Radiological Findings in COVID-19 and Adaptive Approaches for Radiology Departments: Literature Review and Experience Sharing
SPECIAL ARTICLE AND INVITED REVIEW CME
Radiological Findings in COVID-19 and Adaptive Approaches for Radiology Departments: Literature Review and Experience Sharing
LFH Ng1, HHC Tsang2, FHY Wong3, MWC Law4, WH Chong5, CHN Ho6, JKJ Fung7, CCY Chan8, LSK Li1, KT Wong4, JCX Chan5, SHY Lam3, KH Wong9, PL Kwok7, L Xu2,
TKK Lai6, KK Cheng8, TYW Hon10, JYH Hui10, SKY Kwok5, JKF Ma1
1 Department of Radiology, Princess Margaret Hospital, Laichikok, Hong Kong
2 Department of Radiology and Organ Imaging, Queen Elizabeth Hospital, Jordan, Hong Kong
3 Department of Radiology, Queen Mary Hospital, Pokfulam, Hong Kong
4 Department of Imaging and Interventional Radiology, Prince of Wales Hospital, Shatin, Hong Kong
5 Department of Radiology, Tuen Mun Hospital, Tuen Mun, Hong Kong
6 Department of Radiology, Tseung Kwan O Hospital, Tseung Kwan O, Hong Kong
7 Department of Radiology, Pamela Youde Nethersole Eastern Hospital, Chai Wan, Hong Kong
8 Department of Diagnostic and Interventional Radiology, Kwong Wah Hospital, Yaumatei, Hong Kong
9 Department of Radiology, North District Hospital, Sheung Shui, Hong Kong
10 Department of Radiology, United Christian Hospital, Kwun Tong, Hong Kong
Correspondence: Dr LFH Ng, Department of Radiology, Princess Margaret Hospital, Laichikok, Hong Kong. Email: ngphonehim@gmail.com
Submitted: 24 Apr 2020; Accepted: 13 May 2020.
Contributors: LFHN, JCXC, SKYK and JKFM designed the study. All authors contributed to the acquisition of data. LFHN and JCXC analysed the data and drafted the manuscript. All authors had critical revision of the manuscript for important intellectual content. All authors had full
access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics Approval: This study was approved by the Hong Kong Hospital Authority Central Institutional Review Board (Ref CCO-2020-0010).
Abstract
Radiological investigations play an important role in the treatment course of patients with coronavirus disease 2019
(COVID-19) and radiologists should be familiar with the imaging characteristics. Being an integral component of the healthcare system, radiology departments have made adaptations to enhance infection control and strengthen the service. In this article, we review the radiological features of COVID-19 on chest radiography and computed tomography, and share experiences on the adaptive approach of radiology departments amidst the COVID-19 pandemic.
Key Words: Coronavirus; COVID-19; Radiography; Severe acute respiratory syndrome coronavirus 2
中文摘要
2019冠狀病毒病的放射學發現和放射科的調整處理方法:文獻綜述和經驗分享
吳豐謙、曾凱晴、黃皓源、羅穎聰、莊永豪、何灝、馮喬政、陳卓忻、李思琪、黃嘉德、陳積聖、林曉燕、黃健開、郭寶琳、徐璐、賴國強、鄭加勁、韓予偉、許懿馨、郭啓欣、馬嘉輝
放射學檢查在2019冠狀病毒病(COVID-19)患者的治療過程中起著重要作用,放射科醫生應熟悉其成像特徵。作為醫療系統不可或缺的組成部分,放射科已經做出調整以增強感染控制和提高服務質量。本文回顧COVID-19在胸部X光和電腦斷層掃描上的放射學特徵,並分享COVID-19大流行中放射科調整處理方法的經驗。
INTRODUCTION
In late December 2019, the World Health Organization
(WHO) was informed of a cluster of cases of pneumonia
of unknown cause detected in Wuhan City, Hubei
Province, China. Subsequent deep sequencing analysis
from lower respiratory tract samples indicated a novel
coronavirus as the causative organism. The International
Committee on Taxonomy of Virus named the virus
severe acute respiratory syndrome coronavirus 2
(SARS-CoV-2).[1] In February 2020, the WHO
officially named the disease coronavirus disease 2019
(COVID-19). The disease spread rapidly, resulting in an
epidemic throughout China, followed by other countries
around the world. On 11 March 2020, the WHO
officially declared COVID-19 a pandemic.[2] According
to data published by the WHO on 19 April 2020
(10:00 CEST), more than two million cases of COVID-19
have been confirmed worldwide, including more than
150 000 deaths from the disease.[3]
The diagnosis of SARS-CoV-2 infection largely
depends on clinical and epidemiological history
with subsequent laboratory confirmation by realtime
reverse-transcription polymerase chain reaction
(RT-PCR) tests. Radiological investigations also play
an important role in the treatment course of patients
with COVID-19. To provide radiologists with the
latest information, herein we review the radiological
investigations and imaging features of COVID-19
and share the experiences of radiology departments in
Hong Kong.
RADIOLOGICAL INVESTIGATIONS
FOR COVID-19
Chest Radiography
Plain chest radiography (CXR) is readily available and
is commonly performed in patients presenting with
respiratory symptoms of various causes. In patients
with COVID-19, an initial CXR helps not only to detect
features of pneumonia, but also to identify or rule out
differential diagnoses, such as pneumothorax or heart
failure. In countries facing resource constraints with
limited availability and long turnaround time of RT-PCR
tests, CXR is recommended for medical triage of patients
who present with moderate to severe clinical features
and a high pre-test probability of COVID-19.[4]
Depending on the clinical setting and time of presentation,
CXR abnormalities are reported in 33.3% to 95% of
patients with COVID-19.[5] [6] [7] [8]] [9] [10] In a study on 64 patients with
RT-PCR-confirmed SARS-CoV-2 infection from four
hospitals in Hong Kong, 69% of patients demonstrated
abnormalities on baseline CXR, with 80% of patients
exhibiting CXR abnormalities at some point during
their disease course.[7] Because the sensitivity of CXR
could be related to the time of imaging and severity of
pulmonary involvement, a normal CXR cannot exclude
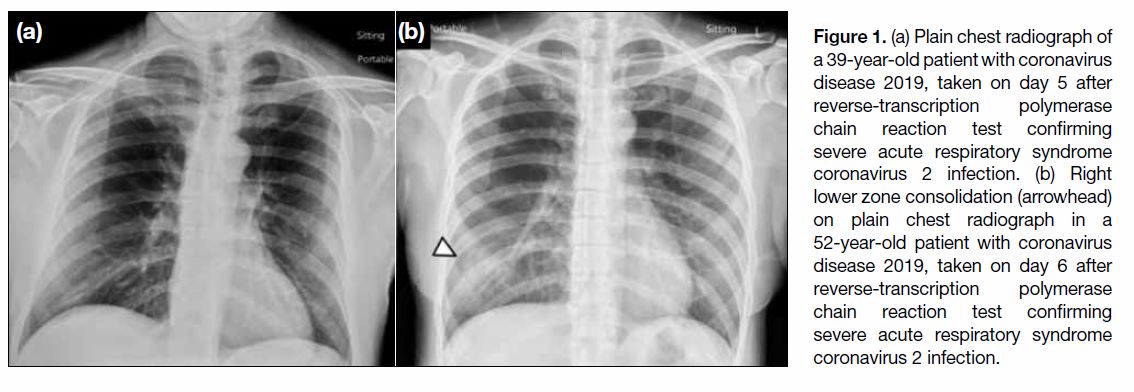
the diagnosis of COVID-19 (Figure 1a).
