Medical Pulmonary Diseases That Cause Neonatal Respiratory Distress: A Radiological Pictorial Essay
PICTORIAL ESSAY
Medical Pulmonary Diseases That Cause Neonatal Respiratory Distress: A Radiological Pictorial Essay
WP Cheung1, KC Wong2, WS Mak1, CYY Kwong1, EYL Kan2
1 Department of Diagnostic and Interventional Radiology, Kwong Wah Hospital, Yaumatei, Hong Kong
2 Department of Radiology, Hong Kong Children’s Hospital, Kowloon Bay, Hong Kong
Correspondence: Dr WP Cheung, Department of Diagnostic and Interventional Radiology, Kwong Wah Hospital, Yaumatei, Hong Kong. Email: cwp461@ha.org.hk
Submitted: 14 Nov 2018; Accepted: 27 Nov 2018.
Contributors: All authors contributed to the concept of study, acquisition and analysis of data, drafting of the manuscript, and had critical revision of the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This pictorial essay received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics Approval: Informed consent was obtained from patients’ carers for all tests and procedures.
INTRODUCTION
Respiratory distress is a common condition affecting up
to 7% of all neonates.[1] It is a common manifestation of
different underlying conditions that include pulmonary,
cardiovascular, metabolic, and systemic diseases. It
is recognised as the presence of signs of increased
respiratory difficulty, including tachypnoea, nasal
flaring, retraction, and abnormal breathing sound.
Neonates are at risk of respiratory failure and subsequent
cardiopulmonary arrest if the increased respiratory effort
cannot be sustained. Early recognition of the presence
and the cause of respiratory distress is important to
enable prompt and appropriate treatment and to reduce
morbidity and mortality.
Medical pulmonary diseases account for most cases of
neonatal respiratory distress; the more common ones
include hyaline membrane disease (HMD), transient
tachypnoea of the newborn (TTN), meconium aspiration
syndrome (MAS), and pneumonia. They primarily relate
to saccular (25-36th week of gestation) and alveolar
(≥37th week of gestation) stages of lung development.[2]
This pictorial essay reviews these common pulmonary conditions that cause neonatal respiratory distress and
demonstrates the typical radiological findings.
When approaching imaging for neonatal respiratory
distress, most commonly chest radiographs (CXRs), it
is important to understand the distinctive clinical and
radiological features of common conditions that cause
neonatal respiratory distress, and to communicate with
the clinician about the detailed clinical information and
findings.
First, some conditions, especially surgical ones such
as congenital pulmonary airway malformation and
congenital lobar overinflation, may have been detected
by antenatal ultrasonography or magnetic resonance
imaging.[3] Review of any antenatal imaging is essential
to guide further evaluation and management.
Second, antenatal, perinatal, and postnatal history is
important to identify risk factors for different conditions.
For example, a history of maternal chorioamnionitis
is a significant risk factor for congenital pneumonia,
perinatal fetal distress increases the risk of MAS and mechanical ventilation is a common cause of air leak
including pneumothorax.
Third, knowing the maturity of the affected neonate is
vital because various degrees of lung maturity predispose
neonates to different pathologies. Preterm neonates are
at higher risk of HMD and neonatal pneumonia, whereas
term and post-term neonates are at higher risk of TTN
and MAS.
Finally, an appreciation of the pathophysiology
of different conditions will help understand their
radiological appearance and distinctive features.
Presence of a mass lesion, mediastinal shift, an abnormal
lung that is radio-opaque or radiolucent, lung volume,
and characteristics of lung opacities are all important
features for distinguishing medical conditions.
Combined with clinical information and laboratory
results, a CXR is usually sufficient to diagnose a medical
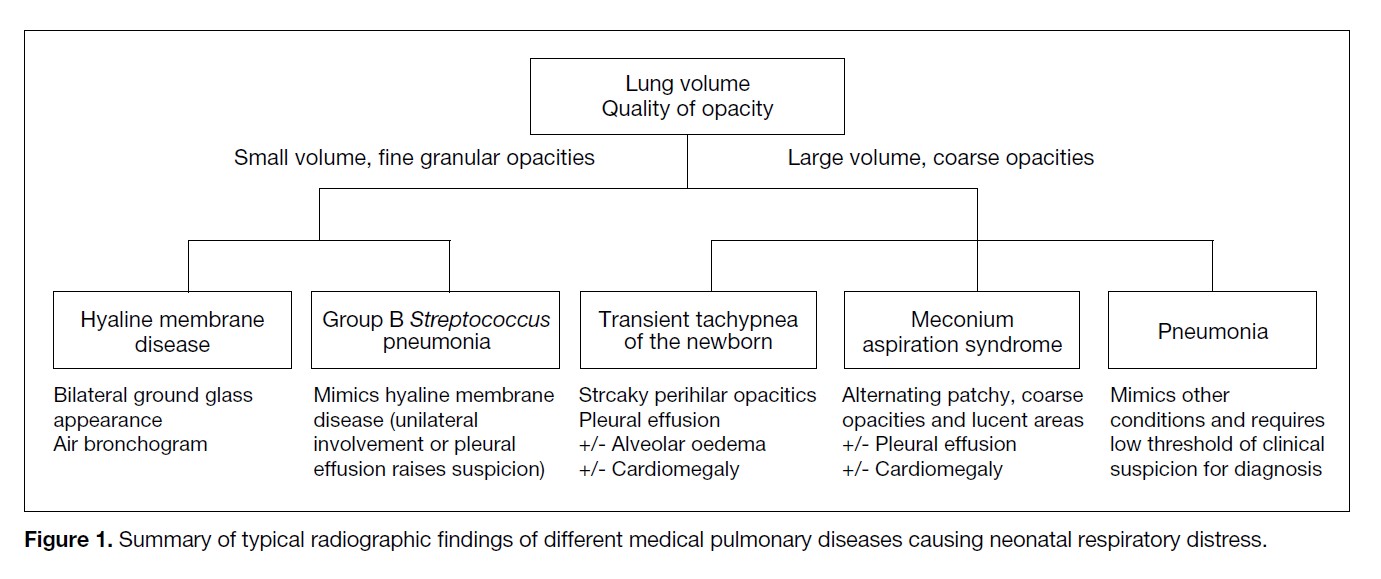
pulmonary condition. Lung volume and lung opacity
are important features to distinguish these conditions
(Figure 1).
Figure 1. Summary of typical radiographic findings of different medical pulmonary diseases causing neonatal respiratory distress.
HYALINE MEMBRANE DISEASE
In preterm neonates, HMD is the leading cause of
respiratory distress. The immature lungs and deficiency
of surfactant underlie the disease mechanism. Affected
neonates present with respiratory distress immediately or
within a few hours of birth and usually require ventilatory
support and exogenous surfactant. As the lungs mature
with increased endogenous surfactant production after
birth, respiratory distress often improves or resolves
after 3 to 4 days.[1,4,5]
Pathophysiology and Risk Factors
In immature lungs, there is reduced alveolisation
and excessive connective tissue matrix. In addition,
surfactant synthesis by type II pneumocytes is not
mature until the 35th week of gestation. As a result,
there is increased alveolar surface tension leading to
alveolar microatelectasis and poor lung compliance.
Epithelial injury and oliguria in the first few days
of postnatal life contribute to pulmonary oedema.
