Comparison of Risk Stratification Systems for Predicting Clinical Outcomes in Patients with Endometrial Carcinoma
ORIGINAL ARTICLE CME
Comparison of Risk Stratification Systems for Predicting Clinical Outcomes in Patients with Endometrial Carcinoma
CYY Yip1, H Pang2, LLK Chan1, PY Wu1, ATY Chang3,4, SI Soong1
1 Department of Clinical Oncology, Pamela Youde Nethersole Eastern Hospital, Chai Wan, Hong Kong
2 School of Public Health, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Pokfulam, Hong Kong
3 Department of Clinical Oncology, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Pokfulam, Hong Kong
4 The University of Hong Kong–Shenzhen Hospital, Shenzhen, China
Correspondence: Dr CYY Yip, Department of Clinical Oncology, Pamela Youde Nethersole Eastern Hospital, Chai Wan, Hong Kong. Email: chloeyip@gmail.com
Submitted: 13 Aug 2018; Accepted: 12 Nov 2018.
Contributors: CYYY and SIS designed the study; CYYY, PYW and ATYC acquired the data; CYYY, HP, LLKC and SIS analysed the data;
CYYY drafted the manuscript. CYYY and SIS critically revised the manuscript for important intellectual content. All authors had full access to
the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: The authors have no conflicts of interest to disclose.
Funding/Support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics Approval: The research protocol was approved by the Hong Kong East Cluster Research Ethics Committee (Ref HKECREC-2018-013).
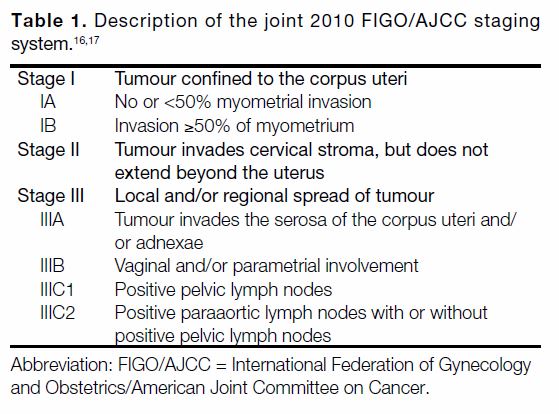
Abstract
Objectives
We sought to compare three risk stratification systems (RSSs) in terms of ability in predicting recurrence
and survival in endometrial cancer: the joint 2010 International Federation of Gynecology and Obstetrics/American Joint Committee on Cancer (FIGO/AJCC) staging system, the 2013 European Society for Medical Oncology (ESMO) classification system, and the 2016 European Society for Medical Oncology, European Society of Gynaecological Oncology and European Society for Radiotherapy & Oncology (ESMO-ESGO-ESTRO) classification system.
Methods
Data of patients with FIGO stage I to III endometrial carcinoma requiring adjuvant oncological treatment from 1 January 2005 to 31 December 2014 in a single institution in Hong Kong were retrospectively reviewed. The three systems were evaluated in terms of accuracy of predicting recurrence, cancer-specific survival, and overall survival using Harrell’s concordance index (C-index).
Results
Data from 128 patients were analysed. Recurrences occurred in 22 (17%) and cancer-related deaths occurred in 18 (14%). The joint 2010 FIGO/AJCC staging system had the highest C-index of 0.75 (95% confidence interval [CI] = 0.65-0.86) for recurrence and 0.76 for overall survival (95% CI = 0.65-0.88). In terms of predicting cancer-specific survival, the ESMO-ESGO-ESTRO subgroup classification had the highest C-index of 0.80 (95% CI = 0.58-1.00).
Conclusion
We demonstrated the discriminative abilities of the joint 2010 FIGO/AJCC staging system, the ESMO classification, and the ESMO-ESGO-ESTRO classification in predicting disease-free survival, cancer-specific survival, and overall survival using Harrell’s C-index. The ESMO-ESGO-ESTRO classification has potential in guiding clinical decision making and patients’ risk assignment in studies. Integration of molecular classification may represent the way forward in classifying endometrial carcinoma and instituting personalised treatment algorithms.