Figure 1. (a) Plain chest radiograph of a 39-year-old patient with coronavirus disease 2019, taken on day 5 after reverse-transcription polymerase chain reaction test confirming severe acute respiratory syndrome coronavirus 2 infection. (b) Right lower zone consolidation (arrowhead) on plain chest radiograph in a 52-year-old patient with coronavirus disease 2019, taken on day 6 after reverse-transcription polymerase chain reaction test confirming severe acute respiratory syndrome coronavirus 2 infection.
For patients with confirmed SARS-CoV-2 infection,
CXR has the advantage of being portable for imaging
within the isolation rooms, thereby reducing the risk
of disease transmission during patient transportation. Portable CXR is invaluable to assess disease progression
and rule out complications. However, in patients with
confirmed SARS-CoV-2 infection, CXR should only be
performed when there is appropriate clinical need, such
as when there is clinical deterioration, rather than as a
daily routine. Avoidance of non–value-added imaging
is particularly important in patients with COVID-19, to
minimise radiation exposure, to reduce the risk of disease
transmission to radiographers, and to conserve personal
protective equipment.
Imaging Features on Chest Radiography
The most common findings on CXR are consolidation
and ground-glass opacities (GGOs), found more often
in a bilateral, peripheral, and lower zone distributions
(Figure 1b).[5] [7] Pleural effusion is uncommon, found only in 3% of patients.[7] A study in Hong Kong
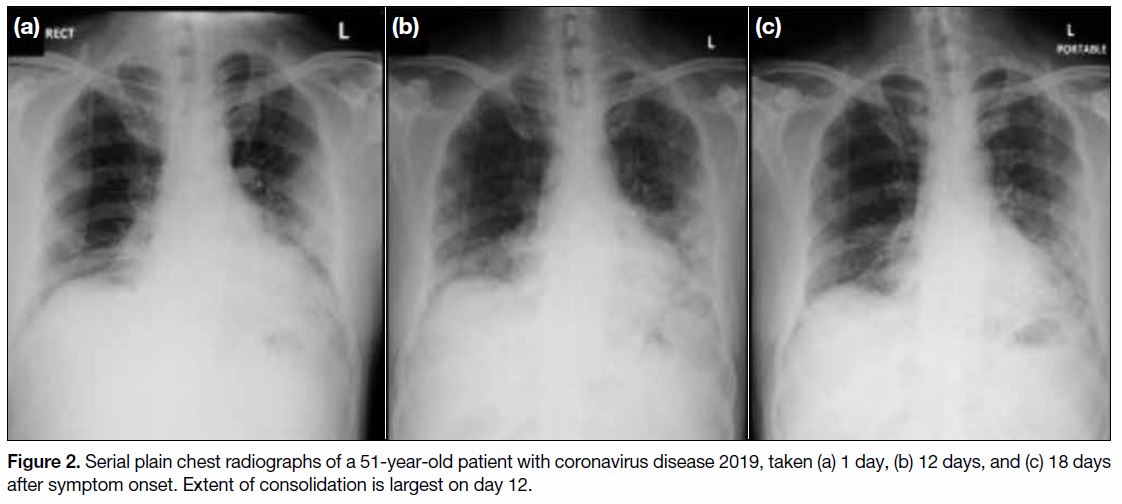
found that CXR findings reach peak severity at 10 to
12 days from symptom onset (Figure 2).[7] This is in
concordance with the earlier peak severity at 6 to 11
days from symptom onset,[11] reported for computed
tomography (CT), which is more sensitive. This is
also in concordance with the clinical course; sepsis
and acute respiratory distress syndrome have been
found to occur at 9 to 12 days from symptom onset.[12]
Nevertheless, CXR findings in COVID-19 are not
organism-specific and can overlap with other viral
infections including influenza, severe acute respiratory
syndrome (SARS), and Middle East respiratory
syndrome (MERS). Furthermore, co-infection with
other respiratory pathogens can occur in patients with
confirmed SARS-CoV-2 infection.[13]
Figrue 2. Serial plain chest radiographs of a 51-year-old patient with coronavirus disease 2019, taken (a) 1 day, (b) 12 days, and (c) 18 days after symptom onset. Extent of consolidation is largest on day 12.
Computed Tomography
Compared with CXR, chest CT is less readily available
and results in greater radiation exposure. Nevertheless,
it provides superior delineation of the pulmonary
involvement caused by COVID-19. Chest CT is
reported to have higher sensitivity for the diagnosis of
SARS-CoV-2 infection when compared with initial
RT-PCR test results. The sensitivity of chest CT has
been reported in various studies as 98%, 97%, 93%,
and 61%.[14] [15] [16] [17] However, chest CT has limited specificity
as low as 25%,[15] and should not be used as the sole
method for the diagnosis of COVID-19. Discordance
between results of RT-PCR and chest CT is commonly
encountered and may lead to diagnostic confusion.[18] [19]
A multidisciplinary approach, involving a combination
of clinical history, clinical manifestations, imaging
features, and laboratory results, is therefore desirable
to achieve a timely and accurate diagnosis. According
to a multinational consensus statement from the
Fleischner Society, chest CT is not routinely indicated
as a screening test for COVID-19 in asymptomatic
individuals. Imaging is also not indicated for patients
with mild features of COVID-19, unless they are at risk
for disease progression. In contrast, chest CT is indicated
for patients showing worsening respiratory status and/or
moderate to severe features of COVID-19.[4]
CT scans can be used to evaluate complications related to COVID-19. Pleural effusion, multiple tiny pulmonary
nodules, and mediastinal lymphadenopathy are atypical
in COVID-19 pneumonia and their appearance should
raise concern for bacterial superinfection or alternative
diagnosis.[20] In addition, CT can readily detect other
complications especially in patients with COVID-19
who have been admitted under intensive care unit, to
confirm or rule out pulmonary embolism during the
acute setting.