Inflammation and necrosis of lung epithelium occurs,
and the accumulation of fibrin and cellular debris form
the hyaline membrane.[4,6] The resultant effects lead
to impaired gaseous exchange with development of
hypoxia and acidosis.
Prematurity is the most important risk factor, with risk
increasing with decreasing gestational age. Antenatal
corticosteroids promote fetal lung surfactant synthesis
and reduce the incidence of HMD. Other risk factors
include maternal factors, especially diabetes mellitus,
fetal factors such as low birth weight and male sex, and
labour factors such as Caesarean section.[1]
Radiological Findings
Classic CXR findings include reduced lung volume,
bilateral symmetrical diffuse fine granular infiltrates
with ground glass appearance or white-out of the lungs
in more severe cases, and air bronchogram. Pleural
effusion is typically absent (Figure 2).[4]
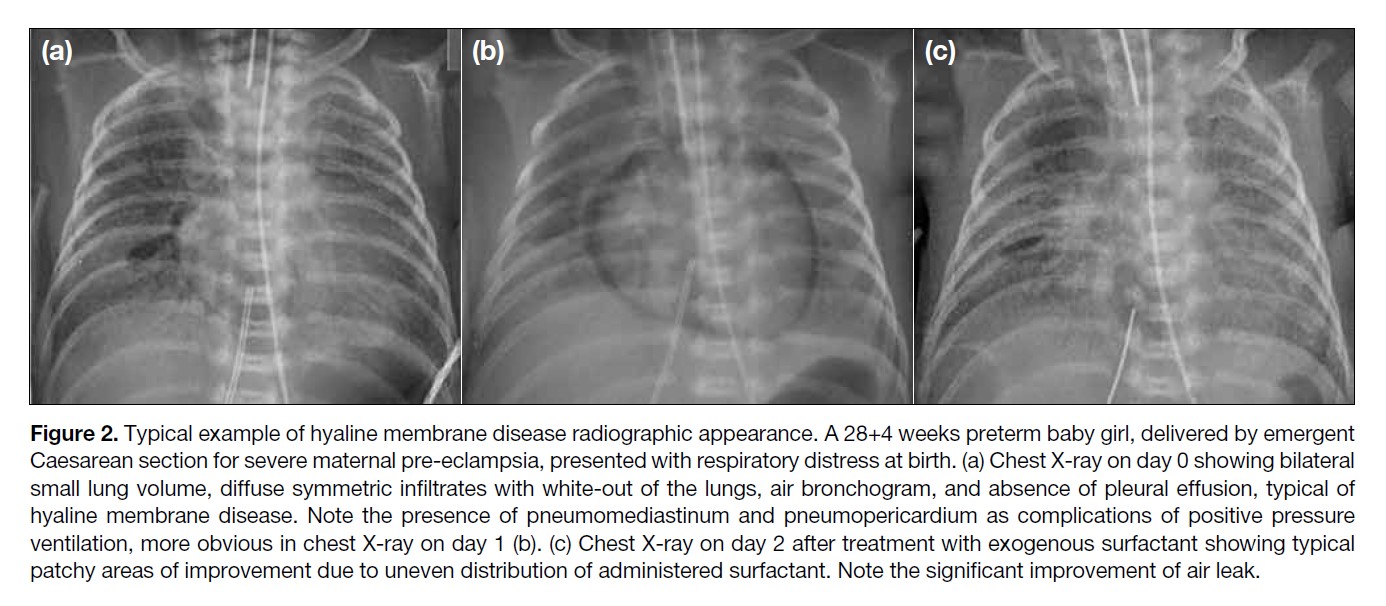
Figure 2. Typical example of hyaline membrane disease radiographic appearance. A 28+4 weeks preterm baby girl, delivered by emergent
Caesarean section for severe maternal pre-eclampsia, presented with respiratory distress at birth. (a) Chest X-ray on day 0 showing bilateral
small lung volume, diffuse symmetric infiltrates with white-out of the lungs, air bronchogram, and absence of pleural effusion, typical of
hyaline membrane disease. Note the presence of pneumomediastinum and pneumopericardium as complications of positive pressure
ventilation, more obvious in chest X-ray on day 1 (b). (c) Chest X-ray on day 2 after treatment with exogenous surfactant showing typical
patchy areas of improvement due to uneven distribution of administered surfactant. Note the significant improvement of air leak.
With assisted ventilation, application of exogenous
surfactant and gradual production of endogenous
surfactant, the CXR appearance improves in 3 to 4 days,
showing increased lung volume and patchy reduction of air space opacities, related to uneven distribution of
exogenous surfactant (Figure 2c).[6]
In the absence of expected improvement, concomitant
patent ductus arteriosus or other congenital heart
diseases should be considered. The typical radiographic
appearance of pulmonary plethora in patent ductus
arteriosus resulting from left to right shunt may not be
obvious in the presence of HMD. Clinical assessment
and echocardiogram play important roles for diagnosis
(Figure 3).[4,7] Deterioration after initial improvement
raises the possibility of superimposed nosocomial
infection. Other complications including air leak and
pulmonary haemorrhage are not uncommon (Figure 4).
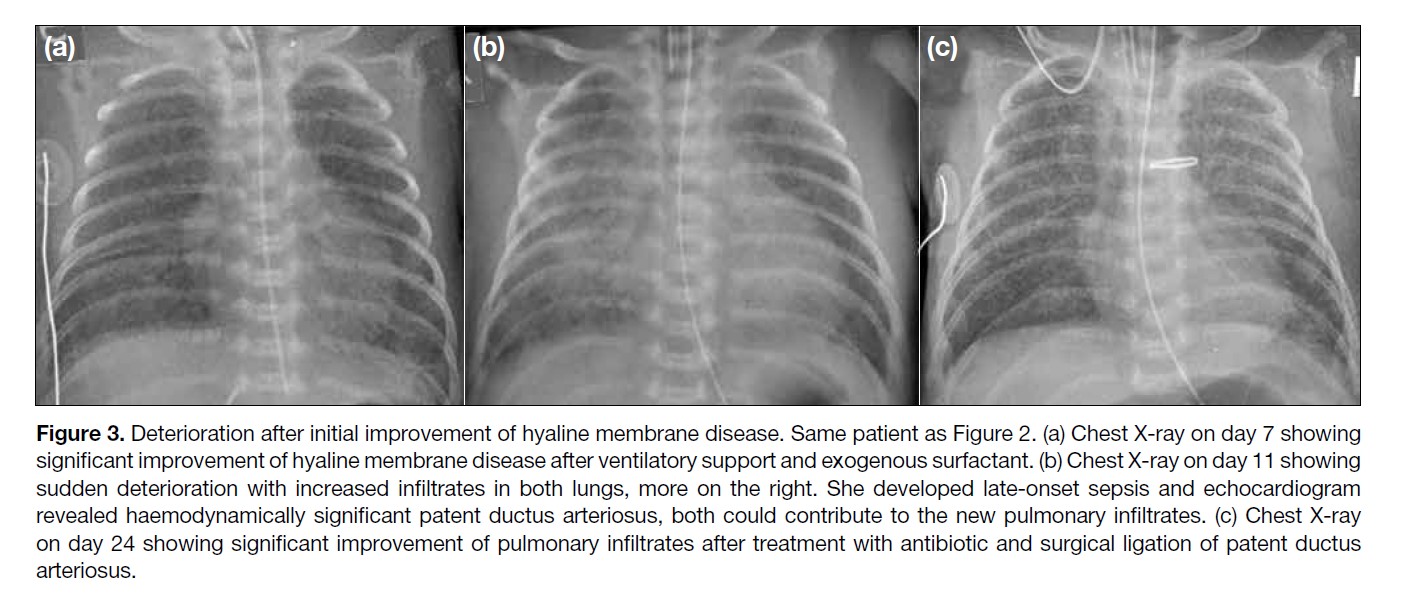
Figure 3. Deterioration after initial improvement of hyaline membrane disease. Same patient as Figure 2. (a) Chest X-ray on day 7 showing
significant improvement of hyaline membrane disease after ventilatory support and exogenous surfactant. (b) Chest X-ray on day 11 showing
sudden deterioration with increased infiltrates in both lungs, more on the right. She developed late-onset sepsis and echocardiogram
revealed haemodynamically significant patent ductus arteriosus, both could contribute to the new pulmonary infiltrates. (c) Chest X-ray
on day 24 showing significant improvement of pulmonary infiltrates after treatment with antibiotic and surgical ligation of patent ductus
arteriosus.
