Metastatic Clear Cell Renal Cell Carcinoma with Hyperprogressive Disease after Anti–Programmed Death-1 Immunotherapy: A Case Report
CASE REPORT
Metastatic Clear Cell Renal Cell Carcinoma with Hyperprogressive Disease after Anti–Programmed Death-1 Immunotherapy: A Case Report
KKS Wong, HCK Sze
Department of Clinical Oncology, Pamela Youde Nethersole Eastern Hospital, Chai Wan, Hong Kong
Correspondence: Dr HCK Sze, Department of Clinical Oncology, Pamela Youde Nethersole Eastern Hospital, Chai Wan, Hong Kong. Email: hcksze@hku.hk
Submitted: 29 May 2019; Accepted: 17 Jul 2019.
Contributors: All authors designed the study, drafted the manuscript, and critically revised the manuscript for important intellectual content.
KKSW acquired and interpreted the data. All authors had full access to the data, contributed to the study, approved the final version for
publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: The authors have no conflict of interest to declare.
Acknowledgement: We thank radiologist Dr CY Chu for acknowledging the computed tomography reports and the radiotherapist in our
department for importing computed tomography images into the radiotherapy planning system.
Funding/Support: This case report received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics Approval: This study was conducted in accordance with the Declaration of Helsinki. The patient provided informed consent for all procedures.
INTRODUCTION
Immunotherapy has recently achieved dramatic success
in the treatment of a wide variety of cancers. With the
identification of programmed death-1 (PD-1) and its
ligand PD-L1 playing a key role in tumour immune
escape and cancer growth, immune checkpoint inhibitors
that block the PD-1/PD-L1 pathway have been studied
and demonstrated significant benefit in clinical trials.
Renal cell carcinoma (RCC) has long been recognised
as an immunologically sensitive tumour with a
distinctive response to high-dose interleukin-2 and
interferon-α. In the CheckMate025 randomised phase 3
study, nivolumab, an immunoglobulin G4 monoclonal
antibody against PD-1, was shown to improve the
response rate and overall survival in patients with
advanced RCC who were previously treated with
one or more antiangiogenic agents compared with
everolimus.[1] [2] [3] Heterogeneous post-treatment response
patterns are observed during immunotherapy.[4]
In addition to conventional response patterns,
hyperprogressive disease (HPD) is a novel observation.
We report a case of metastatic clear cell RCC (ccRCC)
presenting with this novel aggressive phenomenon during the initial phase of immunotherapy.
CASE REPORT
A 64-year-old woman with good past health presented
with gross haematuria in March 2016. Computed
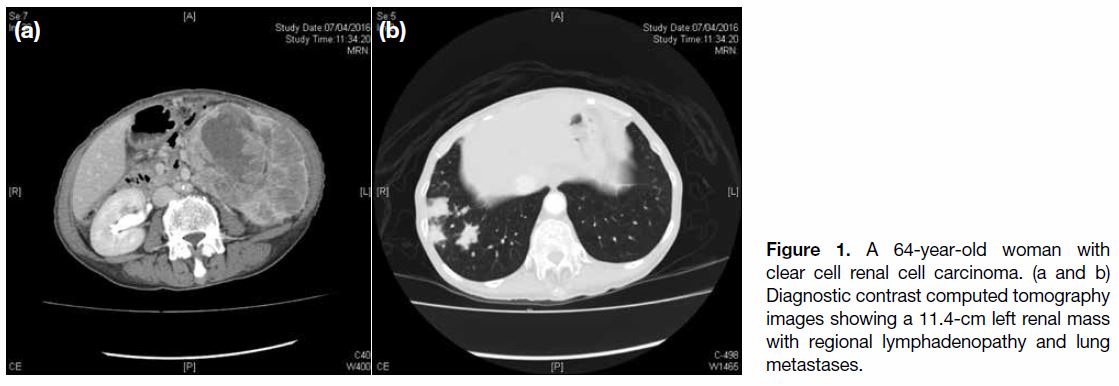
tomography (CT) urogram revealed a left renal mass
measuring 11.4 cm with heterogeneous contrast
enhancement. There were also multiple enlarged lymph
nodes over the left renal hilum and para-aortic region as
well as multiple lung nodules (Figure 1). CT-guided fine
needle aspiration of the lung nodule in the right lower
lobe confirmed ccRCC with immunostaining positive
for AE1/3 and CD10, and negative for CK7, CD117,
34BE12, and TTF-1.
