Risk of Radiation Pneumonitis after Post-lobectomy Thoracic Radiotherapy for Non-small-cell Lung Cancer
ORIGINAL ARTICLE
Risk of Radiation Pneumonitis after Post-lobectomy Thoracic Radiotherapy for Non-small-cell Lung Cancer
W Chan, JSF Nyaw, WWY Tin, EKC Lee, SH Lo, ACH Liu, FCS Wong
Department of Clinical Oncology, Tuen Mun Hospital, Tuen Mun, Hong Kong
Correspondence: Dr W Chan, Department of Clinical Oncology, Tuen Mun Hospital, Hong Kong. Email: cw698@ha.org.hk
Submitted: 14 Feb 2019; Accepted: 29 Jul 2019.
Contributors: All authors contributed to the concept of study, acquisition and analysis of data, drafting of the manuscript, and had critical revision of the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: As an editor of the journal, FCS Wong was not involved in the peer review process. Other authors have disclosed no conflicts of interest.
Funding/Support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics Approval: This retrospective study was approved by the New Territories West Research Ethics Committee (Ref NTWC/CREC/18053).
Abstract
Introduction
Patients who had lobectomy prior to thoracic radiotherapy may be more prone to developing radiation pneumonitis (RP) given their smaller remaining lung volume. There is no consensus on the normal lung dose constraints in this population. V20 ≤30% to 35% and mean lung dose (MLD) ≤20 Gy are common dose constraints used in definitive radiotherapy for lung cancer. We aimed to determine whether V20 ≤33% and mean MLD ≤20 Gy are safe constraints with an acceptable risk of RP in post-lobectomy patients.
Methods
The data of all patients who received post-lobectomy thoracic radiotherapy for non-small-cell lung cancer at a single centre in Hong Kong from 2011 to 2018 were analysed retrospectively. The endpoint was Common Terminology Criteria for Adverse Events (CTCAE) grade ≥3 RP. Statistical analysis was performed to investigate whether V10 and V20 of whole lung were predictive factors for RP.
Results
Fifty-five patients had been treated using the dose constraints of V20 ≤33% and MLD ≤20 Gy. Three patients developed grade 5 RP. No patients had grade 3 or 4 RP. There was no significant association between the V10, V20 and grade ≥3 RP.
Conclusion
Our data showed that the risk of grade ≥3 RP was 5.5% in post-lobectomy patients using dose constraints of V20 ≤33% and MLD ≤20 Gy. Further prospective studies are necessary to clarify the optimal dose constraints in this population.
Key Words: Carcinoma, non-small-cell lung; Radiation pneumonitis; Radiotherapy, adjuvant
中文摘要
肺葉切除術後接受胸部放療的非小細胞肺癌患者出現放射性肺炎的風險
陳偉、饒仕鋒、佃穎恩、李家齊、魯勝雄、廖卓謙、黃志成
引言
在胸部放療前接受肺葉切除術的患者,由於其剩餘肺體積較小,可能更易出現放射性肺炎。對於正常肺部劑量限制尚未有共識。V20 ≤30%至35%及平均肺部劑量(MLD)≤20 Gy是用於肺癌最終放療的常見劑量限制。本研究旨在確定在肺葉切除術後患者中,V20 ≤33%和MLD ≤20 Gy是否安 全及可接受放射性肺炎的風險範圍。
方法
回顧分析2011年至2018年在香港單中心進行肺葉切除術後接受胸部放療的非小細胞肺癌患者的數據。終點為根據毒性標準(CTCAE)定義的≥3級放射性肺炎。進行統計分析以檢視全肺中使用V10和V20是否放射性肺炎的預測因素。
結果
55名患者接受V20 ≤33%和MLD ≤20 Gy的劑量限制。3例患者出現5級放射性肺炎。沒有患者出現3或4級放射性肺炎。V10、V20和≥3級放射性肺炎間無顯著關聯。
結論
研究結果顯示使用劑量限制為V20 ≤33%和MLD ≤20 Gy的肺葉切除術後患者,其≥3級放射性肺炎的風險為5.5%。需要進一步前瞻性研究闡明最佳劑量限制。
BACKGROUND
External beam radiotherapy plays an important role
in the treatment of thoracic malignancy. Radiation
pneumonitis (RP) resulting from radiation-induced
injury of normal lung tissue is an important concern
for thoracic oncologists. Radiotherapy after tumour
resection has been found to be detrimental to survival
in some early-stage lung cancer.[1] It has been suggested
that any improvement of local control by radiotherapy
might be offset by fatal RP in these patients.[2] Predictive