Imaging Features on Chest Computed Tomography
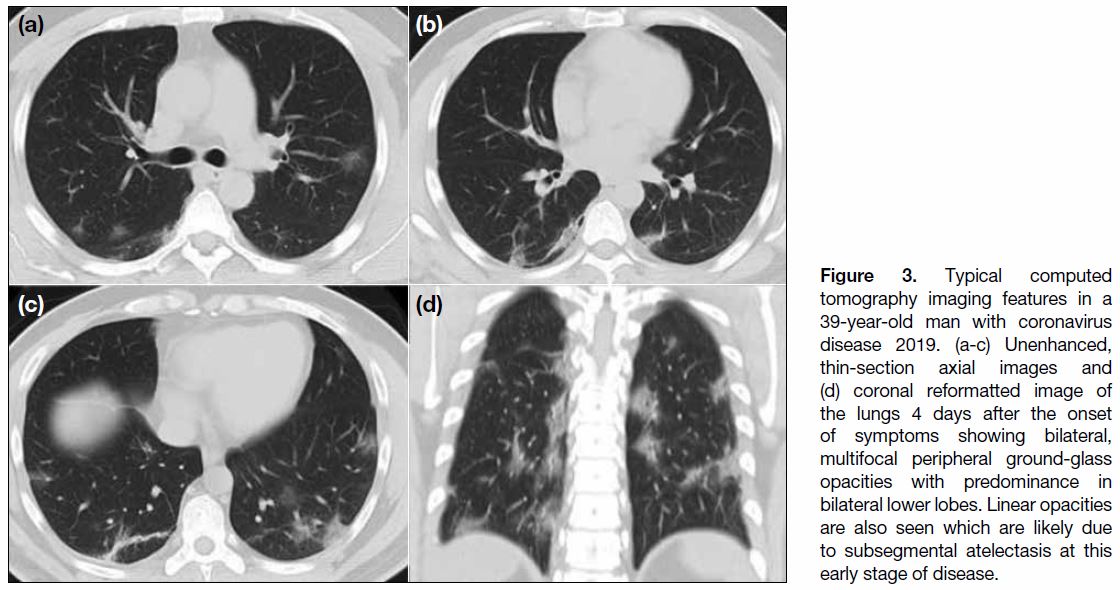
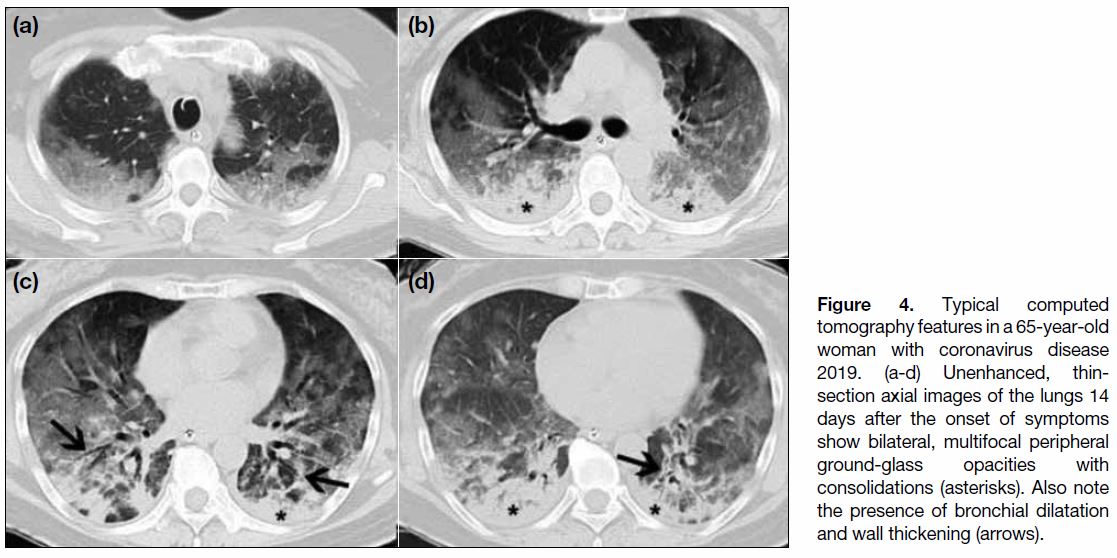
The cardinal hallmark of COVID-19 pneumonia on
chest CT is bilateral GGOs with or without consolidation
in peripheral and posterior lungs (Figures 3 and 4).[11] [21]
The GGOs often have rounded morphology (Figure 5)
or are present with interlobular septal thickening and
intralobular lines creating a “crazy-paving” pattern
(Figure 6).[20] GGO together with small areas of
consolidation may suggest an organising pneumonia
pattern of lung injury.[21]
Figure 3. Typical computed
tomography imaging features in a 39-year-old man with coronavirus disease 2019. (a-c) Unenhanced, thin-section axial images and (d) coronal reformatted image of the lungs 4 days after the onset of symptoms showing bilateral, multifocal peripheral ground-glass opacities with predominance in bilateral lower lobes. Linear opacities are also seen which are likely due to subsegmental atelectasis at this early stage of disease.
Figure 4. Typical computed tomography features in a 65-year-old woman with coronavirus disease 2019. (a-d) Unenhanced, thin-section axial images of the lungs 14 days after the onset of symptoms show bilateral, multifocal peripheral ground-glass opacities with consolidations (asterisks). Also note the presence of bronchial dilatation and wall thickening (arrows).
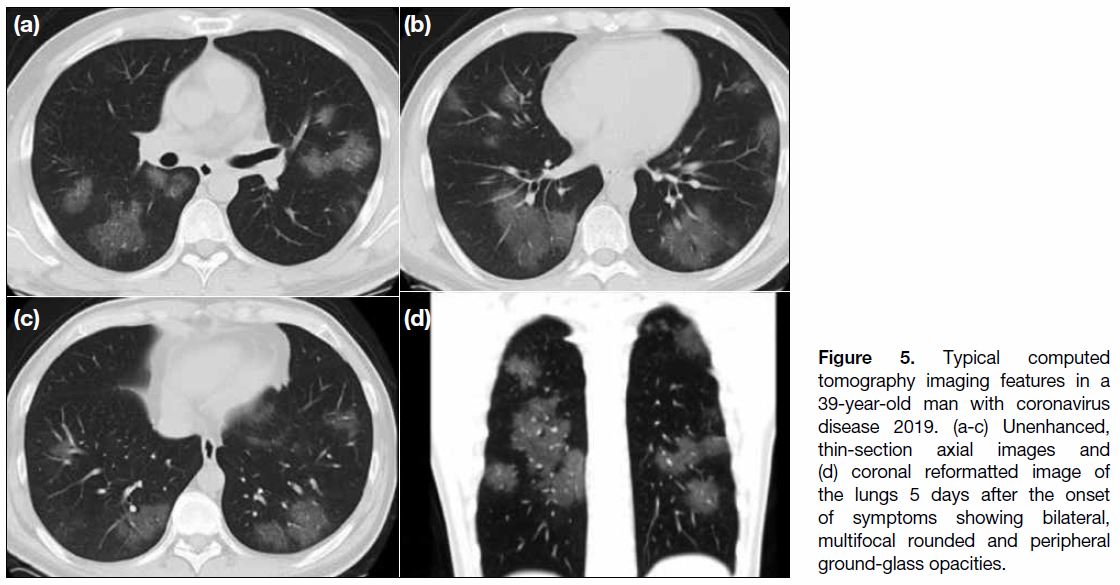
Figure 5. Typical computed tomography imaging features in a 39-year-old man with coronavirus disease 2019. (a-c) Unenhanced, thin-section axial images and (d) coronal reformatted image of the lungs 5 days after the onset of symptoms showing bilateral, multifocal rounded and peripheral ground-glass opacities.