Figure 1. A 64-year-old woman with clear cell renal cell carcinoma. (a and b) Diagnostic contrast computed tomography images showing a 11.4-cm left renal mass with regional lymphadenopathy and lung metastases.
Debulking nephrectomy was not recommended due
to her high operative risk. In June 2016, the patient
was treated with pazopanib and in September 2016,
interval CT scan showed stable disease. In December
2016, interval contrast CT scan revealed mild interval
increase in size of the left renal tumour. Most metastatic
lymphadenopathies and lung nodules also showed
interval enlargement. A new metastasis was seen in
segment VII of the liver and ascites was noted.
Second-line treatment with axitinib was started in
January 2017. Progress contrast CT scan at 9 days
before treatment showed disease progression again with
increase in size of the renal tumour. The lung nodules
had also increased in size and extent, especially over the
bilateral lower lobes and there was interval enlargement
of the multiple metastatic lymphadenopathies. The
liver metastasis in segments VII and VIII were more
conspicuous and new peritoneal deposits were noted.
In April 2017, the patient received 3 mg/kg nivolumab.
At 5 days after nivolumab administration, the patient
reported dyspnoea, lethargy, and poor appetite and was
admitted through the emergency department to a general
medical unit of a hospital near her home. She required
the use of 15-L O2 therapy. On admission, the patient’s
C-reactive protein level was 18.9 mg/L (reference,
≤5 mg/L) and her white cell count was 13.53 × 109/L
(normal range: 3.7-9.3 × 109/L; her baseline white
cell count was normal). At 6 days after nivolumab
administration, urgent contrast CT thorax was performed
to exclude pulmonary embolism. Although no pulmonary
embolism was evident, multiple patchy opacities as
consolidations and multiple nodules in both lung fields
showed dramatic increase in size and extent, especially
over the bilateral lower lobes and the left upper lobe.
Ground-glass opacities in both lungs were also seen
(Figure 2). Given the drastic and rapid change in CT
findings in both lungs (Table 1), the CT findings could
represent pneumonitis associated with the treatment
with a differential diagnosis of disease progression.
The previously seen prominent-to-enlarged metastatic
lymphadenopathies showed interval enlargement and
increase in extent.
Figure 2. The same 64-year-old woman with clear cell renal cell carcinoma. Arrows in contrast computed tomography images show (a,
b) interval development of new lung metastasis, (c, d) interval increase in size of patchy consolidation in sites of metastasis; (e, f) interval
increase in size of lung metastasis and development of new consolidative changes; and (g to l) interval increase in size of lung metastasis.
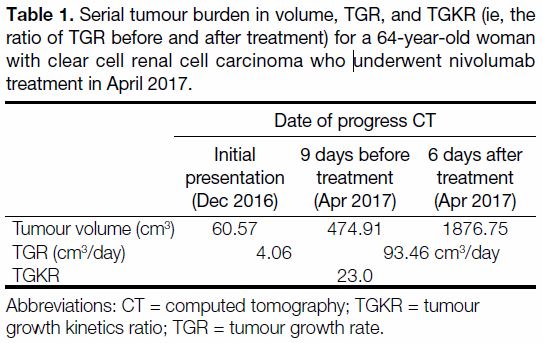
Table 1. Serial tumour burden in volume, TGR, and TGKR (ie, the ratio of TGR before and after treatment) for a 64-year-old woman with clear cell renal cell carcinoma who underwent nivolumab treatment in April 2017.
Her clinical condition deteriorated rapidly despite
administration of intravenous antibiotics and she finally succumbed due to respiratory failure 7 days after
nivolumab administration.
DISCUSSION
Although immune checkpoint blockade has emerged as
a principal treatment modality for many cancers, it has
been recognised that disease response and stabilisation
can occur after an initial paradoxical increase in
tumour burden or appearance of new lesions, indicating
pseudoprogression.
HPD is a newly recognised condition where tumour
growth is accelerated by immunotherapy with consequent
rapid disease progression. Under these circumstances,
continuation of treatment may be considered unsafe.