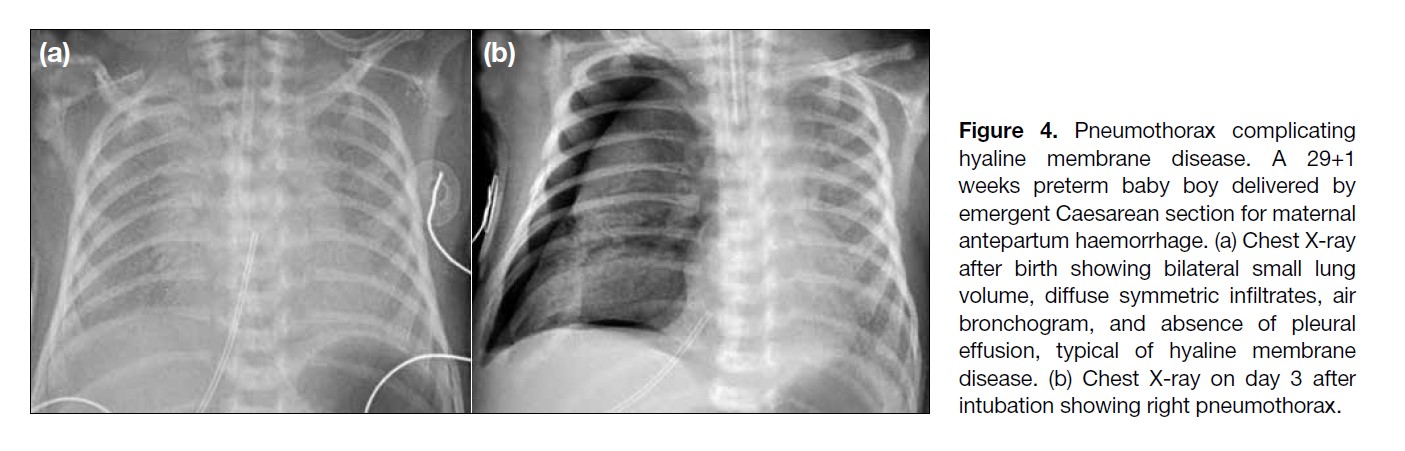
Figure 4. Pneumothorax complicating
hyaline membrane disease. A 29+1
weeks preterm baby boy delivered by
emergent Caesarean section for maternal
antepartum haemorrhage. (a) Chest X-ray
after birth showing bilateral small lung
volume, diffuse symmetric infiltrates, air
bronchogram, and absence of pleural
effusion, typical of hyaline membrane
disease. (b) Chest X-ray on day 3 after
intubation showing right pneumothorax.
TRANSIENT TACHYPNOEA OF THE
NEWBORN
In term neonates, TTN is the most common cause of
respiratory distress. It is caused by failed mechanism
of fetal lung fluid clearance with consequent excessive
retained fluid that impairs gaseous exchange. Neonates
with TTN usually develop symptoms within the first few
hours of delivery. Fortunately, the presence of a normal
surfactant system in term neonates helps protect the lungs
from injury by maintaining alveolar capillary membrane
integrity and allowing gradual absorption of lung fluid.
TTN is usually a self-limiting condition lasting for up to
about 3 days, requiring minimal to modest respiratory
support.
Pathophysiology and Risk Factors
In utero, fetal lungs are filled with fluid for normal lung
development. In term foetuses, there is a physiological
reduction in fluid secretion by lung epithelia at least a
few days before labour onset. There is also increased
fluid absorption by active transport of Na+ and Cl-,
followed by water. This mechanism is also enhanced
by an increased fetal catecholamine level in response
to labour stress. A resultant net absorption of fetal lung
fluid is therefore achieved during labour.[8] Squeezing of
the fetal lungs during passage through the birth canal
during labour is also important for expelling fetal lung
fluid through the airways. Bypassing or impairment of
these mechanisms can result in excessive retained fetal
lung fluid.
Elective Caesarean section before the 39th week of
gestation is the most important risk factor for TTN
because of the lack of normal mechanisms for fetal
lung fluid absorption and expulsion. Other common
risk factors include maternal factors such as maternal
diabetes mellitus, fetal factors such as low birth weight or macrosomia, and labour factors such as precipitous
delivery or maternal sedation.[5]
Radiological Findings
Frontal CXR typically shows hyperinflated lungs
secondary to air trapping, streaky perihilar opacities
due to prominent vascular and lymphatic structures, and
evidence of interstitial oedema, such as pleural effusion
which may extend along pulmonary fissures (with right
horizontal fissure as the best seen one in frontal CXR)
or Kerley’s B lines. Alveolar oedema is a less common
finding; transient cardiomegaly has also been reported.
Follow-up CXR in 1 to 2 days typically shows rapid
improvement or resolution (Figures 5 and 6).
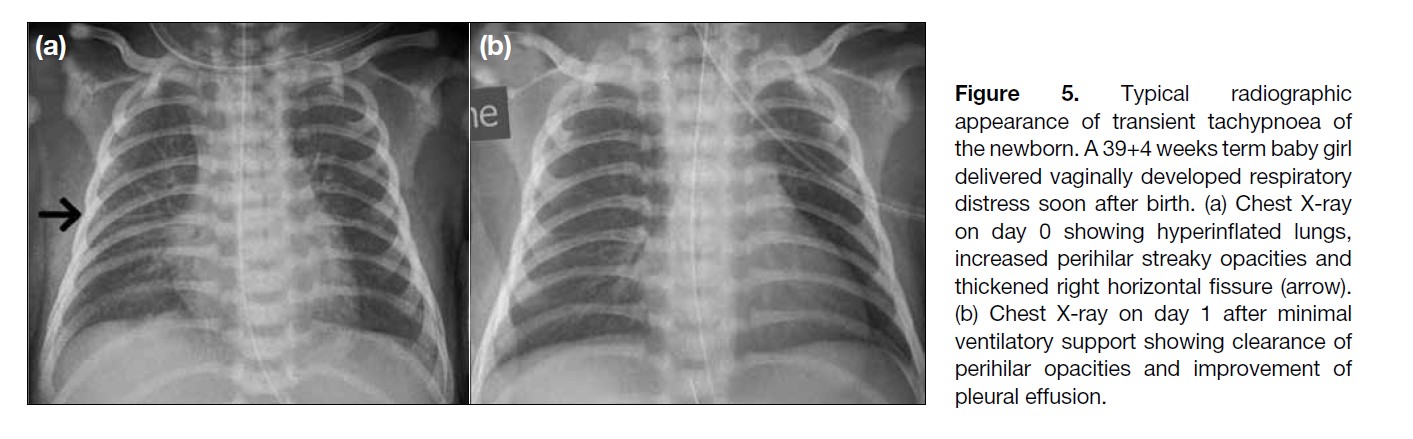
Figure 5. Typical radiographic
appearance of transient tachypnoea of
the newborn. A 39+4 weeks term baby girl
delivered vaginally developed respiratory
distress soon after birth. (a) Chest X-ray
on day 0 showing hyperinflated lungs,
increased perihilar streaky opacities and
thickened right horizontal fissure (arrow).