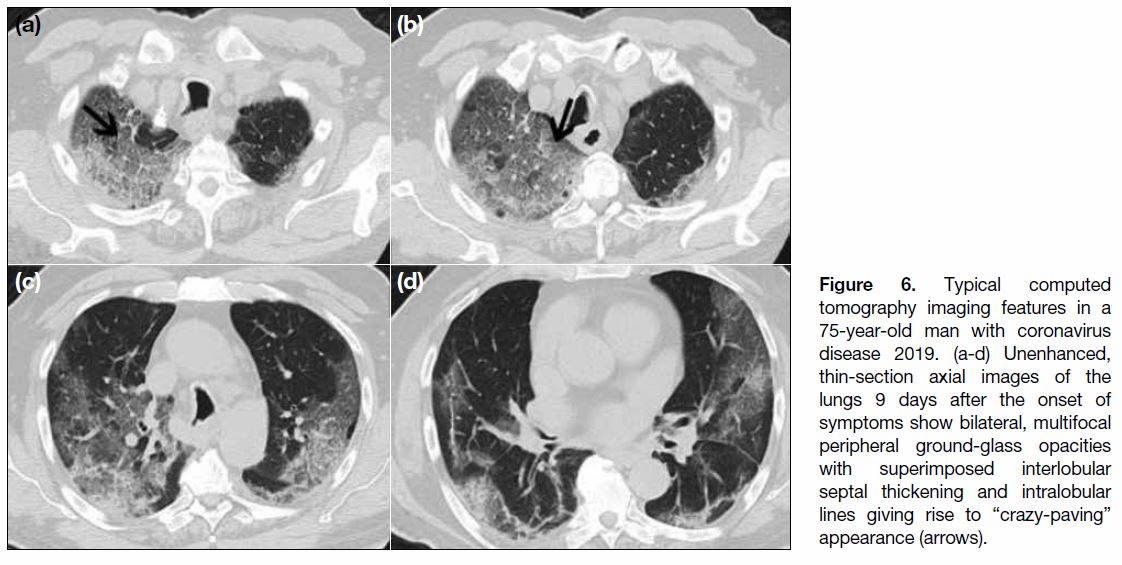
Figure 6. Typical computed tomography imaging features in a 75-year-old man with coronavirus disease 2019. (a-d) Unenhanced, thin-section axial images of the lungs 9 days after the onset of symptoms show bilateral, multifocal peripheral ground-glass opacities with superimposed interlobular septal thickening and intralobular lines giving rise to “crazy-paving” appearance (arrows).
Aligned by the Hong Kong College of Radiologists
and with the cooperative effort of all radiology centres
managed by the Hong Kong Hospital Authority, chest
CT images of patients with confirmed SARS-CoV-2
infection scanned from 22 January 2020 to 16 April 2020
in six public hospitals in Hong Kong were reviewed by qualified radiologists of the Hong Kong College of
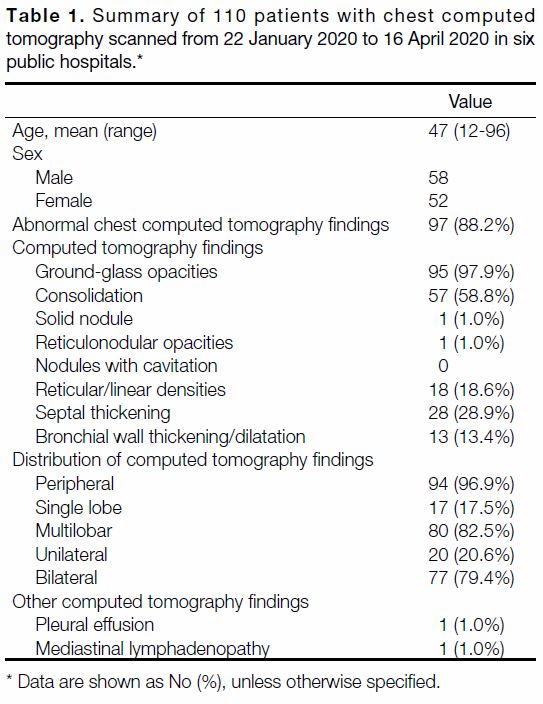
Radiologists (Table 1). Total 110 patients with RT-PCR-confirmed
SARS-CoV-2 infection were included in this
evaluation, 97 (88.2%) of whom had abnormal findings
on their first chest CT after admission. Among the
radiological findings, GGO (Figures 3 4 5 6 7) was the most
common, occurring in 95 (97.9%) patients. Consolidation
(Figure 4) was recorded in 57 (58.8%) patients, septal thickening in 28 (28.9%) patients, and reticular/linear densities in 18 (18.6%) patients. Bronchial wall
thickening and dilatation (Figure 4) were less common,
present in 13 (13.4%) patients. Reticulonodular opacities
and nodules were rare, and were found in the same one
(1.0%) patient. None of the patients had nodules with
cavitation. In terms of lesion distribution, 94 (96.9%)
patients had predominantly peripheral distribution, 77 (79.4%) patients had bilateral involvement, and 80
(82.5%) patients had multilobar involvement. Other
imaging findings were considered atypical, such as pleural effusion in one (1.0%) patient and mediastinal
lymphadenopathy in one (1.0%) patient. The observed
imaging findings and patterns are consistent with the
results from other published studies.[11] [15] [17] [18] [19]
Table 1. Summary of 110 patients with chest computed tomography scanned from 22 January 2020 to 16 April 2020 in six public hospitals.
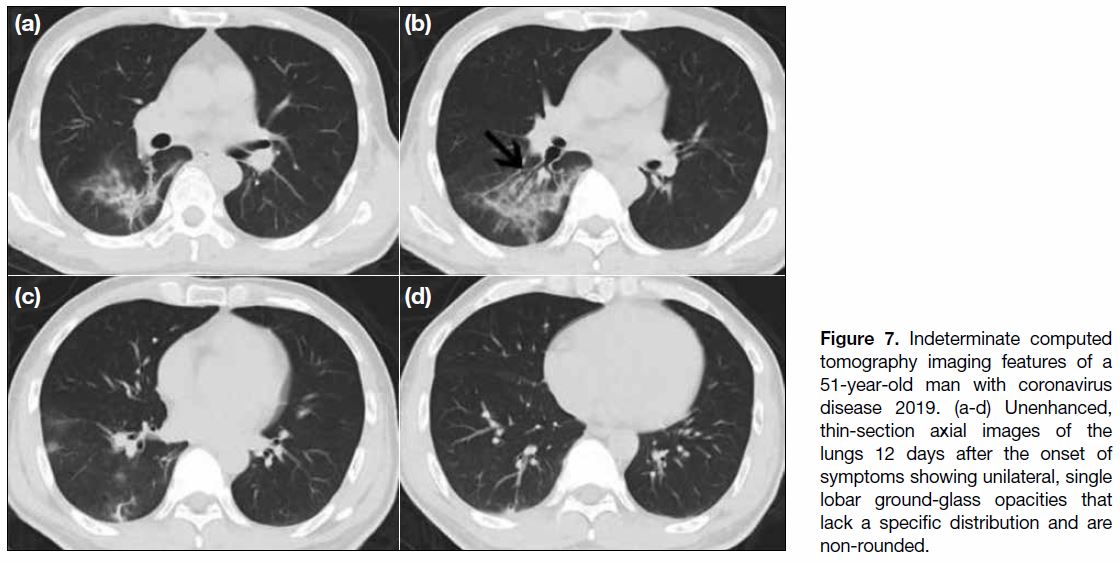
Figure 7. Indeterminate computed tomography imaging features of a 51-year-old man with coronavirus disease 2019. (a-d) Unenhanced, thin-section axial images of the lungs 12 days after the onset of symptoms showing unilateral, single lobar ground-glass opacities that lack a specific distribution and are non-rounded.
In March 2020, the Radiological Society of North
America proposed, with endorsement by the Society of
Thoracic Radiology and American College of Radiology,
structured CT reporting for patients with COVID-19
pneumonia in order to disseminate common findings of
the disease and decrease reporting variability.[20] Findings
are classified into four categories: (1) typical appearance
with (i) peripheral, bilateral GGOs with or without
consolidation or intralobular lines (“crazy-paving”
pattern), or (ii) multifocal GGOs of rounded morphology
with or without consolidation or intralobular lines, or
(iii) reverse halo sign or other findings of organising
pneumonia; (2) indeterminate appearance with
multifocal, diffuse, perihilar, or unilateral GGOs with or
without consolidation lacking a specific distribution or
few small GGOs that are non-rounded or non-peripheral;
(3) atypical appearance; and (4) negative for pneumonia.
