorafenib is associated with significant rates of
AEs when used at full dose, the optimisation of dosing
and schedule is a widely discussed topic. From our data,
intercycle delay was common, with >70% of patients
experiencing a mean of 4.9 days of delay per treatment
cycle due to AEs, but without a statistically significant
impact on survival outcome. The PFS and OS for lower
starting doses were shorter than those of the usual
starting dose, but not significantly shorter. Outcomes also
appeared to be independent of dosing strategies (interval
dose reduction or interval dose escalation). In ReDOS,
a randomised phase II study, patients were randomised
to receive a starting dose of 80 mg with subsequent
dose escalation, or a standard starting dose of 160 mg.
The survival outcomes were not significantly different.[9]
While the small sample size limits robust statistical
inference, our data concur with the latest evidence. As the
slow dose escalation approach appears to result in better
tolerability and safety, we expect it to gain popularity
in the near future. Other strategies on management of
AEs have also been explored, though efficacy is limited.
For example, for treatment-related fatigue, one phase II
study investigated the effect of dexamethasone on these
patients but failed to show any improvement.[10]
Currently there is no established marker to predict the
treatment response towards regorafenib. In our study,
patients with left-sided tumours enjoyed longer OS,
which concurs with the findings of many other studies
that left-sided tumours carry a better prognosis regardless
of treatment, stage, race of patients, or length of study.[11]
Some retrospective studies also suggest that in addition
to its prognostic implications, primary tumour location
may be a predictive factor for treatment response in the
first-line setting.[12] Whether such predictive value also
applies to regorafenib warrants further study.
Although not significant, our results showed a marginal
association between drop in CEA and PFS. The median
time to CEA nadir was found to be 3 weeks. There is
no strong evidence on how the degree of CEA decrease
correlates with clinical response, and most studies have
used arbitrary cut-offs for CEA analysis. We used 50% as an arbitrary cut-off, which was a value also used in
larger studies.[13] As most of the CEA responders only
achieved stable disease in radiological assessment, it may
be suggested that the benefit observed in patients with
a drop in CEA of >50% is independent of radiological
response. The absolute reading of CEA is known to
reflect tumour burden and carries a prognostic value in
patients with mCRC, and was reported to have a role
in predicting treatment failure in the absence of readily
measurable disease response.[14] Further verification is
needed in a prospective manner to evaluate its prognostic
value.
Treatment beyond regorafenib is limited and no clinical
guidelines suggest an agreed-upon next line of treatment,
although trifluridine-tipiracil (Lonsurf), the other drug
licensed for use in refractory mCRC, is a frequent
treatment of choice. In our study, only two patients
underwent further treatment with trifluridine-tipiracil,
and the survival time was considerably long (median,
400 days). Trifluridine-tipiracil consists of a nucleoside
analogue (trifluridine) and a thymidine phosphorylase
inhibitor (tipiracil) which causes DNA strand breaks.[15]
The different mode of action may explain the longer
disease control in patients who have failed regorafenib.
Treatment after regorafenib rather than best supportive
care is therefore a reasonable option provided that
patients are still fit for systemic treatment.
A number of limitations of this study should be
acknowledged. First, the eligible population was small
(n = 14) as only a proportion of patients remained
fit for further treatment after failing multiple lines of
chemotherapy. The considerable cost of regorafenib (a
self-financed item) also limited the number of eligible
patients. As a result, it can only be deduced that there was
a tendency suggesting that good response of CEA and a
left-sided primary tumour were favourable prognostic
factors in patients using regorafenib, and this should be
verified in a larger prospective study. Second, the cut-off
used for CEA analysis was arbitrary, without verification
by prospective data. Finally, the report of toxicity
outcomes is also compromised by its retrospective nature
and the subjectivity of toxicities like fatigue.
With the advancements in molecular studies, more
options will be available for patients with mCRC who
have run out of treatment choices. The BRAF mutation
is found in approximately 5% to 10% of patients with
mCRC.[16] It is known to carry a poor prognosis,[17] and
is a predictive factor for poor response to anti-EGFR therapy in RAS wild-type patients,[18]18 so much so that
established international guidelines do not recommend
anti-EGFR therapy in patients who harbour the BRAF
mutation.[19] Among these patients, monotherapy with the
BRAF inhibitor vemurafenib failed to show a meaningful
activity in BRAFV600E-mutated mCRC.[20] In a phase I/II
open-label study, more than half of the study population
achieved a stable disease after a combination of the BRAF
inhibitor daBRAFenib, and the MEK inhibitor trametinib.