(b) Chest X-ray on day 1 after minimal
ventilatory support showing clearance of
perihilar opacities and improvement of
pleural effusion.
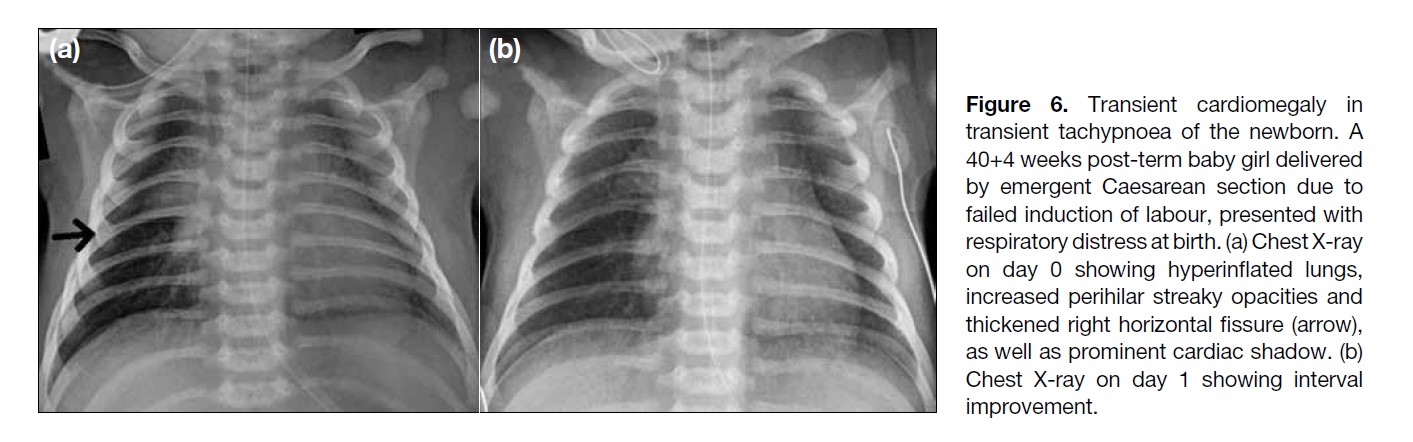
Figure 6. Transient cardiomegaly in
transient tachypnoea of the newborn. A
40+4 weeks post-term baby girl delivered
by emergent Caesarean section due to
failed induction of labour, presented with
respiratory distress at birth. (a) Chest X-ray
on day 0 showing hyperinflated lungs,
increased perihilar streaky opacities and
thickened right horizontal fissure (arrow),
as well as prominent cardiac shadow. (b)
Chest X-ray on day 1 showing interval
improvement.
MECONIUM ASPIRATION
SYNDROME
Meconium-stained liquor is encountered in about 12% of
all deliveries, of which 5% are complicated by MAS. In
neonates with MAS, 4% will die, accounting for about
2% of all perinatal deaths.9 MAS is defined as respiratory
distress in an infant born through meconium-stained liquor with characteristic CXR changes and whose
symptoms cannot be otherwise explained.[10] As MAS
can result in significant morbidities, e.g. air leak and
superimposed infection and mortality, prevention, early
recognition and prompt treatment are essential.
Pathophysiology and Risk Factors
Meconium is viscous fetal colonic content comprised of
a mixture of desquamated epithelial cells, bile, pancreatic
enzymes, swallowed amniotic fluid, lanugo (fetal hair),
and vernix caseosa (waxy fetal skin coating). Meconium
does not pass to the lower descending colon until the 34th
week of gestation, hence MAS is seldom seen before the
37th week of gestation and affects mainly term and post-term
neonates.[5]
Advanced fetal maturity and fetal distress are the most
important risk factors for MAS. With advanced fetal
maturity, there is a higher risk of passing meconium
in utero. In the presence of fetal distress, e.g. hypoxia,
fetal neuronal stimulation causes fetal anal sphincter
relaxation and meconium passage.
Aspirated meconium causes partial or complete
obstruction of medium to small airways due to its high
viscosity, leading to air trapping and atelectasis; chemical
pneumonitis with epithelial injury due to acidity, bile
and pancreatic enzymes; and inactivation of surfactant
by the presence of bile, contributing to atelectasis. These
compromise gaseous exchange and predispose to a wide
range of complications from air leak and superimposed
infection to persistent pulmonary hypertension.[11]
Radiological Findings
The appearance of MAS on CXR depends on its severity.
Mild disease can manifest as perihilar streakiness and reticular opacities that may be difficult to distinguish
from TTN. The classic CXR appearance of MAS includes
hyperinflation due to air trapping and patchy coarse
irregular or band-like opacities with intervening lucent
areas, corresponding to alternating areas of atelectasis
and hyperinflation. Pleural effusion and cardiomegaly
(possibly related to persistent pulmonary hypertension)
are also possible findings. Absence of air bronchogram
is typical (Figures 7 and 8). Complications, especially air
leak, including pneumothorax and pulmonary interstitial
emphysema, should be carefully sought (Figure 9). Radiographic changes may show gradual resolution over time, ranging from 7 to 10 days to weeks, as the aspirated meconium is cleared by macrophages (Figure 10).[10,11]
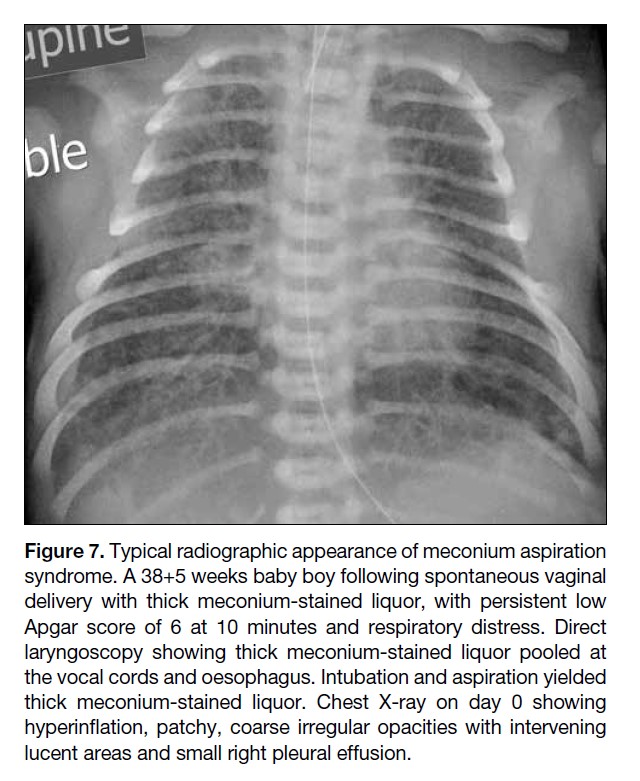
Figure 7. Typical radiographic appearance of meconium aspiration
syndrome. A 38+5 weeks baby boy following spontaneous vaginal
delivery with thick meconium-stained liquor, with persistent low
Apgar score of 6 at 10 minutes and respiratory distress. Direct
laryngoscopy showing thick meconium-stained liquor pooled at
the vocal cords and oesophagus. Intubation and aspiration yielded
thick meconium-stained liquor. Chest X-ray on day 0 showing
hyperinflation, patchy, coarse irregular opacities with intervening
lucent areas and small right pleural effusion.
