Atypical Langerhans Cell Histiocytosis: A Case Report
CASE REPORT
Atypical Langerhans Cell Histiocytosis: A Case Report
C Lo1, WK Chan2; G Lo3
1 Department of Radiology, Queen Mary Hospital, Pokfulam, Hong Kong
2 Department of Pathology, Hong Kong Sanatorium & Hospital, Happy Valley, Hong Kong
3 Department of Radiology, Hong Kong Sanatorium & Hospital, Happy Valley, Hong Kong
Correspondence: Dr C Lo, Department of Radiology, Queen Mary Hospital, Pokfulam, Hong Kong. Email: chrissy.lo@gmail.com
Submitted: 13 Aug 2017; Accepted: 11 Sep 2017.
Contributors: All authors designed the study, acquired and analysed the data, drafted the manuscript, and had critical revision of the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This case report received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics Approval: Patient consent was waived by the ethics board (Ref REC-2019-16).
INTRODUCTION
Langerhans cell histiocytosis (LCH) is the most common
dendritic cell disorder. It is named for the similarity of
the lesional cells to the Langerhans cells found in the
skin and mucosa. LCH can be divided into three forms
based on number of lesions and systems involved.[1] The
clinical manifestation of LCH depends on the number of
sites and systems involved. Most (70%) cases of LCH
are the unifocal (localised) form, which is limited to
a single bone or few bones and may involve the lung.
Patients with this form of LCH are usually aged 5 to 15
years. Another 20% of LCH cases are the multifocal
unisystem (chronically recurring) form, which involves
multiple locations in bones and the reticuloendothelial
system (liver, spleen, lymph nodes and skin). This form is
often accompanied by diabetes insipidus when pituitary
gland is involved. Patients with this form of LCH are
usually aged 1 to 5 years. The remaining 10% of cases
of LCH are the multifocal multisystem (fulminant) form,
which is often fatal. This form of LCH is characterised