factors of RP in definitive radiotherapy with or without
concurrent chemotherapy include V10 and V20 of whole
lung (both ipsilateral and contralateral lungs),[3] higher
daily dose,[4] concurrent chemotherapy,[4] old age,[5] pre-radiotherapy
forced expiratory volume,[6] and female sex.[6]
The Quantitative Analysis of Normal Tissue Effects in
the Clinic (QUANTEC) suggested limiting V20 (whole
lung) to ≤30% to 35% and mean lung dose (MLD) to
≤20 Gy to 23 Gy in patients with non-small-cell lung
cancer who are treated with definitive radiotherapy.[7]
This dose constraint may not be applicable in patients
who have reduced lung volume as a result of surgical
lung resection. A retrospective study showed that
mesothelioma patients undergoing radiotherapy after
pneumonectomy had a significantly higher risk of RP.[8]
This is not surprising, given their much smaller residual
lung volume. The QUANTEC recommended more
stringent dose constraints of V20 <4% to 10%, V5 <60%,
and MLD <8 Gy for post-pneumonectomy patients.[7]
What remains unclear is whether patients who had
lobectomy, with its associated reduced lung volume,
also require more stringent dose constraints. Suggested
dose constraints in the post-lobectomy setting include
V20 ≤35% and MLD <20 Gy.[9] It is unknown whether
post-lobectomy patients could be treated using the dose
constraints employed for definitive radiotherapy (V20
to ≤30%-35% and MLD ≤20-23 Gy) with an acceptable
risk of RP.
A recently published retrospective study by the MD
Anderson Cancer Center (MDACC) has shown that in
patients who received radiotherapy after lobectomy,
pneumonectomy or wedge resection, V10 >30% and V20
>20% were predictive of grade ≥ 2 RP.[10] The predictive
dosimetric parameters of RP specific to post-lobectomy
patients receiving radiotherapy are less clear.10
We performed a retrospective study to investigate the
incidence of grade ≥3 RP in post-lobectomy patients
treated with the dose constraints of V20 ≤33% and MLD
≤20 Gy. We hypothesised that V10 and V20 of whole
lung are predictive factors of grade ≥3 RP.
METHODS
We retrospectively reviewed the clinical records of all
patients who underwent postoperative radiotherapy for
non-small-cell lung cancer from January 2011 to January
2018 in Tuen Mun Hospital, Hong Kong. Only data from
patients that had undergone lobectomy were included.
These patients were identified using the MOSAIQ® Radiation Oncology information system and the Clinical
Data Analysis and Reporting System of the Hong Kong
Hospital Authority.
Clinical factors including age, disease stage,
performance status, smoking status, specific lobe(s)
resected, tumour histology, indications for radiotherapy,
dose fractionation of radiotherapy, radiotherapy
technique (three-dimensional [3D] conformal or
intensity-modulated radiotherapy [IMRT]), and the use
of concurrent chemotherapy were recorded. Dosimetric
parameters including V5, V10 and V20 of whole lung,
MLD, mean heart dose, and planning target volume
(PTV) size were recorded as well.
The primary endpoint of the study was grade ≥3 RP
according to the Common Terminology Criteria for
Adverse Events (CTCAE) version 4.03.[11] The diagnosis
of RP was made by two clinical oncologists either at the
time of presentation or retrospectively upon reviewing
the clinical record. Grade 2 RP was not included as the
primary endpoint, because its retrospective diagnosis can
be difficult. For instance, cough or shortness of breath
after radiotherapy could be attributed to underlying
co-morbidities such as chronic obstructive pulmonary
disease (COPD).