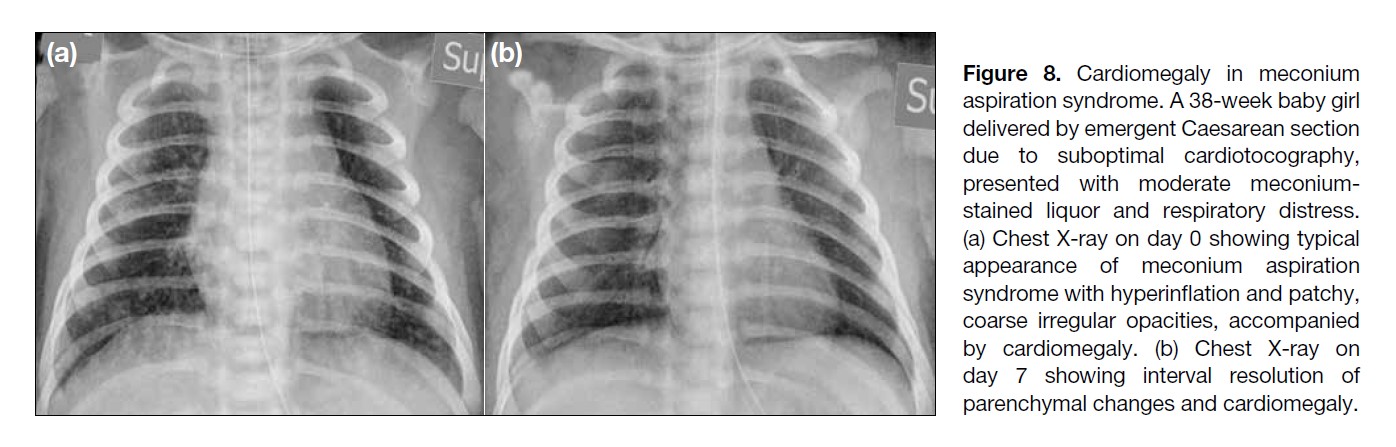
Figure 8. Cardiomegaly in meconium
aspiration syndrome. A 38-week baby girl
delivered by emergent Caesarean section
due to suboptimal cardiotocography,
presented with moderate meconium-stained
liquor and respiratory distress.
(a) Chest X-ray on day 0 showing typical
appearance of meconium aspiration
syndrome with hyperinflation and patchy,
coarse irregular opacities, accompanied
by cardiomegaly. (b) Chest X-ray on
day 7 showing interval resolution of
parenchymal changes and cardiomegaly.
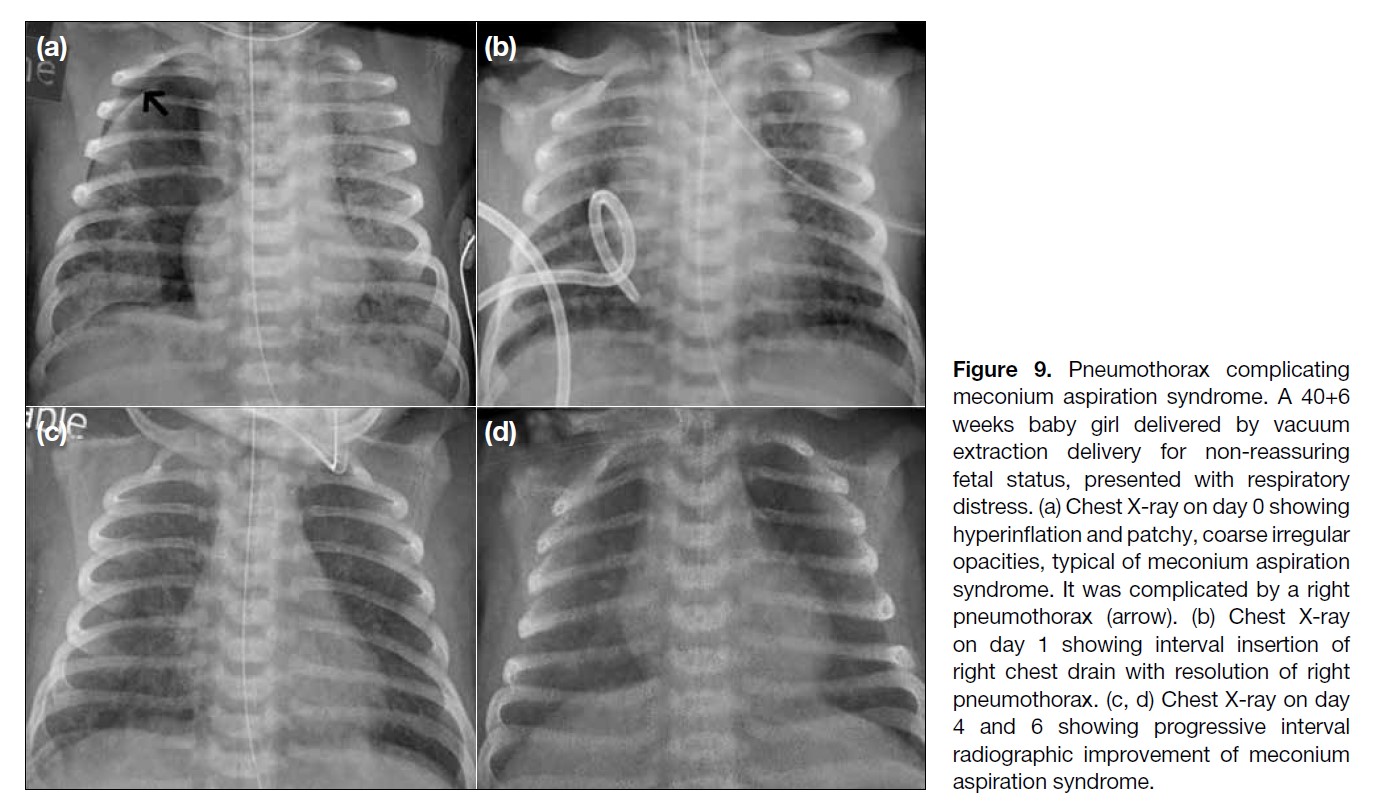
Figure 9. Pneumothorax complicating
meconium aspiration syndrome. A 40+6
weeks baby girl delivered by vacuum
extraction delivery for non-reassuring
fetal status, presented with respiratory
distress. (a) Chest X-ray on day 0 showing
hyperinflation and patchy, coarse irregular
opacities, typical of meconium aspiration
syndrome. It was complicated by a right
pneumothorax (arrow). (b) Chest X-ray
on day 1 showing interval insertion of
right chest drain with resolution of right
pneumothorax. (c, d) Chest X-ray on day
4 and 6 showing progressive interval
radiographic improvement of meconium
aspiration syndrome.