Based on this the Radiological Society of North America
proposed reporting language for COVID-19 pneumonia,
the majority of our patients had typical (69%) or at least
indeterminate appearances (18%) on chest CT, whereas
few had atypical appearance (0.9%) or were negative for
pneumonia (11.8%) [Table 2[20]].
Table 2. Radiological Society of North America proposed reporting language for computed tomography findings related to coronavirus disease 2019.[20]
Follow-up Chest Computed Tomography
When necessary, serial chest CT can reflect disease
evolution and monitor treatment effects.[22] In patients
with COVID-19 who deteriorate, initial findings of
small GGOs on chest CT become more extensive,
and might grow larger with crazy-paving patterns and consolidation.[22] [23] [24] In severe and critical patients, the
occurrence rates of consolidation, linear opacities, crazypaving,
and bronchial thickening increase, as well as the
extent of lung involvement.[23] Eventually the disease
proceeds to ‘white lung’ appearance, seriously affecting
the patient’s lung function.[24]
For patients who eventually recover from pneumonia,
the most severe features are found on CT imaging
at approximately 10 days after the initial onset of
symptoms. At approximately 14 days, radiological signs
of improvement are seen.[22] Ai et al[15] observed that chest
CT improvement precedes negative RT-PCR test results
in some patients.
Recent studies have revealed that COVID-19 shares
similar CT features with organising pneumonia, most
notably atoll signs and peripheral multilobar GGOs.
Such similarities suggest the presence of secondary
organising pneumonia in some patients with COVID-19.
Secondary organising pneumonia was also documented
in viral pneumonia caused by SARS-CoV, MERS-CoV,
and influenza. Future histological correlations are
required to confirm these associations. Because a small
proportion of patients with organising pneumonia
progress to pulmonary fibrosis (fibrosing organising
pneumonia), follow-up CT imaging may be advisable
for patients showing features of organising pneumonia.
Organising pneumonia can be effectively managed by
corticosteroids to prevent progression to fibrosis.[25]
According to a multinational consensus statement
from the Fleischner Society, follow-up CT imaging is
indicated for patients with functional impairment and
hypoxaemia after recovery from COVID-19. The purpose
of the follow-up CT imaging is to differentiate between
causes for such pulmonary function impairment after
recovery, whether it is due to sequelae of infection and
mechanical ventilation or from a potentially treatable
cause (e.g., organising pneumonia as mentioned
above).[4]
As stated above, not all patients with COVID-19
will undergo follow-up CT imaging. A single-centre
review on the features of follow-up chest CT imaging
revealed that among 70 patients who had chest CT scan
after confirmation of SARS-CoV-2 infection, and
15 patients had a second chest CT scan. The mean
(±standard deviation) interval of the second chest CT
after the first positive RT-PCR test was 21.9±5.8 days.
The mean interval between the first and second CT scan was 18.1±5.4 days. The mean age of the patients
was 56.1±16.7 years. All patients had GGOs and
consolidative changes with peripheral distribution on the
initial scan. One (6.7%) patient had complete resolution
on the second CT, whereas other patients showed
varying degrees of interval reductions in the GGOs and
consolidative changes. All patients who underwent serial
CT scans survived and were subsequently discharged
home. Two patients underwent a third chest CT scan.
One of them demonstrated features of secondary
organising pneumonia (Figure 8). The other showed
complete resolution on the third CT scan (Figure 9).
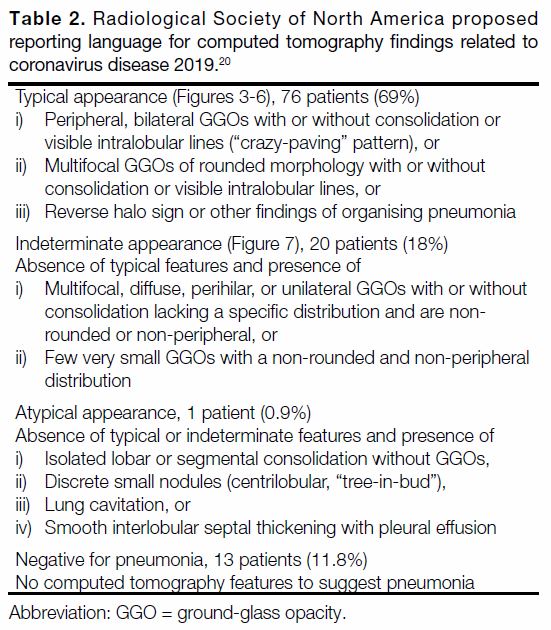
Figure 8. Serial chest computed tomography (CT) scans in a 56-year-old man with coronavirus disease 2019. Scan obtained on day 2 (a)
after reverse-transcription polymerase chain reaction test confirmed severe acute respiratory syndrome coronavirus 2 infection chest CT
scan showed peripheral ground-glass opacities and consolidations without zonal predominance. Scan obtained on day 14 (b) showed
interval reduction of the mixed ground-glass opacities and consolidative changes. Scan obtained on day 22 (c) showing the consolidations
became arched bands with shaded margins. It was likely the result of perilobular inflammation, which could represent features of perilobular
fibrosis found in secondary organising pneumonia. The patient was discharged home on day 32 after two consecutive negative reverse-transcription
polymerase chain reaction tests.
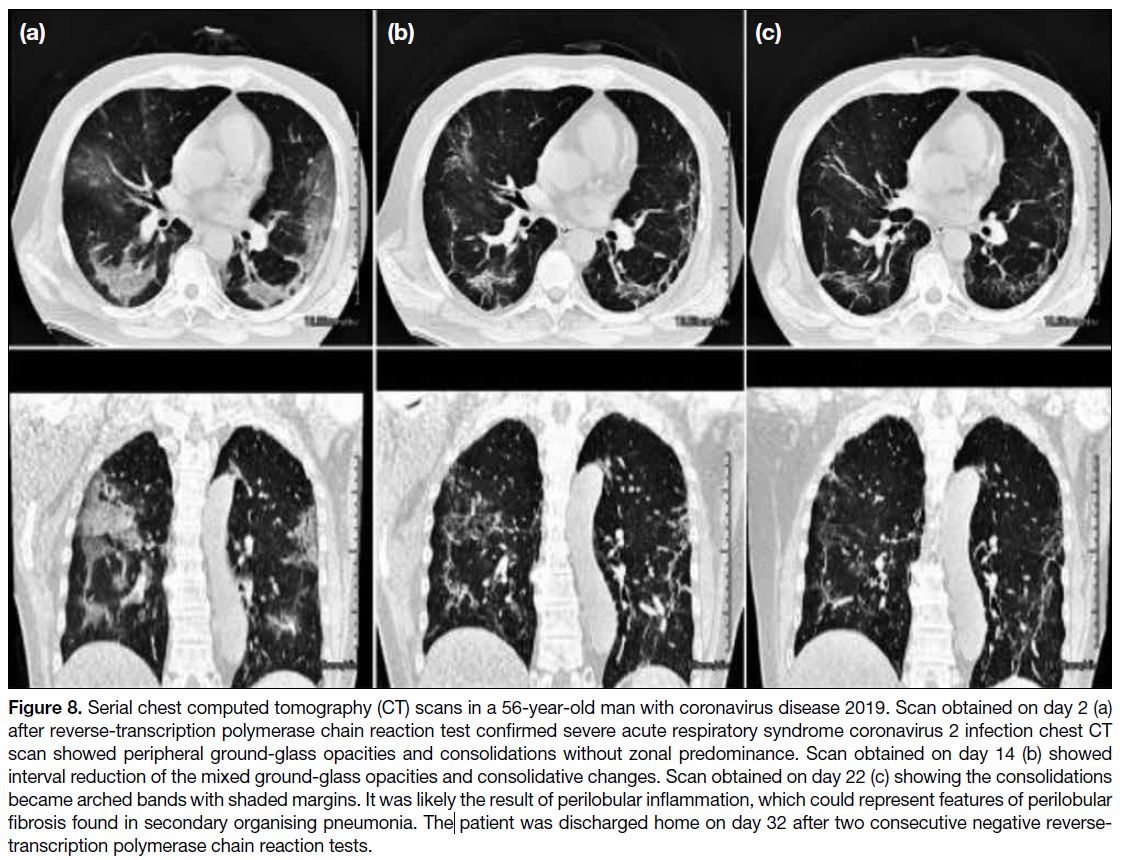
Figure 9. Serial chest computed tomography scans in a 39-year-old man with coronavirus disease 2019. Scan obtained on day 2 (a) after positive reverse-transcription polymerase chain reaction test for severe acute respiratory syndrome coronavirus 2 showed small peripheral consolidation and ground-glass opacities scattered in both middle and lower lobes. Scan obtained on day 14 (b) showing significant reduction of the consolidation and ground-glass opacities. Scan obtained on day 28 (c) showed complete resolution. The patient was discharged home on day 33 after two consecutive negative reverse-transcription polymerase chain reaction tests.