by disseminated involvement of the reticuloendothelial
system with anaemia and thrombocytopenia. Patients
with this form of LCH are usually aged 1 to 2 years.
We present a case of atypical LCH and describe the
pathological and radiographical features.
CASE REPORT
A boy, aged 2 years 10 months, presented with a
rapidly enlarging right supraorbital soft tissue mass with
inflammatory signs for 4 weeks. Plain film radiographs
were not obtained, but magnetic resonance imaging
(MRI) was ordered, to rule out orbital cellulitis or orbital
tumour including rhabdomyosarcoma.
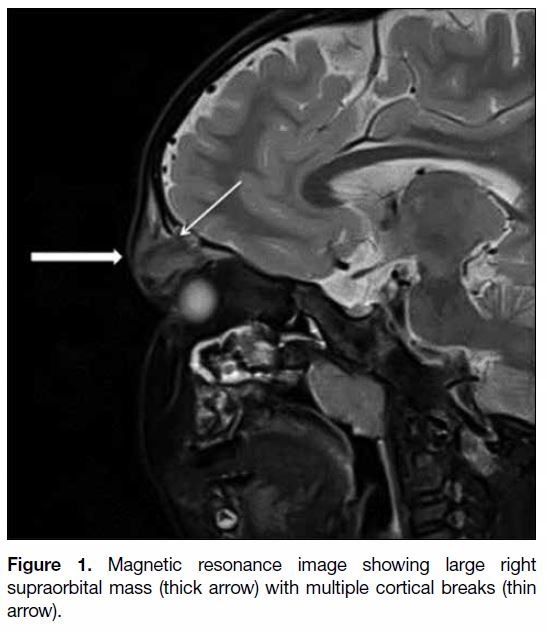
MRI scan showed a large expansile lytic lesion in the
right orbital ridge. Multiple cortical breaks noted in the
bone were associated with a soft tissue mass measuring
3.3 × 1.6 × 3.4 cm (transverse × anteroposterior ×
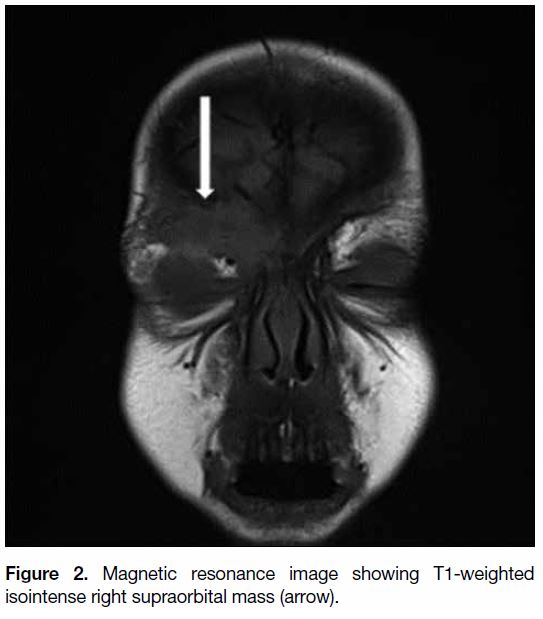
craniocaudal; Figure 1). This lesion was T1 isointense
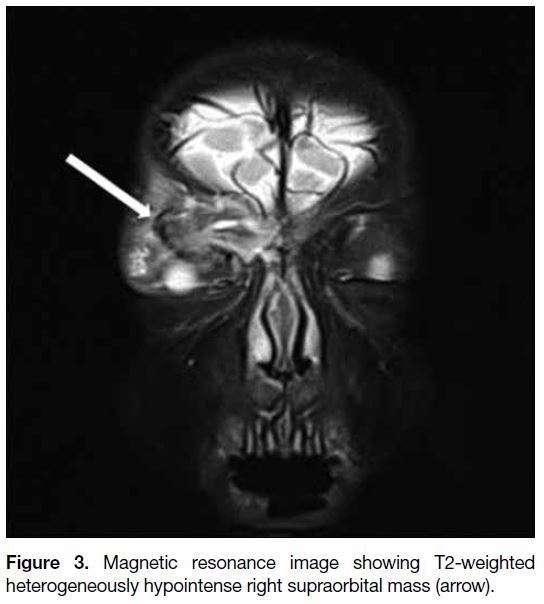
(Figure 2) and T2 heterogeneously hypointense with small
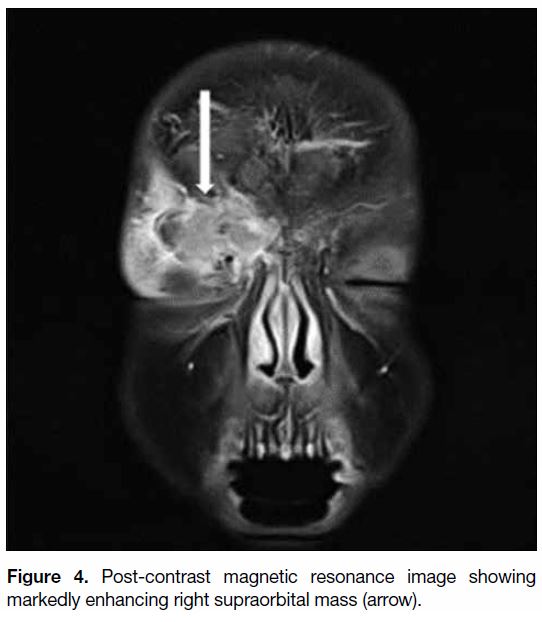
T2 hyperintense foci (Figure 3). Marked enhancement
of the soft tissue mass was noted after contrast injection
(Figure 4). The orbital globe, extraocular muscles,
lacrimal gland, optic nerve, and optic chiasm were normal.
Figure 1. Magnetic resonance image showing large right
supraorbital mass (thick arrow) with multiple cortical breaks (thin
arrow).
Figure 2. Magnetic resonance image showing T1-weighted isointense right supraorbital mass (arrow).
Figure 3. Magnetic resonance image showing T2-weighted heterogeneously hypointense right supraorbital mass (arrow).
Figure 4. Post-contrast magnetic resonance image showing markedly enhancing right supraorbital mass (arrow).
Biopsy of the mass was then performed by an
ophthalmologist. The biopsy results showed atypical
LCH. BRAF mutation analysis was negative, indicating
a low risk of recurrence.