Fisher’s exact test was used to determine if V10 ≥30%
and V20 ≥20% were predictive of grade ≥3 RP. The
Mann-Whitney U test was used to determine whether
V5, V10 and V20 of whole lung, mean heart dose, MLD,
and PTV size were associated with the risk of grade ≥3
RP. Receiver operating characteristic curve analysis was
used to establish the optimal cut-off points for dosimetric
parameters identified to be associated with grade ≥3 RP.
This retrospective study was approved by the New
Territories West Research Ethics Committee (Ref
NTWC/CREC/18053).
RESULTS
A total of 58 patients were identified. Three patients were
excluded from analysis because of the following reasons:
declined further radiotherapy after only six fractions
given (n = 1), only palliative lobectomy performed for
obstructive pneumonia prior to radiotherapy (n = 1),
and death from extrathoracic recurrence shortly after
completion of radiotherapy (n = 1). The patient with
disease recurrence was found to have extensive liver
metastases 2 weeks after completion of radiotherapy.
He developed liver failure and succumbed to it 2 weeks
later.
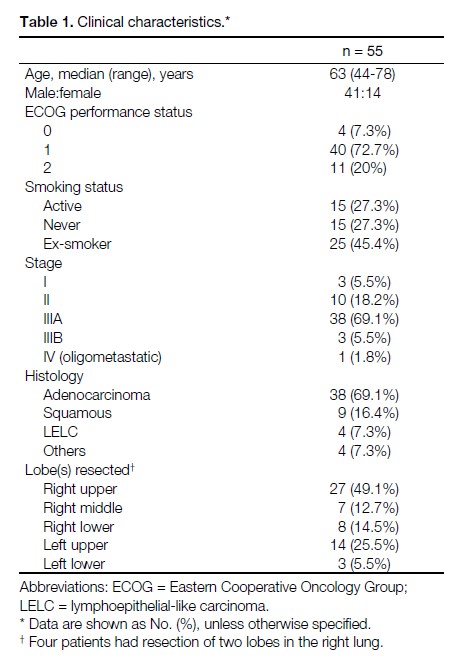
Among the 55 patients available for analysis, the
median age was 63 years. 69.1% had adenocarcinoma;
16.4% had squamous cell carcinoma; and 7.3% had
lymphoepithelial-like carcinoma. Their demographics,
stage, histology and the specific lobe(s) resected are
listed in Table 1.
Table 1. Clinical characteristics.
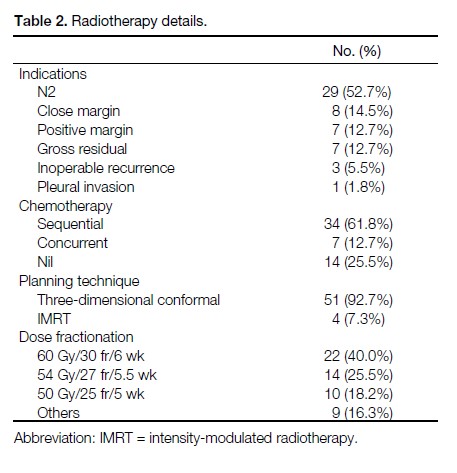
Most patients received radiotherapy for N2 disease
(52.7%). Other indications included positive / close
margin, gross residual tumour, and inoperable recurrence
after initial curative surgery. The mean radiotherapy
dose was 54 Gy (range, 50 Gy-66 Gy). The common
dose fractionations were 60 Gy/30 fr/6 weeks (40.0%),
54 Gy/27 fr/5.5 weeks (25.5%), and 50 Gy/25 fr/5 weeks
(18.2%). Other dose fractionation used for patients
with confirmed recurrence after lobectomy included
66 Gy/33 fr/6.5 weeks. The details of radiotherapy are
listed in Table 2.
Table 2. Radiotherapy details.
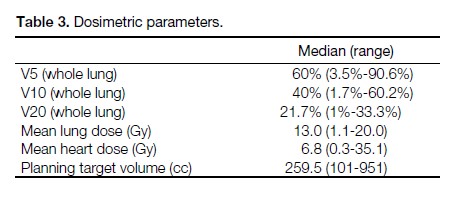
All but two patients were treated using the dose constraint
of V20 ≤33% and MLD ≤20 Gy. One patient had a V20
of 33.2% and one patient had a V20 of 33.3%. The V5,
V10, V20 of whole lung, MLD, mean heart dose, and
PTV size of all patients are listed in Table 3.