A recent report by Wang et al[25] found that the majority
of patients discharged home had residual disease on final
CT scans. The number of days from symptom onset
to discharge was median 24 days (range, 10-44 days).
The CT changes peaked during illness days 6 to 11
after symptom onset. The increase in GGOs observed
on serial scans by Wang et al[25] was not observed in our reviewed patients, which could be related to the longer
median interval between scans of 17 days (range, 11-32
days) versus 6 days (range, 2-19 days).
COMPARING IMAGING FEATURES
AMONG COVID-19, SARS, AND MERS
The COVID-19 pandemic in 2020, SARS outbreak in
2003, and MERS outbreak in 2012, are all caused by
viruses belonging to the same Coronaviridae family.
It is understandable that they can mimic each other
on clinical grounds. Radiologically they share some
common features but also have noticeable differences.
In the largest series of COVID-19 patients in China,
abnormalities on radiographs were detected in up to
59.1% of patients at presentation.[26] This is less than
the reported radiographic detection rate of SARS
and MERS (about 83%).[27] [28] Among patients with
radiological abnormalities, the distribution of changes
most commonly involves the peripheral part of the
lungs for all three infectious conditions. Unilateral
abnormalities are more common in SARS and MERS
than in COVID-19, where changes more frequently involve both lungs. Progression from focal unilateral
peripheral lung involvement to bilateral multifocal
lesions with upper lobes and perihilar involvement are
associated with poor prognosis of severe cases in SARS
(Figure 10) and MERS, whereas the prognostic value of
radiographic changes remains unclear for COVID-19.
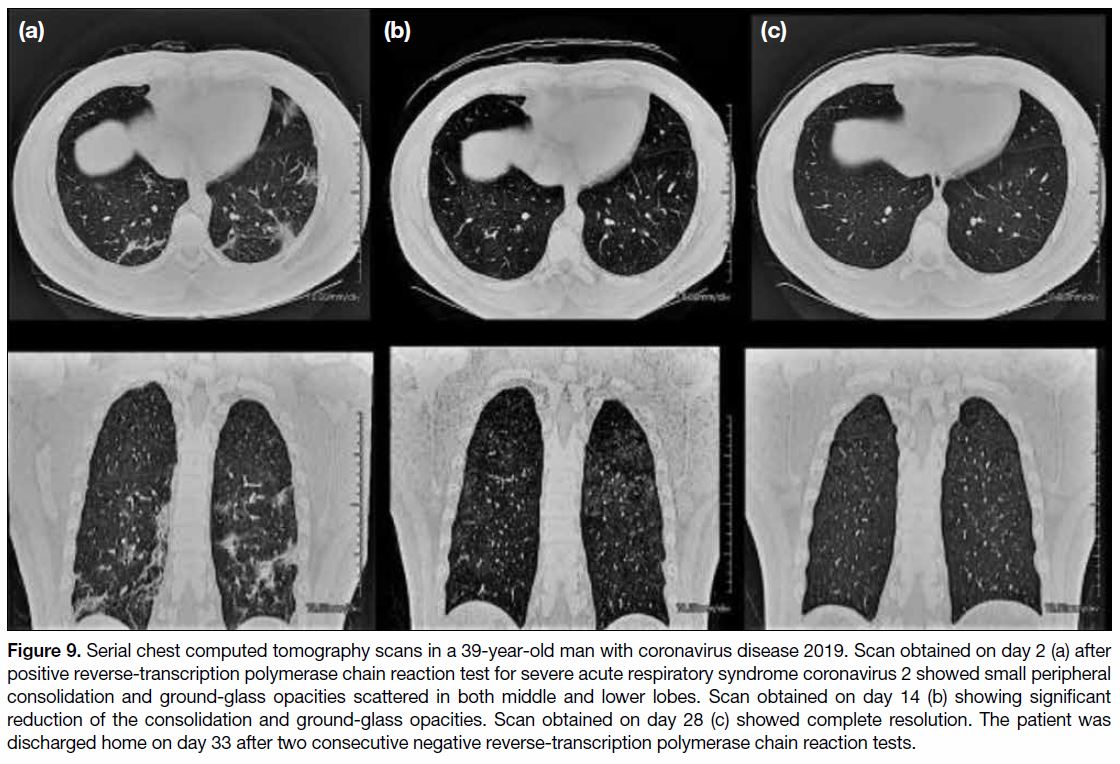
Figure 10. A 42-year-old patient with severe acute respiratory syndrome. (a) Plain chest X-ray taken on presentation, showing unilateral focal peripheral air space opacity at right lower zone. (b) Plain chest X-ray 5 days after presentation showing progression into bilateral multifocal air space opacities.
As with all viral pneumonias, the possible radiological
patterns of parenchymal lung changes of these
coronavirus infections are very similar. These include
GGOs, consolidation, or a mixture of both. On CT
imaging, interlobular septal thickenings and intralobular
lines within the parenchymal changes are also well-described
features, with possible progression into a
crazy-paving pattern.[27] [29]
It is also important to consider the important
negative radiological findings of these infections.
Cavitation, centrilobular nodules, mediastinal or hilar
lymphadenopathy, and pleural effusion are not typical
features in COVID-19 and SARS.[29] In patients with
MERS, pleural effusion was common (33%) and is
reported to be associated with poorer prognosis.[27]
In long-term follow-up imaging for SARS patient with
pneumonia, areas of air trapping due to damaged ciliated
respiratory epithelium and lung fibrosis have been
reported.[30] Likewise, fibrosis is a relatively common
(33%) feature among survivors of MERS.[27] As it has only
been a few months since COVID-19 was first reported,
the long-term lung changes remain to be investigated
with longer follow-up.
POTENTIAL ROLE OF ARTIFICIAL
INTELLIGENCE IN COVID-19 RESPONSE
Recently, Li et al[31] reported a deep-learning algorithm
which can detect COVID-19 on chest CT images and
differentiate it from community-acquired pneumonia.