There is no consensus on the definition of HPD but
quantifying the progression rate with tumour growth
rate (TGR) may enable more objective elucidation of
the condition. HPD has been previously been defined as
RECIST (response evaluation criteria in solid tumours)
disease progression with ≥2-fold increase in TGR from
baseline before treatment.[5] [6] [7]
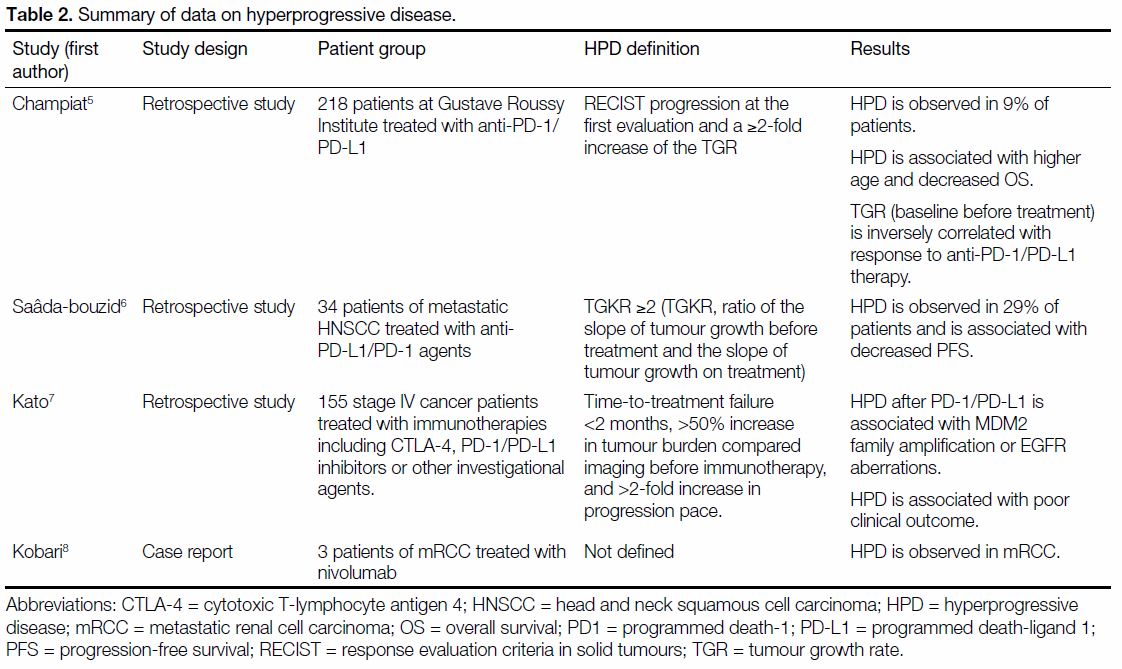
A study that included 131 evaluable patients treated
with anti-PD-1/PD-L1 reported a 9% incidence of HPD.
Among the nine patients diagnosed with renal cancer,
none developed HPD.[5] In another study that included 34
head and neck cancer patients treated with PD-1/PD-L1
inhibitors, the incidence of HPD was 29%.[6] Table 2
summarises the findings of clinical studies reporting
HPD following use of anti-PD-1/PD-L1+/- CTLA4
(cytotoxic T-lymphocyte associated antigen 4). HPD
was consistently associated with worse clinical outcomes.[5] [6] [7] [8]
Table 2. Summary of data on hyperprogressive disease.
Previous studies used the sum of the longest diameter of
target lesions to calculate TGR. In this case report, we
used three-dimensional parameters to record TGR. We imported serial progress CT images into the radiotherapy
planning system Varian™ and contoured all the intrathoracic
measurable target lesions. Measurable target
lesions were defined as tumour deposits with diameter
>1 cm. Tumour volume was calculated by computer.
Table 1 summarises the change in tumour volume and
TGR before and after treatment. A 23-fold increase in TGR after nivolumab administration was evident. This
phenomenon of significant disease progression with more
than doubling of tumour growth rate is known as HPD.
The mechanism of HPD is unclear. Kato et al[7] identified
the potential role of genomic analysis and demonstrated
that MDM2 amplification or EGFR aberrations may be
associated with poor clinical outcome.