Key Words: Endometrial neoplasms; Recurrence; Risk; Survival
中文摘要
比較不同風險分級系統預測子宮內膜癌臨床結果的能力
葉欣怡、彭希文、陳麗君、吳宇光、張天怡、宋崧
目的
對比2010年FIGO/AJCC(國際婦產科聯盟/美國癌症聯合委員會)分期系統、2013年ESMO(歐洲醫學腫瘤學會)分類系統及2016年ESMO-ESGO-ESTRO(歐洲醫學腫瘤學會-歐洲婦科腫瘤學會-歐洲放射腫瘤學會)分類系統的三種不同風險分級系統針對子宮內膜癌復發和存活率的預測能力。
方法
回顧性研究2005年1月至2014年12月期間於香港單一中心接受輔助性抗癌治療的FIGO分期I-III子宮內膜癌患者的臨床資料。利用Harrell的一致性指數(Harrell’s C-index)評估上述三種風險分級系統預測預測復發率、腫瘤相關存活率和整體存活率的能力。
結果
128名患者納入分析,當中22名患者(17%)復發,而18名患者(14%)因子宮內膜癌死亡。2010年FIGO/AJCC分期系統針對預測復發和整體存活率得出的C-index分別是 0.75(95%置信區間0.65-0.86)和 0.76(95%置信區間0.65-0.88),均為三種風險分級系統中最高。ESMO-ESGO-ESTRO亞組分類系統針對預測腫瘤相關存活率則為最高,即C-index為0.80(95%置信區間0.58-1.00)。
結論
利用Harrell一致性指數,證實2010年FIGO/AJCC分期系統、ESMO分類系統及ESMO-ESGO-ESTRO分類系統均有能力預測無病存活率、腫瘤相關存活率和整體存活率。ESMO-ESGO-ESTRO分類系統具有指導臨床決策和在研究中對患者風險區分的潛力。結合分子分型是對子宮內膜癌進行分類並建立個性化治療的重要未來發展趨勢。
BACKGROUND
Endometrial cancer is the most common gynaecological
malignancy in developed countries.[1] In Hong Kong, it represented the fourth most common cancer and was
ranked eleventh in causes of mortality in females in 2015,
with a median age of 55 at diagnosis. The proportions of
patients found to have stage I, II, III, and IV disease were
64.4%, 8.2%, 10.1%, and 7.1%, respectively, while the
remaining 10.2% were unstaged.[2] [3]
Although the majority of endometrial cancers are
diagnosed at an early stage (I and II), the stage does not
always accurately predict the prognosis, with 5-year
survivals ranging from 75% to 90%.[4] The prognosis is
governed by stage, histological subtype, grade, depth
of myometrial invasion, and lymphovascular space
invasion (LVSI).[5] [6] [7] [8] In clinical practice, these factors, in
addition to the results of recent studies of external beam
radiotherapy and brachytherapy, including GOG-99,[9]
PORTEC-1,[10] ASTEC/EN.5,[11] PORTEC-2,[12] as well as
chemotherapy (GOG-122,[13] pooled analysis of NSGO-EC-9501/EORTC-55991 and MaNGO ILIADE-III[14]) and chemoradiotherapy (PORTEC-3[15]), strongly influence
the choice of single or combined adjuvant therapies.
Against this background, several risk stratification
systems (RSSs) have been proposed, with the aim of
guiding adjuvant treatment, formulating a prognosis,
and determining treatment appropriateness and efficacy
in clinical studies. Currently, three widely used RSSs are
the joint 2010 International Federation of Gynecology
and Obstetrics/American Joint Committee on Cancer
(FIGO/AJCC) staging system,[16] [17] the European Society
for Medical Oncology (ESMO) classification,[18] and the
joint European Society for Medical Oncology, European
Society of Gynaecological Oncology and European
Society for Radiotherapy & Oncology (ESMO-ESGO-ESTRO)
classification.[19]
However, there is a lack of literature that examines
the external validity or performance of the RSSs. The
objective of this study was to evaluate the discriminative
ability of the aforementioned three RSSs in predicting
recurrence and survival.
METHODS
Study Population
Data from patients with FIGO stage I to III endometrial
carcinoma requiring postoperative adjuvant oncological
treatment from 1 January 2005 to 31 December 2014 in
the Department of Clinical Oncology of Pamela Youde
Nethersole Eastern Hospital, Hong Kong were included
in this retrospective study. Cases with macroscopic
residual disease were excluded. Clinical data were
collected retrospectively from medical records. Clinical
and pathological characteristics, including tumour and
nodal stage, grade, histological subtype, presence or
absence of myometrial invasion, LVSI, treatment details,
and clinical outcome were recorded.