Covering up to 4000 multicentred chest CT
examinations, this algorithm generated promising per-examination
sensitivity and specificity of 90% and 96%,
respectively, with an average test time of 4.5 s.
Artificial intelligence algorithms have assisted in early warning, diagnosing, triaging patients, and monitoring
treatment response. For example, RADLogics allows
quantification of opacities and provides a scoring system
for patients with COVID-19 that correlates with disease
severity.[32] The Dutch University of Delft triages patients
with COVID-19 based on CXR findings.[33] For artificial
intelligence to be an effective tool in responding to the
COVID-19 pandemic, high-quality large population-based
datasets and worldwide concerted efforts are
required for further development.
ADAPTATION OF RADIOLOGY
DEPARTMENTS DURING COVID-19 PANDEMIC
The first confirmed case of SARS-CoV-2 infection in
Hong Kong occurred on 23 January 2020 and the Hospital
Authority raised the response level in public hospitals
from “Serious” to “Emergency” on 25 January 2020.[34]
The first 20 patients with confirmed SARS-CoV-2
infection in Hong Kong were treated in an Infectious
Disease Centre (IDC). Subsequent patients were treated
in various acute public hospitals in the territory.
Modification and adaptation of the radiology department
is essential in response to the COVID-19 pandemics.[35] [36]
High-level infection control measures were implemented
in radiology departments throughout Hong Kong. All
patients with suspected and confirmed SARS-CoV-2
infection were treated in isolation wards in the IDC.
Imaging facilities were provided in the IDC, including
general radiography, CT, ultrasonography, and portable
C-arm radiography. The examination rooms were
equipped with negative pressure to prevent the spread of
infection. Most radiological examinations and procedures
could be performed in the IDC without the need to
transport the patient to main radiology department, thus
reducing the risk of cross-infection. Portable radiographs
were taken in the isolation wards with designated gown
up and gown down areas for radiographers. The chest CT
scans of patients with COVID-19 were grouped together
in specific sessions with allowance of air exchange time
(15 minutes for IDC CT with air change rate of 12 ACH; 30 minutes for main CT with air change rate of 6 ACH)
and room decontamination time between each patient.
Infection control measures were also implemented in
the main radiology departments, including temperature
screening of outpatients at hospital or department
entrances and mask wearing for all patients, visitors,
and staff. The radiological appointments for patients
with COVID-19 awaiting RT-PCR results were deferred
until the results were available. For patients with
confirmed SARS-CoV-2 infection requiring radiological
examinations for various clinical indications, CT was the
preferred modality, rather than ultrasonography, owing
to the shorter examination time and lower degree of
physical contact between staff and patient.
Radiology service prioritisation, re-organisation, and
manpower re-deployment were initiated to enhance
the care of patients with COVID-19 in terms of
imaging diagnosis, assessment of disease severity, and
monitoring of progress and complication, as well as to
maintain other essential diagnostic and interventional
radiology services. This was achieved by rescheduling
outpatient elective appointments and deploying more
manpower to perform inpatient and urgent reporting and
interventional procedures to speed up patient discharge.
Examinations with higher risks such as barium enema
and modified barium swallow were suspended or
reduced. Face-to-face clinical radiological meetings
and educational meetings were suspended and replaced
by video conferencing, email, or telephone discussion.
Some radiology departments also segregated radiologists
and radiographers into "clean" and "dirty" teams (“dirty”
being the colloquial term for “high risk for potential
contamination or exposure to COVID-19”) to limit
cross-infection.
Staff training and engagement in infection control was
another key measure. Personal infection prevention
measures such as social distancing and vigilant
handwashing were advocated. There was close
liaison between the radiology department and the
infection control team to ensure the adequate supply
and distribution of personal protective equipment,
enhancing infection control and staff morale. Timely
communication with staff and other stakeholders
including hospital management and infection control
team was mediated through emails and social media
apps to provide knowledge sharing and updates on
information and policies.[37] [38]
CONCLUSION
Radiological investigations play an important role in
the diagnosis and management of COVID-19. The main
imaging features on CXR include consolidation and
GGOs, more often found in a bilateral, peripheral, and
lower zone distribution. Compared with CXR, chest CT
is more sensitive and provides superior delineation of the
pulmonary involvement. The typical imaging findings
of COVID-19 on chest CT are bilateral peripheral
distribution of GGOs with or without consolidation.
Interlobular septal thickening and intralobular lines
creating a “crazy-paving” pattern may also be seen. In
contrast, pleural effusion, lymphadenopathy and lung
nodules are considered atypical. As it has only been a few
months since the first case of COVID-19 was reported,
further follow-up would be necessary to delineate the
long-term radiological outcome in recovered patients
with COVID-19.
Radiology departments of the public health sector have
made adaptations and enhanced infection control. As
more experience is accumulated, we are confident that
radiology departments will continue to function well and
assist the whole medical profession to respond to the
COVID-19 pandemic.
REFERENCES
1. Wang L, Wang Y, Ye D, Liu Q. Review of the 2019 Novel
Coronavirus (SARS-CoV-2) based on current evidence. Int J
Antimicrob Agents. 2020 Mar 19. Epub ahead of print. Crossref
2. World Health Organization. Coronavirus disease 2019 (COVID-19)
situation report–51. Available from: https://www.who.int/docs/default-source/coronaviruse/situation-reports/2.... Accessed 11 Mar 2020.
3. World Health Organization. Coronavirus disease 2019 (COVID-19)
situation report–90. Available from: https://www.who.int/docs/default-source/coronaviruse/situation-reports/2.... Accessed 19 Apr 2020.
4. Rubin GD, Ryerson CJ, Haramati LB, Sverzellati N, Kanne JP,
Raoof S, et al. The role of chest imaging in patient management
during the COVID-19 pandemic: A multinational consensus
statement from the Fleischner Society. Chest. 2020 Apr 7. Epub
ahead of print. Crossref
5. Yoon SH, Lee KH, Kim JY, Lee YK, Ko H, Kim KH, et al. Chest
radiographic and CT findings of the 2019 Novel Coronavirus
Disease (COVID-19): analysis of nine patients treated in Korea.
Korean J Radiol. 2020;21:494-500. Crossref
6. Ng MY, Lee EY, Yang J, Yang F, Li X, Wang H, et al. Imaging
profile of the COVID-19 infection: radiologic findings and literature
review. Radiol Cardiothorac Imaging. 2020 Feb 13. Epub ahead of
print. Crossref
7. Wong HY, Lam HY, Fong AH, Leung ST, Chin TW, Lo CS, et
al. Frequency and distribution of chest radiographic findings in
COVID-19 positive patients. Radiology. 2020 Mar 27. Epub ahead
of print. Crossref
8. Arentz M, Yim E, Klaff L, Lokhandwala S, Riedo FX,
Chong M, et al. Characteristics and outcomes of 21 critically
ill patients with COVID-19 in Washington State. JAMA.
2020;323:1612-4. Crossref
9. Bandirali M, Sconfienza LM, Serra R, Brembilla R, Albano D,
Pregliasco FE, et al. Chest radiograph findings in asymptomatic
and minimally symptomatic quarantined patients in Codogno, Italy
during COVID-19 pandemic. Radiology. 2020;295:E7. Crossref
10. Kim ES, Chin BS, Kang CK, Kim NJ, Kang YM, Choi JP, et al.
Clinical course and outcomes of patients with severe acute
respiratory syndrome coronavirus 2 infection: a preliminary report
of the first 28 patients from the Korean cohort study on COVID-19.