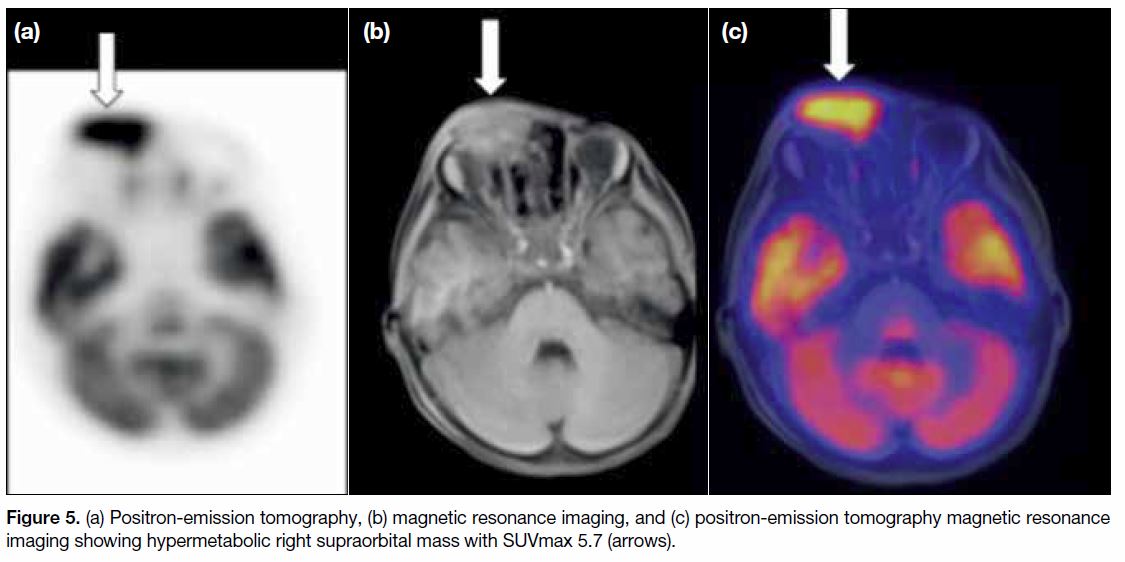
Positron-emission tomography with MRI (PET-MRI) was then chosen to evaluate the whole body because
of the lower radiation dose reduction compared with
PET-CT. PET-MRI scan showed the right orbital lesion
to be hypermetabolic. SUVmax was 5.7 (Figure 5).
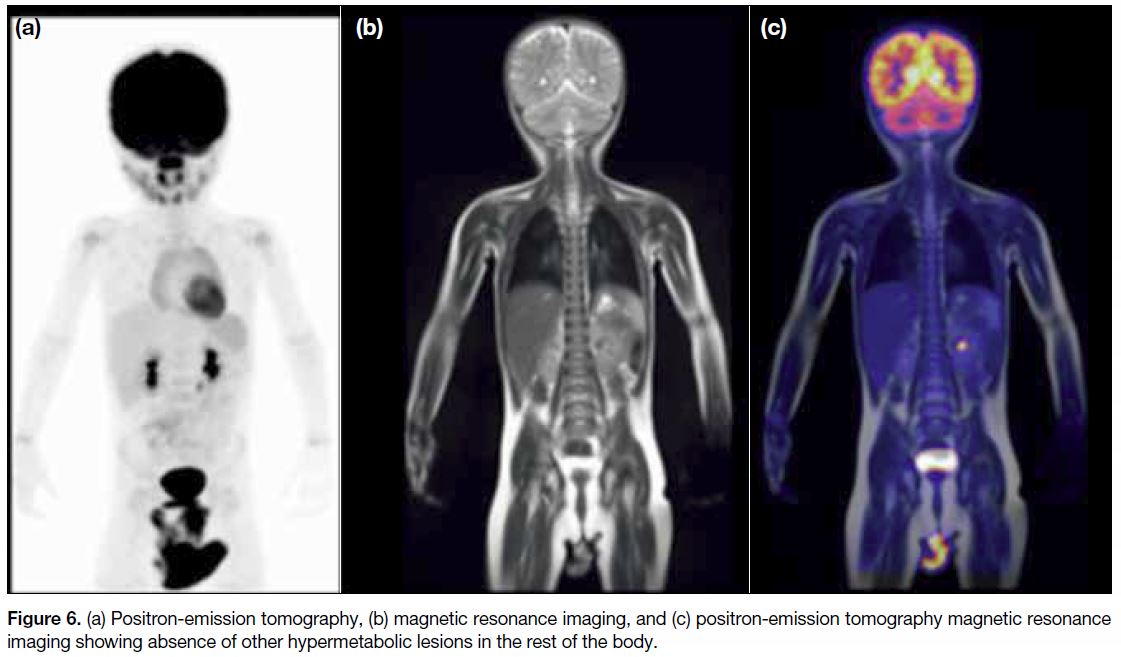
No other hypermetabolic lesion was seen in the rest of the body (Figure 6). As part of the PET-MRI protocol
in our hospital, a complimentary low-dose computed
tomography (CT) of the thorax was also obtained. The
CT scan did not show any centrilobular nodules or lung
cysts (Figure 6).