Since anti-PD1 immunotherapy reactivates cytotoxic
T cells to enhance their tumouricidal action, massive
infiltration of immune cells into the tumour may explain
the rapid radiological disease progression, as evidenced
by biopsy results of metastatic melanoma in another case
report that showed similar findings.[9] Tumour necrosis,
deemed to be one of the causes of pseudoprogression,
may also play a role in hyperprogression.[10]
In this case report, there are some limitations to our
analysis. First, there was no subsequent imaging after disease progression to fulfil the definition of
“progressive disease” under irRC. Second, there was no
pathological correlation since no biopsy was performed
to demonstrate radiological pneumonitic changes and
disease progression. Third, TGR was calculated based
only on intrathoracic tumour burden since only CT
thorax was performed after nivolumab administration.
Fourth, the time between baseline scan and the previous
scan was 5 months. As such, TGR before treatment may
have been underestimated since rapid tumour growth
may have occurred just prior to the baseline scan.
HPD is a newly recognised disease response pattern
during immunotherapy. It is characterised by accelerated
TGR during the initial phase of treatment. Clinicians
should be aware of this potentially lethal phenomenon,
particularly in patients with extensive visceral metastases.
Currently there are few studies of HPD. The phenomenon
is observed across metastatic non-small cell lung cancer,
head and neck squamous cell carcinoma, and ccRCC.
More studies are needed to clarify the implications of this
phenomenon and the associated underlying biogenetics.
This may provide insight into more personalised
oncological treatment in future.
REFERENCES
1. Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ,
Srinivas S, et al. Nivolumab versus everolimus in advanced renalcell
carcinoma. N Engl J Med. 2015;373:1803-13. Crossref
2. McDermott DF, Motzer RJ, Atkins MB, Plimack ER, Sznol M,
George S, et al. Long-term overall survival (OS) with nivolumab
in previously treated patients with advanced renal cell carcinoma
(aRCC) from phase I and II studies. J Clin Oncol. 2016;34(15
Suppl):4507. Crossref
3. Escudier B, Sharma P, McDermott DF, George S, Hammers HJ,
Srinivas S, et al. CheckMate 025 randomized phase 3 study:
outcomes by key baseline factors and prior therapy for nivolumab
versus everolimus in advanced renal cell carcinoma. Eur Urol.
2017;72:962-71. Crossref
4. de Velasco G, Krajewski KM, Albiges L, Awad MM, Bellmunt J,
Hodi FS, et al. Radiologic heterogeneity in responses to anti-PD-1/PD-L1 therapy in metastatic renal cell carcinoma. Cancer Immunol
Res. 2016;4:12-7. Crossref
5. Champiat S, Dercle L, Ammari S, Massard C, Hollebecque A,
Postel-Vinay S, et al. Hyperprogressive disease is a new pattern
of progression in cancer patients treated by anti-PD-1/PD-L1. Clin
Cancer Res. 2017;23:1920-8. Crossref
6. Saâda-Bouzid E, Defaucheux C, Karabajakian A, Coloma VP,
Servois V, Paoletti X, et al. Hyperprogression during anti-PD-1/PD-L1 therapy in patients with recurrent and/or metastatic head
and neck squamous cell carcinoma. Ann Oncol. 2017;28:1605-11. Crossref
7. Kato S, Goodman A, Walavalkar V, Barkauskas DA, Sharabi A,
Kurzrock R. Hyperprogressors after immunotherapy: analysis of
genomic alterations associated with accelerated growth rate. Clin
Cancer Res. 2017;23:4242-50. Crossref
8. Kobari Y, Kondo T, Takagi T, Omae K, Nakazawa H, Tanabe K.
Rapid progressive disease after nivolumab therapy in three patients
with metastatic renal cell carcinoma. In Vivo. 2017;31:769-71. Crossref
9. Cohen JV, Alomari AK, Vortmeyer AO, Jilaveanu LB, Goldberg SB,
Mahajan A, et al. Melanoma brain metastasis pseudoprogression
after pembrolizumab treatment. Cancer Immunol Res. 2016;4:179-82. Crossref
10. Chiou VL, Burotto M. Pseudoprogression and immune-related
response in solid tumors. J Clin Oncol. 2015;33:3541-3. Crossref