Treatment and Follow-up
Preoperative workups consisted of magnetic resonance
imaging of the pelvis and/or computed tomography
(CT) of the abdomen and pelvis, chest X-ray and
CT of the thorax or whole-body positron emission
tomography–computed tomography. Patients with
endometrioid (type 1) adenocarcinoma underwent
total abdominal hysterectomy with bilateral salpingo-oophorectomy
(TAHBSO) with or without pelvic ± paraaortic lymphadenectomy at the operating surgeons’
discretion, while patients with non-endometrioid (type 2)
carcinoma, i.e., serous and clear-cell carcinoma,
underwent TAHBSO as well as lymphadenectomy,
omentectomy, and peritoneal biopsies.
Proposed adjuvant treatment varied depending on stage
of disease, tumour pathology, age, performance status,
and the time of treatment commencement. Stage IB
cases with at least one of the following three risk factors:
grade 3, age >60 years or LVSI, and those with stage II
and III disease were generally treated with whole pelvic
irradiation (WPI) and intravaginal brachytherapy.
With the advent of PORTEC-2,[12] from October
2010, patients were administered brachytherapy
alone (in contrast to previous treatment with WPI and
brachytherapy combined) if they had the following
characteristics: stage IA grade 2 with either age >60 years
or LVSI, stage IA grade 3, and stage IB without the
aforementioned three risk factors.
Since September 2010, adjuvant chemotherapy was
administered to patients with non-endometrioid
carcinomas of all stages and stage III endometrioid
carcinomas post-radiotherapy, based on the pooled
analysis of NSGO-EC-9501/EORTC-55991 and MaNGO ILIADE-III as mentioned above.[14]
Patients were followed up every 3 to 4 months during
the first 2 years, every 6 months for the third to fifth year,
and annually thereafter.
Disease-free survival (DFS), cancer-specific survival
(CSS), and overall survival (OS) were evaluated. DFS
was defined as date of diagnosis to date of relapse or
death related to endometrial cancer. CSS was defined as
date of diagnosis to date of death related to endometrial
cancer. OS was defined as date of diagnosis to date of
death from any cause.
Risk Stratification Systems Description
The RSSs evaluated in this study included the joint 2010 FIGO/AJCC staging system, the ESMO classification,
and the ESMO-ESGO-ESTRO classification. They were
selected in view of their recognised clinical applicability.
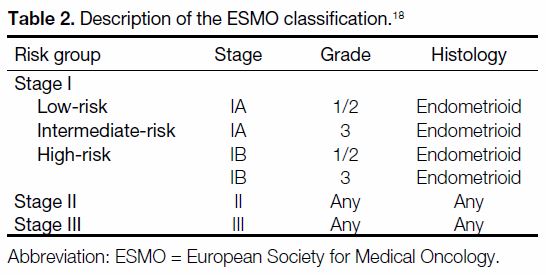
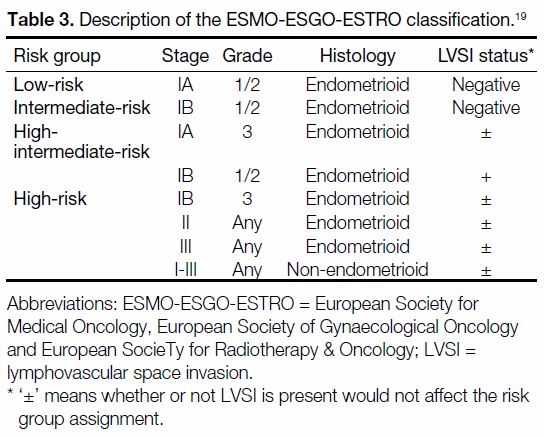
Tables 1 2 3[16] [17] [18] [19] describe the categories in each RSS.
The performance of the three RSSs, when classified
into large risk groups (shown in bold), was evaluated
and compared when such risk groups could be further
divided into subgroups.