The overall response rate was 12% and the median PFS
was 3.5 months (95% CI = 3.4-4.0 months).[21] Immune
checkpoint inhibitors have been shown to benefit
patients with deficient mismatch repair (dMMR), which
is characterised by a high number of DNA replication
errors and high levels of DNA microsatellite instability.
The dMMR tumours are present in approximately 5%
of patients with mCRC[22] and are known to carry a poor
prognosis, which is driven by its association with the
BRAF mutation.[17] In a phase II study looking at the use
of pembrolizumab in patients with mCRC, an objective
response rate (ORR) of 50% was achieved in patients
with dMMR, while none was achieved in patients with
proficient mismatch repair.[23] OS and PFS have not
been reached for patients with dMMR, whereas the
OS was 7.6 months and the PFS was 2.3 months for
patients with proficient mismatch repair. As a result,
pembrolizumab has been granted an indication by the
FDA for its use in colorectal cancer that has progressed
following treatment with a fluoropyrimidine, oxaliplatin,
and irinotecan without satisfactory alternative treatment
options. Nivolumab, a monoclonal antibody against
programmed cell death protein 1, is another option
for patients with dMMR. In the CheckMate 142 trial
that looked at nivolumab ± ipilimumab, a monoclonal
antibody against cytotoxic T-lymphocyte antigen 4, in
heavily pretreated patients with mCRC, the ORR with
nivolumab monotherapy was 31.1%.[22] It was even
higher in the nivolumab plus ipilimumab group with an
ORR of 55%. Grade 3 to 4 toxicities were observed in
20% in the monotherapy group, and 29% in the doublet
group.[24] Based on these results, FDA indications have
been granted for the use of nivolumab in patients with
dMMR who have failed a fluoropyrimidine, oxaliplatin,
and irinotecan; and for the use of nivolumab plus
ipilimumab for patients with previously treated dMMR
mCRC.
In patients with unclear dMMR status, addition of
nivolumab to regorafenib has been investigated in
REGONIVO, a phase IB study. This strategy yielded
an ORR of 38% in the unselected population, and an even higher response in patients with microsatellite
instability–high CRC (44%).[25] When used with
nivolumab, reduction of the starting dose of regorafenib
to 80 mg rendered this regimen more tolerable.
CONCLUSION
Treatments for mCRC after oxaliplatin- and irinotecanbased
chemotherapy remain limited. Our institutional
experience with regorafenib was generally consistent
with the available literature. Our study also found that
there is a tendency towards a longer duration of stable
disease in patients with an initial drop of CEA after
starting regorafenib and a left-sided primary tumour.
Treatment beyond regorafenib in those who remain
medically fit and are able to afford more treatment
resulted in a favourable OS. With the promise of
novel agents shown to be highly effective in selected
populations, and their overall favourable toxicity profile,
further prospective studies are warranted.
REFERENCES
Hong Kong Cancer Registry, Hospital Authority, Hong Kong SAR Government. 2016. Available from: https://www3.ha.org.hk/cancereg Accessed 24 Apr 2019.
2. National Cancer Institute, Department of Health and Human Service, US Government. Surveillance, Epidemiology, and End Results (SEER) Program Research Data (1975-2016), National Cancer Institute, DCCPS, Surveillance Research Program. Available from: https://www.seer.cancer.gov. Accessed 1 Jun 2019.