The median follow-up duration was 25 months. Patients
who had undergone post-lobectomy radiotherapy in our
hospital had regular follow-up by both a cardiothoracic
surgeon and a clinical oncologist. History taking,
physical examination, and chest X-ray were done during
these clinic visits. Computed tomography (CT) scan
was performed only if there was clinical suspicion for
any pathology. The follow-up interval was every 3 to
4 months during the first year.
Table 3. Dosimetric parameters.
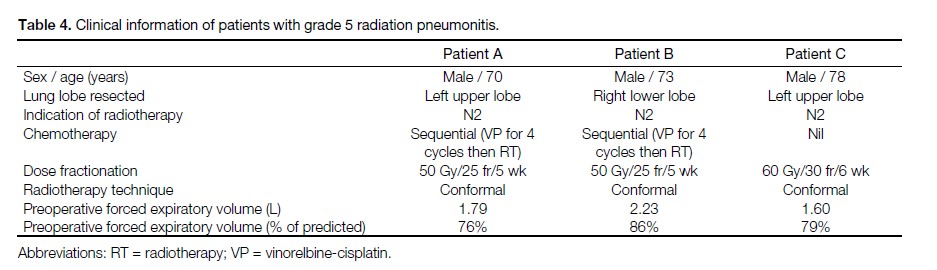
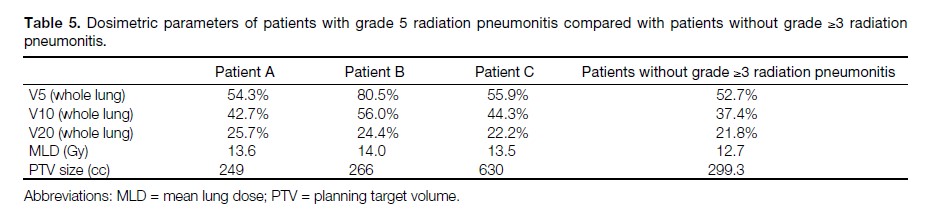
Three patients developed grade 5 RP. All three of them
had V10 ≥30% and V20 ≥20%. No patient with grade
3 or 4 RP was identified. The clinical information of
these three patients are listed in Table 4. The dosimetric
parameters of these three patients and the remaining
patients in our study are listed in Table 5.
Table 4. Clinical information of patients with grade 5 radiation pneumonitis.
Table 5. Dosimetric parameters of patients with grade 5 radiation pneumonitis compared with patients without grade ≥3 radiation
pneumonitis.
Among the 55 patients included in the analysis, 33 had
V10 ≥30% and V20 ≥20%. The risk of grade ≥3 RP was
5.5% (95% confidence interval [CI] = 1.4%-16.1%). The
mortality from RP among patients with V10 ≥30% and
V20 ≥20% was 9.1% (95% CI = 2.4%-25.5%).
There was no significant association between V10
≥30%, V20 ≥20% and the occurrence of grade ≥3 RP
(p = 0.267). No statistically significant relationship was
found between grade ≥3 RP and V5 (p = 0.393), V10
(p = 0.128), or V20 of whole lung (p = 0.767), MLD
(p = 0.541), or PTV size (p = 0.436).
All three patients with grade 5 RP were hospitalised for
acute shortness of breath within 15 days after completion
of radiotherapy (range, 7-15 days). Two patients
received a short course of corticosteroid treatment for
the presumed diagnosis of acute exacerbation of COPD
in the first admission to the acute medical ward and
were discharged. Both were re-admitted within 10 days
after the last dose of prednisolone and eventually died
during the second hospitalisation. The diagnosis of RP
was made by two clinical oncologists after reviewing the
clinical history, imaging, and laboratory findings. In the
third patient, the diagnosis of RP was made in the first
admission in the oncology ward. He was discharged with
a tapering dose of steroid. This patient was re-admitted
on tapering doses of steroid and died during the second
hospitalisation.
In summary, all three patients had deterioration of
symptoms shortly after steroids were stopped or tapered
to a lower dose.