Table 2. Description of the ESMO classification.[18]
Table 3. Description of the ESMO-ESGO-ESTRO classification.[19]
Statistical Analysis
DFS, CSS, and OS were analysed for each RSS by
generating Kaplan-Meier plots (Figure) with log-rank
significance testing. To assess the discriminative ability
of the models, the Harrell’s C-index was calculated
using the method proposed by Uno et al.[20] It is
interpreted as the probability that a randomly selected
subject who experienced the outcome will have a higher
predicted probability of having the outcome occur
compared to a randomly selected subject who did not
experience the event. A C-index of 1 indicates that the
model can perfectly distinguish between individuals
with discordant events, and a C-index of 0.5 means
no discriminative ability.[21] Statistical analysis was
performed using SPSS (Windows version 22.0; IBM
Corp, Armonk [NY], United States) and R (Version
3.3.3; https://www.r-project.org/).
Figure. (a) Disease-free survival, (b) cancer-specific survival, and (c) overall survival curves according to the FIGO/AJCC, ESMO, and
ESMO-ESGO-ESTRO risk stratification systems.
RESULTS
Study Population
Data from a total of 128 cases were included in the
analysis. Median follow-up time was 83.5 months
(range, 5.5-143.5 months).
In all, 10.9% (n = 14), 67.2% (n = 86) and 13.3% (n = 17)
of the cases underwent preoperative pelvic magnetic
resonance imaging, CT of the abdomen and pelvis, and a
combination of both modalities, respectively, while the
remaining 8.6% (n = 11) underwent positron emission
tomography–computed tomography.
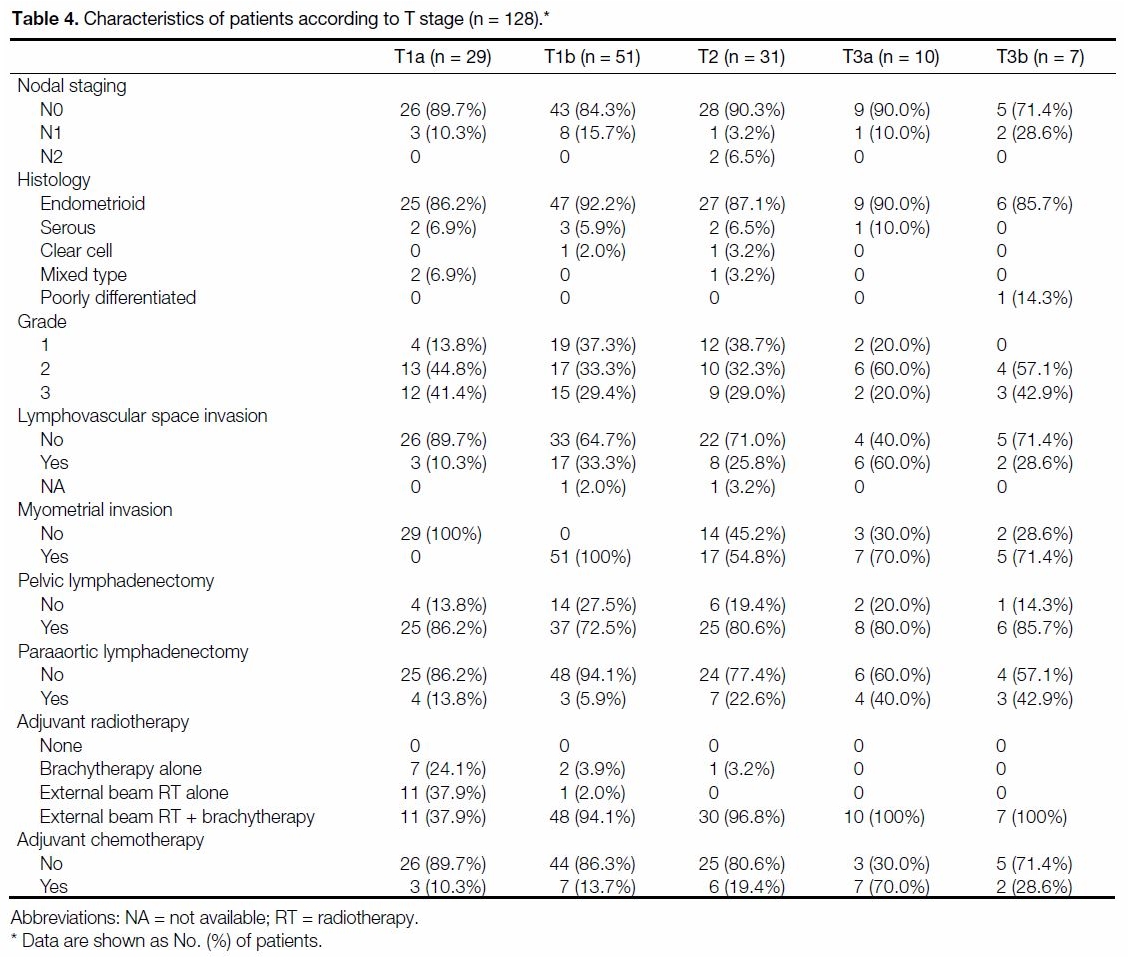
Table 4 shows the distribution of the clinicopathological
characteristics of the study cohort. A total of 114 cases
(89.1%) had endometrioid histology. In all, 100%, 78.9% and 16.4% of the cases underwent TAHBSO, pelvic
lymphadenectomy and paraaortic lymphadenectomy,
respectively. Overall, 11.7% (n = 15) had positive pelvic
nodes, whereas only 1.6% (n = 2) had paraaortic nodal
disease.