J Korean Med Sci. 2020;35:e142. Crossref
11. Salehi S, Abedi A, Balakrishnan S, Gholamrezanezhad A.
Coronavirus disease 2019 (COVID-19): a systematic review of
imaging findings in 919 patients. AJR Am J Roentgenol. 2020 Mar
14. Epub ahead of print. Crossref
12. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical
course and risk factors for mortality of adult inpatients with
COVID-19 in Wuhan, China: a retrospective cohort study. Lancet.
2020;395:1054-62. Crossref
13. Kim D, Quinn J, Pinsky B. Shah NH, Brown I. Rates of co-infection
between SARS-CoV-2 and other respiratory pathogens. JAMA.
2020 Apr 15. Epub ahead of print. Crossref
14. Fang Y, Zhang H, Xie J, Lin M, Ying L, Pang P, et al. Sensitivity
of chest CT for COVID-19: comparison to RT-PCR. Radiology.
2020 Feb 19. Epub ahead of print. Crossref
15. Ai T, Yang Z, How H, Zhan C, Chen C, Lv W, et al. Correlation
of chest CT and RT-PCR testing in coronavirus disease 2019
(COVID-19) in China: a report of 1014 cases. Radiology. 2020
Feb 26. Epub ahead of print. Crossref
16. Inui S, Fujikawa A, Jitsu M, Kunishima N, Watanabe S, Suzuki Y, et al.
Chest CT findings in cases from the cruise ship “Diamond Princess”
with coronavirus disease 2019 (COVID-19). Radiol Cardiothorac
Imaging. 2020 Mar 17. Epub ahead of print. Crossref
17. Wen Z, Chi Y, Zhang L, Liu H, Du K, Li Z, et al. Coronavirus
disease 2019: initial detection on chest CT in a retrospective
multicenter study of 103 Chinese subjects. Radiol Cardiothorac
Imaging. 2020 Apr 6. Epub ahead of print. Crossref
18. Xu X, Yu C, Qu J, Zhang L, Jiang S, Huang D, et al. Imaging and clinical
features of patients with 2019 novel coronavirus SARS-CoV-2.
Eur J Nucl Med Mol Imaging.2020;47:1275-80. Crossref
19. Bernheim A, Mei X, Huang M, Yang Y, Fayad ZA, Zhang N, et al.
Chest CT findings in coronavirus disease-19 (COVID-19):
relationship to duration of infection. Radiology. 2020;295:200463. Crossref
20. Simpson S, Kay FU, Abbara S, Bhalla S, Chung JH, Chung M, et al.
Radiological Society of North America Expert Consensus
Statement on reporting chest CT findings related to COVID-19.
endorsed by the Society of Thoracic Radiology, the American
College of Radiology, and RSNA. Radiol Cardiothorac Imaging.
2020 Mar 25. Epub ahead of print. Crossref
21. Ye Z, Zhang Y, Wang Y, Huang Z, Song B, et al. Chest CT
manifestations of new coronavirus disease 2019 (COVID-19): a
pictorial review. Eur Radiol. 2020 Mar 19. Epub ahead of print. Crossref
22. Pan F, Ye T, Sun P, Gui S, Liang B, Li L, et al. Time course of
lung changes on chest CT during recovery from coronavirus disease
2019 (COVID-19). Radiology. 2020;295:715-21. Crossref
23. Li K, Wu J, Wu F, Guo D, Chen L, Fang Z, et al. The clinical and
chest CT features associated with severe and critical COVID-19 pneumonia. Invest Radiol. 2020;55:327-31. Crossref
24. Pan Y, Guan H, Zhou S, Wang Y, Li Q, Zhu T, et al. Initial
CT findings and temporal changes in patients with the novel
coronavirus pneumonia (2019-nCoV): a study of 63 patients in
Wuhan, China. Eur Radiol. 2020 Feb 13. Epub ahead of print. Crossref
25. Wang Y, Dong C, Hu Y, Li C, Ren Q, Zhang X, et al. Temporal
changes of CT findings in 90 patients with COVID-19 pneumonia:
a longitudinal study. Radiology. 2020 Mar 19. Epub ahead of
print. Crossref
26. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical
characteristics of coronavirus disease 2019 in China. N Engl J Med.
2020;382:1708-20. Crossref
27. Das KM, Lee EY, Langer RD, Larsson SG. Middle East Respiratory
Syndrome coronavirus: what does a radiologist need to know? AJR
Am J Roentgenol. 2016;206:1193-201. Crossref
28. Hosseiny M, Kooraki S, Gholamrezanezhad A, Reddy S, Myers L.
Radiology perspective of coronavirus disease 2019 (COVID-19):
lessons from severe acute respiratory syndrome and Middle East
respiratory syndrome. AJR Am J Roentgenol. 2020;214:1078-
82. Crossref
29. Antonio GE, Wong KT, Hui DS, Lee N, Yuen EH, Wu A, et al.
Imaging of severe acute respiratory syndrome in Hong Kong. AJR
Am J Roentgenol. 2003;181:11-7. Crossref
30. Chang YC, Yu CJ, Chang SC, Galvin JR, Liu HM, Hsiao CH, et al.
Pulmonary sequelae in convalescent patients after severe acute
respiratory syndrome: evaluation with thin-section CT. Radiology.
2005;236:1067-75. Crossref
31. Li L, Qin L, Xu Z, Yin Y, Wang X, Kong B, et al. Artificial
intelligence distinguishes COVID-19 from community acquired
pneumonia on chest CT. Radiology. 2020 Mar 19. Epub ahead of
print. Crossref
32. Gozes O, Frid-Adar M, Greenspan H, Browning PD, Zhang H,
Ji W, et al. Rapid AI development cycle for the coronavirus
(COVID-19) pandemic: Initial results for automated detection &
patient monitoring using deep learning CT image analysis. arXiv.
[Preprint] 2003. Available from: https://arxiv.org/abs/2003.05037.
Accessed 30 Mar 2020.
33. Delft Imaging. Triage for COVID-19 using artificial intelligence on
chest X-rays. Available from: https://www.delft.care/cad4covid/.
Accessed 30 Mar 2020.
34. Hospital Authority, Hong Kong SAR Government. Hospital
Authority activates Emergency Response Level [press release].
2020 Jan 25. Available from: https://www8.ha.org.hk/qmh/index_doc/eng.pdf. Accessed 25 Jan 2020.
35. Mossa-Basha M, Meltzer CC, Kim DC, Tuite MJ, Kolli KP,
Tan BS. Radiology department preparedness for COVID19:
Radiology Scientific Expert Panel. Radiology. 2020 Mar 16. Epub
ahead of print. Crossref
36. Goh Y, Chua W, Lee JK, Leng Ang BW, Liang CR, Tan CA, et al.
Operational strategies to prevent COVID-19 spread in radiology:
experience from a Singapore radiology department after severe
acute respiratory syndrome. J Am Coll Radiol. 2020 Apr 3. Epub
ahead of print. Crossref
37. Huang Z, Zhao S, Li Z. Chen W, Zhao L, Deng L, et al. The
battle against coronavirus disease 2019 (COVID-19): emergency
management and infection control in a radiology department. J Am
Coll Radiol. 2020 Mar 24. Epub ahead of print. Crossref
38. Lai TS, Yu WC. The lessons of SARS in Hong Kong. Clin Med (Lond). 2010;10:50-3. Crossref