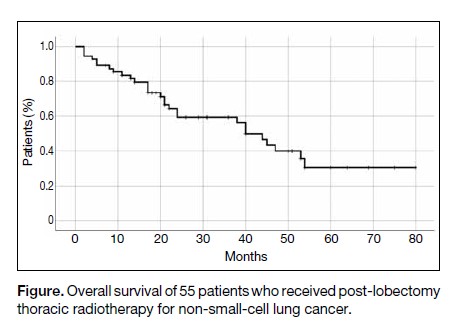
The median overall survival of the 55 patients was
40 months (Figure).
Figure. Overall survival of 55 patients who received post-lobectomy thoracic radiotherapy for non-small-cell lung cancer.
DISCUSSION
Our study demonstrated that even after restricting V20
to ≤33% and MLD to ≤20 Gy, there was still a notable
risk of RP in post-lobectomy patients, with an overall
mortality of 5.5%.
Consistent with the MDACC’s results regarding the
predictive role of V10 >30% and V20 >20%,10 all three
patients with grade 5 RP had V10 >30% and V20 >20%.
However, because of the small sample size, we were not
able to demonstrate the predictive value of any dosimetric
parameters including V10 and V20 of whole lung.
It should be noted that 60% of our patients had V10 ≥30%
and V20 ≥20%. It may not be practicable to regard V10
<30% and V20 <20% as dose constraints. Patients who
have V10 ≥30% and V20 ≥20% may still be permitted to
undergo thoracic radiotherapy, provided that the benefit
in reducing recurrence would outweigh the higher than
normal risk of RP. Nevertheless, efforts should be made
to keep V10 <30% and V20 <20%. Possible strategies to
reduce the risk of RP include the use of four-dimensional
CT ventilation for functional avoidance and IMRT.[12]
The observation that all three patients had deterioration
of symptoms and died after finishing or while tapering
down steroid raised our concern. Two of our patients were
initially managed by physicians as acute exacerbations of
COPD with a short course of prednisolone 30 mg daily.
Steroid prescribed for RP is usually of much higher dose
and longer duration. Prednisone is commonly prescribed
at a starting dose of at least 40 to 60 mg daily (or 1 mg/kg
daily) and is slowly tapered over 8 to 12 weeks while
monitoring patient symptoms.13 We hypothesise that the
prednisolone given in these two patients, for the presumed
diagnosis of COPD, did suppress their pneumonitis and
led to initial improvement of their symptoms. Following
their discharge from hospital, the tapering or cessation of
steroids might have led to rebound of RP, finally leading
to repeat admission and death.
There were no patients with grade 3 or 4 RP. It can
be argued that if some of the patients that ultimately
developed grade 5 RP were correctly diagnosed earlier,
death might not have ensued. However, this would
unlikely alter the rate of grade ≥3 RP. These patients
were all admitted for severe shortness of breath requiring
oxygen. Their clinical conditions in their first admission
already satisfied the criteria for diagnosing grade 3
RP, regardless of the subsequent treatment outcome.
Nevertheless, better cooperation between physicians
in the acute medical ward and oncologists may be
beneficial. Earlier diagnosis of RP and the use of steroid
with adequate dose and duration may potentially alter the
outcome.
Our results compared less favourably with the MDACC
study. In the MDACC study, only 3.3% of their patients
had grade 3 RP, and no patients had grade 4 or 5 RP.[10]
Their lower rate of RP may be explained by their more
advanced radiotherapy technique. Only 34% of their
patients were treated with 3D conformal technique. The
remaining was treated with IMRT or proton therapy. In
our study, 93% of patients were treated with 3D conformal
technique. IMRT has been shown to carry a lower risk of
RP compared with 3D conformal technique.[12] 17% of the
patients in the MDACC study were treated with proton
therapy, which has been shown to significantly reduce
V10 and V20 compared with photon techniques.[14] Our
study results are likely more applicable to patients treated
with conformal radiotherapy, which is still commonly
used worldwide.