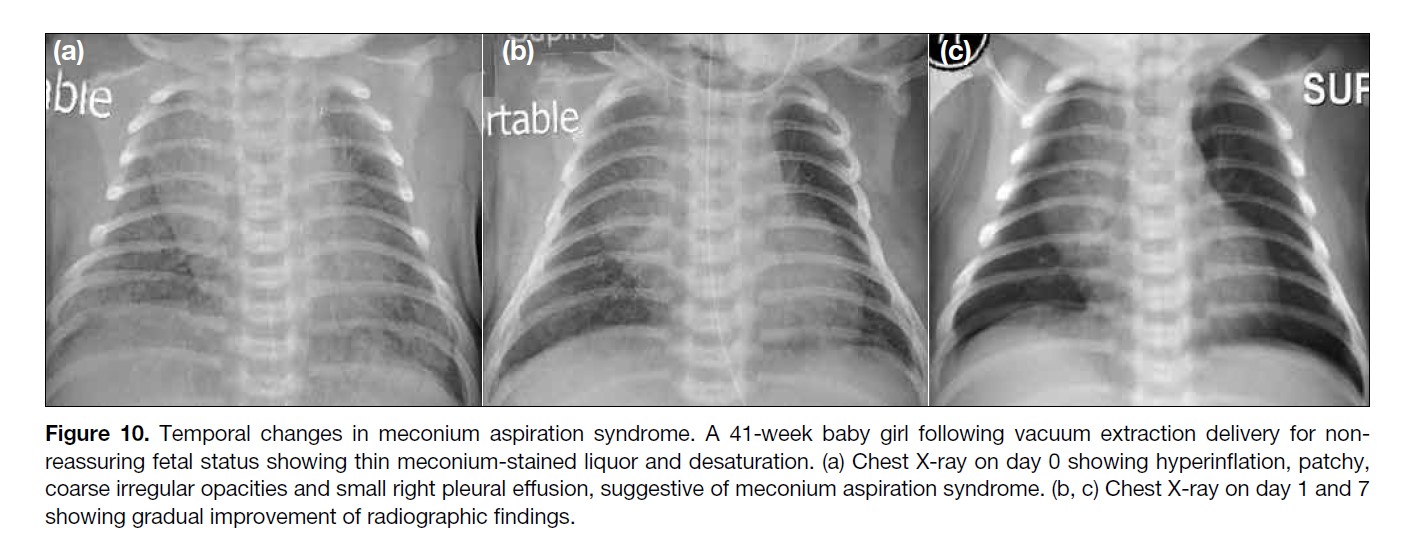
Figure 10. Temporal changes in meconium aspiration syndrome. A 41-week baby girl following vacuum extraction delivery for non-reassuring
fetal status showing thin meconium-stained liquor and desaturation. (a) Chest X-ray on day 0 showing hyperinflation, patchy,
coarse irregular opacities and small right pleural effusion, suggestive of meconium aspiration syndrome. (b, c) Chest X-ray on day 1 and 7
showing gradual improvement of radiographic findings.
PNEUMONIA
The estimated incidence of pneumonia during the
neonatal period is about 1% in term and 10% in
preterm neonates. It accounts for about 10% of global
child mortality, especially in developing countries and
regions.[12] Pneumonia during the neonatal period can be
classified as early-onset (in first week after birth) or late-onset
(after first week after birth).[12] Early- and late-onset pneumonia have different mechanisms of transmission
and spectrums of causative agents. Bacterial infection is
the most common culprit for both early- and late-onset
pneumonia, followed by viral or fungal infection.
The diagnosis of pneumonia in neonates is often
challenging, not only because there is significant overlap
of clinical and radiological findings with other neonatal
respiratory conditions, but also because laboratory
results may be non-specific and causative pathogens
may not be identified.[1] Pneumonia can also co-exist with
other causes of respiratory distress. A low threshold of clinical suspicion and the combination of all clinical,
radiological, and laboratory findings are essential to
reach a diagnosis and provide timely antimicrobial
treatment, so reducing morbidity and mortality.
Pathophysiology and Risk Factors
Early-onset Pneumonia
Early-onset pneumonia includes congenital pneumonia
transmitted by transplacental spread or infected amniotic
fluid, and that contracted perinatally by aspiration of
infected liquor or vaginal organisms during labour.
Common risk factors include maternal factors such as
chorioamnionitis or vaginal colonisation with group B
Streptococcus, fetal factors such as prematurity, and
labour factors such as prolonged labour or premature
rupture of membrane. Most common pathogens
include bacteria, especially group B Streptococcus and
Escherichia coli, viruses (e.g. herpes simplex virus and
adenovirus), and fungi (e.g. Candida species).[1,12,13]
Late-onset Pneumonia
Late-onset pneumonia is usually acquired during the
postnatal period and may be nosocomial or community-acquired.
Exceptions include Chlamydia trachomatis
that is usually acquired during labour by aspiration of the
pathogen from the birth canal and presents 1 to 2 weeks
after birth. Common risk factors include environmental
factors such as prolonged hospitalisation, suboptimal
hand-washing technique of caregivers, or home
environments including infection of family members; and
neonatal factors such as assisted ventilation or conditions
predisposing to aspiration, including neuromuscular
disorders and tracheaoesophageal fistula. Most common
causative agents are bacteria such as Staphylococcus aureus and Streptococcus pneumoniae; viruses such
as respiratory syncytial, influenza and parainfluenza
viruses; and fungi such as Candida species.[1,12,13]
Radiological Findings
CXR findings of pneumonia in neonates show a wide
range of patterns, overlapping with other neonatal
respiratory conditions, such as diffuse parenchymal
infiltrates with air bronchogram similar to HMD (Figure 11), especially for group B Streptococcus infection[14]; perihilar streakiness with or without effusion, mimicking
TTN; and coarse irregular patchy opacities resembling
MAS (Figure 12). Other possible radiographic findings
range from normal, lobar consolidation to central
perihilar airspace opacities mimicking pulmonary
oedema. Complications such as pulmonary interstitial
emphysema, pneumothorax, and pneumomediastinum
may also be encountered (Figure 13).[15]
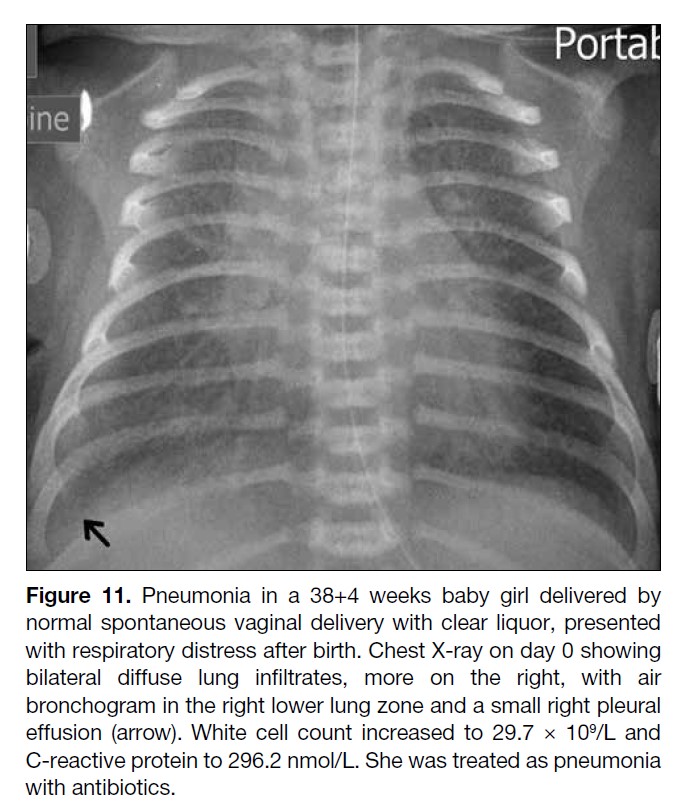
Figure 11. Pneumonia in a 38+4 weeks baby girl delivered by
normal spontaneous vaginal delivery with clear liquor, presented
with respiratory distress after birth. Chest X-ray on day 0 showing
bilateral diffuse lung infiltrates, more on the right, with air
bronchogram in the right lower lung zone and a small right pleural
effusion (arrow). White cell count increased to 29.7 × 109/L and
C-reactive protein to 296.2 nmol/L. She was treated as pneumonia
with antibiotics.