3. Jawed I, Wilkerson J, Prasad V, Duffy AG, Fojo T. Colorectal cancer survival gains and novel treatment regimens: a systematic review and analysis. JAMA Oncol. 2015;1:787-95. Crossref
4. Yoshino T, Arnold D, Taniguchi H, Pentheroudakis G, Yamazaki K, Xu RH, et al. Pan-Asian adapted ESMO consensus guidelines for the management of patients with metastatic colorectal cancer: a JSMO-ESMO initiative endorsed by CSCO, KACO, MOS, SSO and TOS. Ann Oncol. 2018;29:44-70. Crossref
5. Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303-12. Crossref
6. Li J, Qin S, Xu R, Yau TC, Ma B, Pan H, et al. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2015;16:619-29. Crossref
7. Lam KO, Lee KC, Chiu J, Lee VH, Leung R, Choy TS, et al. The real-world use of regorafenib for metastatic colorectal cancer: multicentre analysis of treatment pattern and outcomes in Hong Kong. Postgrad Med J. 2017;93:395-400. Crossref
8. Røed Skårderud M, Polk A, Kjeldgaard Vistisen K, Larsen FO, Nielsen DL. Efficacy and safety of regorafenib in the treatment of metastatic colorectal cancer: A systematic review. Cancer Treat Rev. 2018;62:61-73. Crossref
9. Bekaii-Saab TS, Ou FS, Ahn DH, Boland PM, Ciombor KK, Heying EN, et al. Regorafenib dose-optimisation in patients with refractory metastatic colorectal cancer (ReDOS): a randomised, multicentre, open-label, phase 2 study. Lancet Oncol. 2019;20:1070-82. Crossref
10. Tanioka H, Miyamoto Y, Tsuji A, Assayama M, Shiraishi T, Yuki S, et al. Prophylactic effect of dexamethasone on regorafenib-related fatigue and/or malaise: a randomized, placebo-controlled, double-blind clinical study in patients with unresectable metastatic colorectal cancer (KSCC1402/HGCSG1402). Oncology. 2018;94:289-96. Crossref
11. Petrelli F, Tomasello G, Borgonovo K, Ghidini M, Turati L Dallera P, et al. Prognostic survival associated with left-sided vs right-sided colon cancer: a systematic review and meta-analysis. JAMA Oncol. 2017;3:211-9. Crossref
12. Holch JW, Ricard I, Stintzing S, Modest DP, Heinemann V. The relevance of primary tumour location in patients with metastatic colorectal cancer: A meta-analysis of first-line clinical trials. Eur J Cancer. 2017;70:87-98. Crossref
13. Aggarwal C, Meropol NJ, Punt CJ, Iannotti N, Saidman BH, Sabbath KD, et al. Relationship among circulating tumor cells, CEA and overall survival in patients with metastatic colorectal cancer. Ann Oncol. 2013;24:420-8. Crossref
14. Clinical practice guidelines for the use of tumor markers in breast and colorectal cancer. Adopted on May 17, 1996 by the American Society of Clinical Oncology [editorial]. J Clin Oncol. 1996;14:2843-77. Crossref
15. Lenz HJ, Stintzing S, Loupakis F. TAS-102, a novel antitumor agent: a review of the mechanism of action. Cancer Treat Rev. 2015;41:777-83. Crossref
16. Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949-54. Crossref
17. Tran B, Kopetz S, Tie J, Gibbs P, Jiang ZQ, Lieu CH, et al. Impact of BRAF mutation and microsatellite instability on the pattern of metastatic spread and prognosis in metastatic colorectal cancer. Cancer. 2011;117:4623-32. Crossref
18. Rowland A, Dias MM, Wiese MD, Kichenadasse G, McKinnon RA, Karapetis CS, et al. Meta-analysis of BRAF mutation as a predictive biomarker of benefit from anti-EGFR monoclonal antibody therapy for RAS wild-type metastatic colorectal cancer. Br J Cancer. 2015;112:1888-94. Crossref
19. Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27:1386-422. Crossref
20. Kopetz S, Desai J, Chan E, Hecht JR, O’Dwyer PJ, Maru D, et al. Phase II pilot study of vemurafenib in patients with metastatic BRAF-mutated colorectal cancer. J Clin Oncol. 2015;33:4032-8. Crossref
21. Corcoran RB, Atreya CE, Falchook GS, Kwak EL Ryan DP, Bendell JC, et al. Combined BRAF and MEK inhibition with daBRAFenib and trametinib in BRAFV600-mutant colorectal cancer. J Clin Oncol. 2015;33:4023-31. Crossref
22. Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz HJ, Morse MA, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18:1182-91. Crossref
23. Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509-20. Crossref
24. Overman MJ, Lonardi S, Wong KY, Lenz HJ, Gelsomino F, Aglietta M, et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J Clin Oncol. 2018;36:773-9. Crossref
25. Fukuoka S, Hara H, Takahashi N, Kojima T, Kawazoe A, Asayama M, et al. Regorafenib plus nivolumab in patients with advanced gastric (GC) or colorectal cancer (CRC): An open-label, dose-finding, and dose-expansion phase 1b trial (REGONIVO, EPOC1603). J Clin Oncol. 2019;37(15 suppl):2522. Crossref