In a Chinese study on RP in post-lobectomy patients,
among 85 patients included for analysis, 25.9%
developed grade ≥3 RP.[15] In a subsequent publication
involving 177 patients from the same centre, multivariate
analysis showed that total lung mean dose (>10.8 Gy),
V5 to ipsilateral lung (>64.9%), and concurrent
chemotherapy were significantly associated with grade
≥3 RP.[16] Based on these factors, a nomogram to predict
the risk of grade ≥3 RP was generated. The incidence
of grade ≥3 RP was not reported in the full paper of this
subsequent publication.
A Japanese group published their finding in salvage
radiotherapy in 21 patients with recurrence after
surgery treated between 2000 and 2004.[17] All patients
had undergone lobectomy and mediastinal lymph node
dissection before developing recurrence. The mean
salvage radiotherapy dose was 60 Gy (range, 46-60 Gy).
Three patients developed grade ≥2 RP. Grade 3 RP
developed in one patient only. The predominant beam
arrangement was anteroposterior-posteroanterior
followed by off-cord oblique fields. Their grade ≥3 RP
risk was 4.8%. This slightly lower risk might be related
to the lower dose received by normal lung. Their median
V20 and MLD were 17% and 9.1 Gy, respectively. The
median V20 and MLD in our study were 21.7% and
13 Gy, respectively. Our result is likely more relevant
in the adjuvant setting where there is no documented
disease recurrence.
Our study has some limitations. First, it was a retrospective
study. Most of our patients did not have regular
surveillance CT scans. A better-designed prospective
study with regular CT scans would allow better reporting
of RP of all grades. However, we believe that grade ≥3
RP is an objective and unequivocal outcome compared
with RP of lower grades. For instance, CTCAE grade
3 pneumonitis is defined as either limiting self-care
activities of daily living, causing severe symptoms, or
requiring oxygen. Such clinically significant symptoms
would usually prompt further investigation and lead
to the diagnosis of RP even in the absence of regular
follow-up cross-sectional imaging.
Second, due to the paucity of grade ≥3 RP in our study,
we were not able to conduct a robust statistical analysis
to identify any predictive factors. A multicentre study is
likely required to have enough cases of severe RP for
statistical analysis to identify the predictive factors and
cut-off point.
Despite the limitations, our study has some strengths.
First, our study adds to the scarce literature of RP in postlobectomy
patients receiving radiotherapy. There are few
published data on the safety of post-lobectomy thoracic
radiotherapy and the risk of RP using dose constraints of
V20 ≤33% and MLD ≤20%.
Second, our patients had good follow-up and complete
radiotherapy records. Most of our patients reside locally
and all of them continued follow-up in our hospital. No
patients were excluded from analysis due to incomplete
radiotherapy records, likely because our patients were
treated in recent years with easily accessible electronic
records.
Most studies published so far about RP in post-lobectomy
patients were retrospective in nature and had the inherent
problems of underdiagnosis or incomplete data. The
Lung ART study is an ongoing prospectively designed
multicentre study to evaluate the role of postoperative
thoracic radiotherapy in completely resected N2 non-small-cell lung cancer.[18] The results will likely provide
new insights in the risk of RP and the appropriate dose
constraints in this group of patients.
CONCLUSION
In summary, we demonstrated that the risk of grade ≥3
RP in post-lobectomy patients was 5.5% despite fulfilling
the dose-volume constraints of V20 ≤33% and MLD
≤20 Gy. The mortality from RP among patients with V10
≥30% and V20 ≥20% was as high as 9.1%. This adds to
the scarce literature on the appropriate dose constraint
in post-lobectomy thoracic radiotherapy. A high index
of suspicion and prompt diagnosis of RP is important
for patients receiving post-lobectomy radiotherapy.
Prospectively designed multicentre studies, such as the
Lung ART study, can provide further information on the
risk of RP and the appropriate dose-volume constraints.