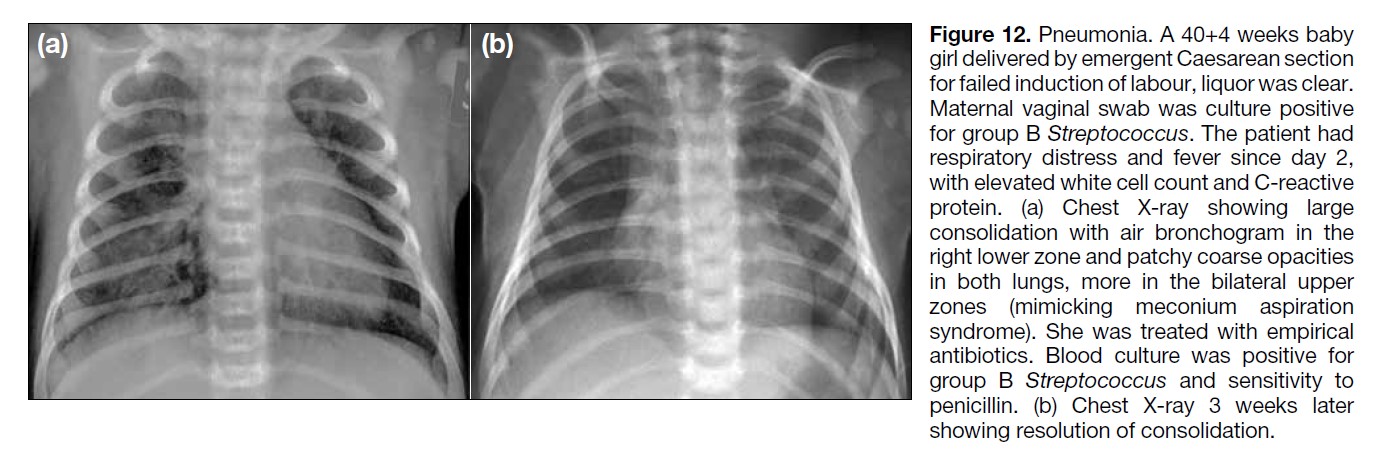
Figure 12. Pneumonia. A 40+4 weeks baby
girl delivered by emergent Caesarean section
for failed induction of labour, liquor was clear.
Maternal vaginal swab was culture positive
for group B Streptococcus. The patient had
respiratory distress and fever since day 2,
with elevated white cell count and C-reactive
protein. (a) Chest X-ray showing large
consolidation with air bronchogram in the
right lower zone and patchy coarse opacities
in both lungs, more in the bilateral upper
zones (mimicking meconium aspiration
syndrome). She was treated with empirical
antibiotics. Blood culture was positive for
group B Streptococcus and sensitivity to
penicillin. (b) Chest X-ray 3 weeks later
showing resolution of consolidation.
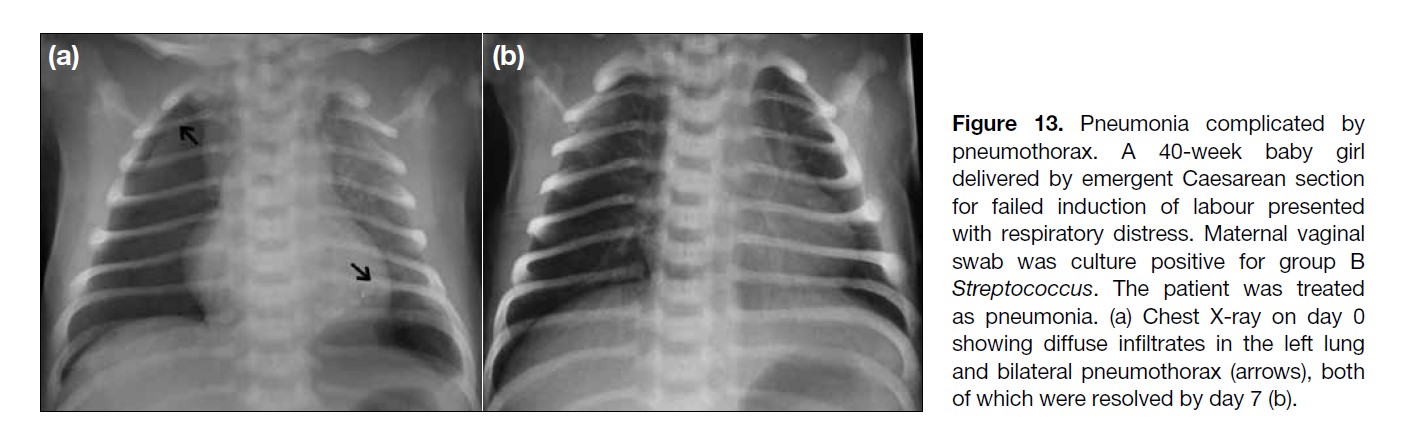
Figure 13. Pneumonia complicated by
pneumothorax. A 40-week baby girl
delivered by emergent Caesarean section
for failed induction of labour presented
with respiratory distress. Maternal vaginal
swab was culture positive for group B
Streptococcus. The patient was treated
as pneumonia. (a) Chest X-ray on day 0
showing diffuse infiltrates in the left lung
and bilateral pneumothorax (arrows), both
of which were resolved by day 7 (b).