Table 4. Characteristics of patients according to T stage (n = 128).
Ten cases received adjuvant brachytherapy alone and
12 received WPI alone. A total of 106 cases received
adjuvant radiotherapy in the form of a combination of
both modalities. A total of 25 cases received adjuvant
chemoradiotherapy.
Survival Analysis
Cancer recurred in 17.2% (n = 22), with first sites of
recurrence being local, regional and distant in 9.1%
(2/22), 4.5% (1/22) and 86.4% (19/22), respectively.
Overall DFS was 82.5% at 5 years and 79.5% at 10 years.
In total, there were 14.1% (n = 18) deaths that were
cancer-related. CSS was 88.6% at 5 years and 81.4% at 10 years.
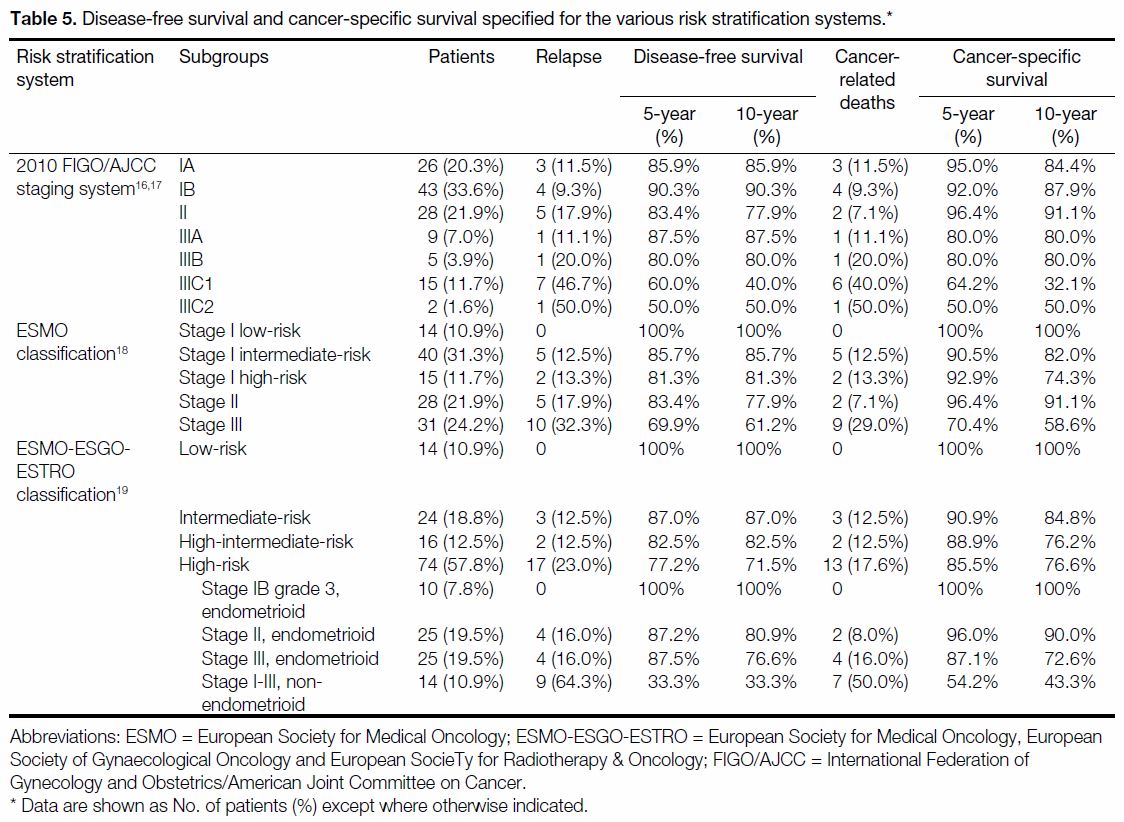
Table 5 and the Figure show the respective DFS and CSS
rates and Kaplan-Meier plots according to each RSS.