REFERENCES
1. Postoperative radiotherapy in non-small-cell lung cancer: systematic review and meta-analysis of individual patient data from nine randomised controlled trials Lancet. 1998;352:257-63. Crossref
2. Munro AJ. What now for postoperative radiotherapy for lung cancer? Lancet. 1998;352:250-1. Crossref
3. Madani I, De Ruyck K, Goeminne H, De Neve W, Thierens H, Van Meerbeeck J. Predicting risk of radiation-induced lung injury. J Thorac Oncol. 2007;2:864-74. Crossref
4. Segawa Y, Takigawa N, Kataoka M, Takata I, Fujimoto N, Ueoka H. Risk factors for development of radiation pneumonitis following radiation therapy with or without chemotherapy for lung cancer. Int J Radiat Oncol Biol Phys. 1997;39:91-8. Crossref
5. Palma DA, Senan S, Tsujino K, Barriger RB, Rengan R, Moreno M, et al. Predicting radiation pneumonitis after chemoradiation therapy for lung cancer: An international individual patient data meta-analysis. Int J Radiat Oncol Biol Phys. 2013;85:444-50. Crossref
6. Robnett TJ, Machtay M, Vines EF, McKenna MG, Algazy KM, McKenna WG. Factors predicting severe radiation pneumonitis in patients receiving definitive chemoradiation for lung cancer. Int J Radiat Oncol Biol Phys. 2000;48:89-94. Crossref
7. Marks LB, Bentzen SM, Deasy JO, Kong FM, Bradley JD, Vogelius IS, et al. Radiation dose-volume effects in the lung. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S70-6. Crossref
8. Miles EF, Larrier NA, Kelsey CR, Hubbs JL, Ma J, Yoo S, et al. Intensity-modulated radiotherapy for resected mesothelioma: the Duke experience. Int J Radiat Oncol. 2008;71:1143-50. Crossref
9. Gomez DR, Komaki R. Postoperative radiation therapy for nonsmall cell lung cancer and thymic malignancies. Cancers (Basel). 2012;4:307-22. Crossref
10. Boonyawan K, Gomez DR, Komaki R, Xu Y, Nantavithya C, Allen PK, et al. Clinical and dosimetric factors predicting grade ≥2 radiation pneumonitis after postoperative radiotherapy for patients with non-small cell lung carcinoma. Int J Radiat Oncol Biol Phys. 2018;101:919-26. Crossref
11. Cancer Therapy Evaluation Program, Division of Cancer Treatment & Diagnosis, National Cancer Institute, US Government.CTCAE_4 @ ctep.cancer.gov. Available from: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_4.03.xlsx. Accessed 25 Jun 2019.
12. Jain V, Berman AT. Radiation pneumonitis: old problem, new tricks. Cancers (Basel). 2018;10. pii: E222. Crossref
13. Bledsoe TJ, Nath SK, Decker RH. Radiation pneumonitis. Clin Chest Med. 2017;38:201-8. Crossref
14. Chang JY, Zhang X, Wang X, Kang Y, Riley B, Bilton S, et al. Significant reduction of normal tissue dose by proton radiotherapy compared with three-dimensional conformal or intensity-modulated radiation therapy in Stage I or Stage III non-small-cell lung cancer. Int J Radiat Oncol. 2006;65:1087-96. Crossref
15. Gong Y, Wang S, Xu Y, Wang J, Sun C, Bai S, et al. 124P: Acute severe radiation pneumonitis in post-operation radiation therapy among patients with lung cancer: An analysis of dose-volume parameters. J Thorac Oncol. 2016;11:S109-10. Crossref
16. Tang X, Li Y, Tian X, Zhou X, Wang Y, Huang M, et al. Predicting severe acute radiation pneumonitis in patients with non-small cell lung cancer receiving postoperative radiotherapy: Development and internal validation of a nomogram based on the clinical and dose-volume histogram parameters. Radiother Oncol. 2019;132:197-203. Crossref
17. Uno T, Isobe K, Kawakami H, Ueno N, Kawata T, Yamamoto S, et al. Dose-volume factors predicting radiation pneumonitis in patients receiving salvage radiotherapy for postlobectomy locoregional recurrent non-small-cell lung cancer. Int J Clin Oncol. 2006;11:55-9. Crossref
18. Faivre-Finn C, Le Pechoux C, Edwards J, Chappel B, Gornall H. 169: Lung ART: Phase III study comparing post-operative conformal radiotherapy to no post-operative radiotherapy in patients with completely resected non-small cell lung cancer and mediastinal N2 involvement. Lung Cancer. 2017;103:S79. Crossref