Certain features should raise a radiological suspicion of
underlying pneumonia, such as unilateral involvement,
normal lung volume, and presence of pleural effusion
in a pattern similar to HMD (Figure 14); lack of an
expected rapid radiographic improvement in a pattern
mimicking TTN; and presence of air bronchogram in a
pattern resembling MAS (Figure 15).[14]
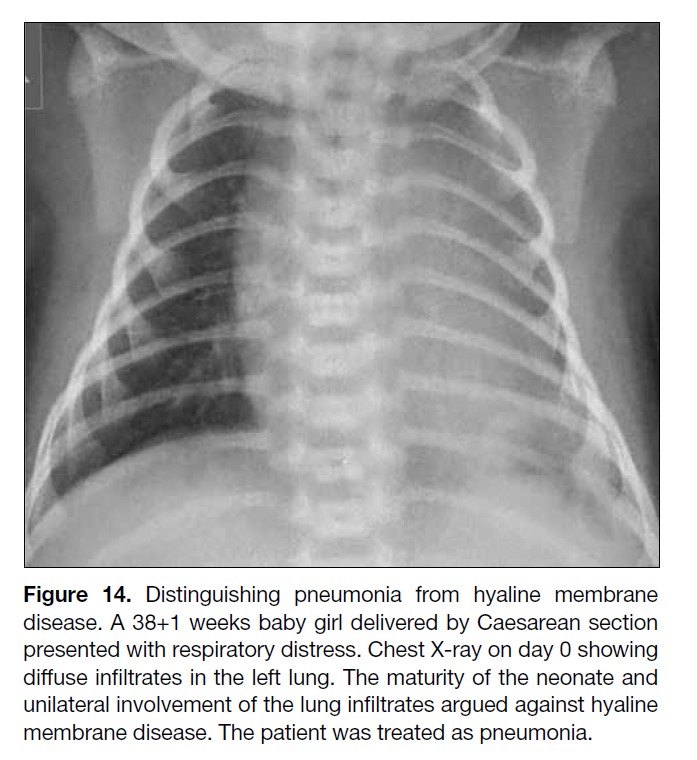
Figure 14. Distinguishing pneumonia from hyaline membrane
disease. A 38+1 weeks baby girl delivered by Caesarean section
presented with respiratory distress. Chest X-ray on day 0 showing
diffuse infiltrates in the left lung. The maturity of the neonate and
unilateral involvement of the lung infiltrates argued against hyaline
membrane disease. The patient was treated as pneumonia.
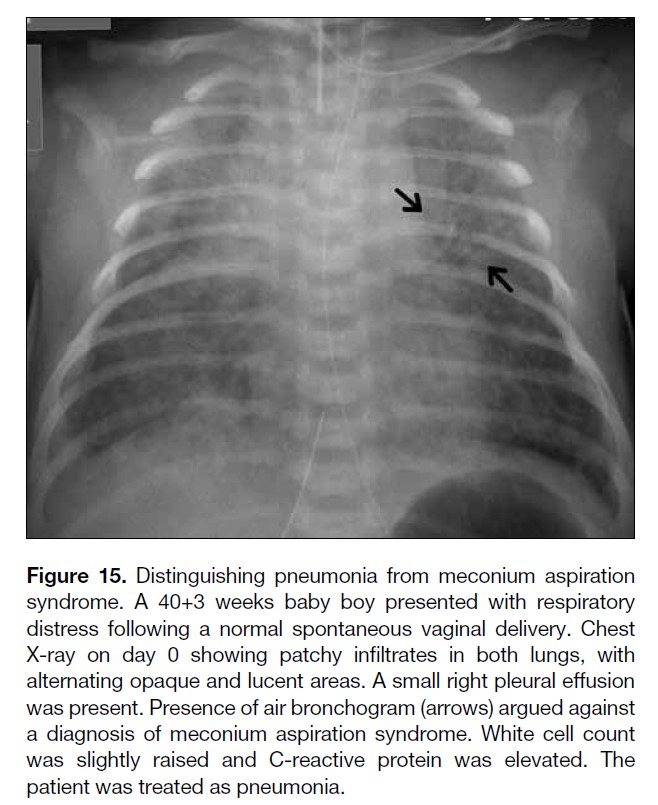
Figure 15. Distinguishing pneumonia from meconium aspiration
syndrome. A 40+3 weeks baby boy presented with respiratory
distress following a normal spontaneous vaginal delivery. Chest
X-ray on day 0 showing patchy infiltrates in both lungs, with
alternating opaque and lucent areas. A small right pleural effusion
was present. Presence of air bronchogram (arrows) argued against
a diagnosis of meconium aspiration syndrome. White cell count
was slightly raised and C-reactive protein was elevated. The
patient was treated as pneumonia.
It is again stressed that the diagnosis of pneumonia in
neonates requires a low threshold of suspicion and
consideration of all clinical, radiological, and laboratory
findings.
CONCLUSION
Understanding the pathophysiology, risk factors, and
radiological appearance of pulmonary diseases that
cause neonatal respiratory distress is essential for timely
diagnosis and treatment.
REFERENCES
1. Edwards MO, Kotecha SJ, Kotecha S. Respiratory distress of the term newborn infant. Paediatric Respir Rev. 2013;14:29-36 Crossref
2. Weisman LE, Hansen TN. Contemporary diagnosis and
management of neonatal respiratory diseases. 3rd edition. Newton
(PA): Handbooks in Health Care Co.; 2003.
3. Biyyam DR, Chapman T, Ferguson MR, Deutsch G, Dighe MK.
Congenital lung abnormalities: embryologic features, prenatal
diagnosis, and postnatal radiologic-pathologic correlation.
Radiographics. 2010;30:1721-38. Crossref
4. Grappone L, Messina F. Hyaline membrane disease or respiratory distress syndrome? A new approach for an old disease. JPNIM.
2014;3:e030263.
5. Reuter S, Moser C, Baack M. Respiratory distress in the newborn. Pediatr Rev. 2014;35:417-28. Crossref
6. Liszeski MC, Lee EY. Neonatal lung disorders: pattern recognition approach to diagnosis. AJR Am J Roentgenol. 2018;210:964-
75. Crossref
7. Slovis TL, Shankaran S. Patent ductus arteriosus in hyaline
membrane disease: chest radiography. AJR Am J Roentgenol.
1980;135:307-9. Crossref
8. Elias Na, O’Brodovich H. Clearance of fluid from airspaces of
newborns and infants. NeoReviews. 2006;7:e88-94. Crossref
9. Ross MG. Meconium aspiration syndrome—more than intrapartum
meconium. N Engl J Med. 2005;353:946-8. Crossref
10. Garcia-Peña P, Guillerman RP, editors. Pediatric Chest Imaging. 3rd ed. New York, NY: Springer; 2014. Crossref
11. TF Yeh. Meconium aspiration syndrome: pathogenesis and current management. NeoRewiews. 2010;11:e503-12. Crossref
12. Nissen MD. Congenital and neonatal pneumonia. Paediatric Respir Rev. 2007;8:195-203. Crossref
13. Speer ME. Neonatal pneumonia. Available from: http://www.uptodate.com/contents/neonatal-pneumonia. Accessed 2 Jun 2018.
14. Yoon HK. Interpretation of neonatal chest radiography. J Korean Soc Radiol. 2016;74:279-90. Crossref
15. Haney PJ, Bohlman M, Sun CC. Radiographic findings in neonatal pneumonia. AJR Am J Roentgenol. 1984;143:23-6. Crossref