Figure 5. (a) Positron-emission tomography, (b) magnetic resonance imaging, and (c) positron-emission tomography magnetic resonance imaging showing hypermetabolic right supraorbital mass with SUVmax 5.7 (arrows).
Figure 6. (a) Positron-emission tomography, (b) magnetic resonance imaging, and (c) positron-emission tomography magnetic resonance imaging showing absence of other hypermetabolic lesions in the rest of the body.
Because the supraorbital lesion was the only lesion and
the prognosis was good, we decided that the patient
would undergo surgery. However, surgery at this age
may cause deformity. Therefore, the patient is now
undergoing 6 months of chemotherapy to shrink the
mass before surgery.
DISCUSSION
Radiology
Typical LCH lesions present with well-defined lytic
lesions with bevelled edges in the skull.[1] MRI scan usually shows a soft tissue mass within the diploic space
that is isointense on T1-weighted images, hyperintense on T2-weighted images,[1,2] and enhances after contrast
injection.1 Decreased T2 signal is seen in the healing
phase.[1]
In the present case, the isointense T1 images and
the markedly enhancing post-contrast images are all
consistent with LCH features. The atypical feature seen
on the MRI scan was the presence of heterogeneous T2
low signal throughout the mass. In typical LCH cases,
decreased T2 low signal indicates healing.[1]
However, in the present case, PET-MRI showed
hypermetabolic activity in the supraorbital mass,
indicating active disease. SUVmax of the supraorbital
mass was 5.7 (reference SUVmax of mediastinal blood
pool: 0.61). The lesion was shown to be unifocal because
the whole-body PET-MRI scan did not reveal any other
tumours. This knowledge of the lesional activity helped
us to manage this case.[3,4] PET-MRI can also be used to
monitor therapy.[3,4]
A study that compared 18F-fluorodeoxyglucose (FDG)-PET with plain film radiographs, CT scans, MRI scans,
and bone scans showed that, overall, FDG-PET was
rated confirmatory or superior in 235 (92%) lesions out
of 256.[4] Whole-body FDG-PET can detect LCH activity
and early response to therapy in bone and soft tissues
with much greater accuracy than other conventional
imaging modalities.[4]
Pathology
Histopathological examination of the incisional biopsy
showed a plump mononuclear cell population with large
lobulated nuclei, some with grooves and others with small
central nucleoli. The cells showed moderately abundant
pale to eosinophilic cytoplasm. Immunostaining results
demonstrated positive staining for CD1a and langerin,
confirming Langerhans cell origin. Because of the
higher than usual mitotic count of up to 22 per 10 high
power fields, and because there was some variation in
nuclear size with some cells having distinct nucleoli, we
diagnosed atypical LCH. The features were insufficient
for a diagnosis of Langerhans cell sarcoma.
CONCLUSION
This case provided a unique opportunity to use PETMRI
to investigate metabolic activity of disease within
the supraorbital mass and throughout the whole body.
Using PET-MRI rather than PET-CT resulted in a
radiation dose reduction of >50%.[3] The choice of PETMRI
is especially appropriate for such a young patient,
because he will need to have periodic follow-up scans
for his disease.
REFERENCES
1. Zaveri J, La Q, Yarmish G, Neuman J. More than just Langerhans cell histiocytosis: a radiologic review of histiocytic disorders. Radiographics. 2014;34:2008-24. Crossref
2. Lim SJ, Lim MK, Park SW, Kim JE, Kim JH, Kim DH, et al.
Langerhans cell histiocytosis in the skull: Comparison of MR
image and other images. JKSMRM. 2009;13:74-80.
3. Kaste SC, Rodriguez-Galindo C, McCarville ME, Shulkin BL.
PET-CT in pediatric Langerhans cell histiocytosis. Pediatr Radiol.
2007;37:615-22. Crossref
4. Phillips M, Allen C, Gerson P, McClain K. Comparison of
FDG-PET scans to conventional radiography and bone scans
in management of Langerhans cell histiocytosis. Pediatr Blood
Cancer. 2009;52:97-101. Crossref