Table 5. Disease-free survival and cancer-specific survival specified for the various risk stratification systems.
Performance of Risk Stratification Systems
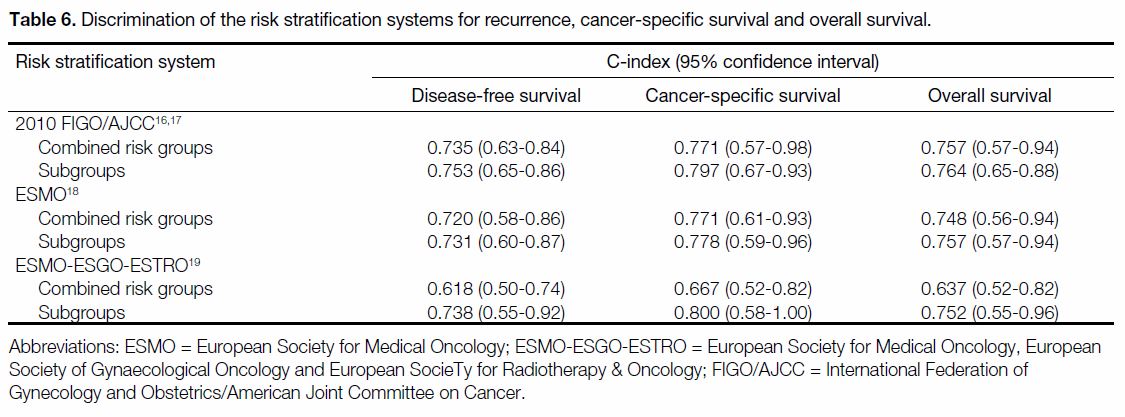
A comparison of the Harrell’s C-index of the RSSs is
shown in Table 6. The joint 2010 FIGO/AJCC staging
system had the highest C-index of 0.75 (95% confidence
interval [CI] = 0.65-0.86) for recurrence and 0.76
for OS (95% CI = 0.65-0.88). In terms of CSS, the
ESMO-ESGO-ESTRO subgroup classification had the
highest C-index of 0.80 (95% CI = 0.58-1.00).
Table 6. Discrimination of the risk stratification systems for recurrence, cancer-specific survival and overall survival.
DISCUSSION
The classification of endometrial carcinoma has evolved
over time. It has been staged surgico-pathologically
since 1988 according to the FIGO staging system.
The revised version in 2009 that resulted from better
understanding of tumour biology is currently the most
widely adopted RSS, and has been externally validated
to improve prediction of prognosis compared to the
earlier version.[22] Being practical and reproducible, it
allows accurate information exchange among centres.
However, its performance is limited by the fact that it
does not distinguish patients with non-endometrioid
(type II) cancers as a separate subgroup, whose outcome
was shown to be inferior, with increased risk of
recurrence and distant metastases[23]; moreover, the role of
grade and LVSI as independent predictors of recurrence
is disregarded.
Stemming from the perceived inadequacy of FIGO, other
risk factors for recurrence were used in the PORTEC and
GOG studies to define subgroups of patients that would
derive the greatest benefit from adjuvant radiotherapy.
Thereafter, ESMO classification and several other RSSs
incorporating different combinations of key prognostic
parameters were developed, with the goal of dividing early-stage cases into low-, intermediate-, and highrisk
groups to improve prognosis prediction and guide
treatment. In December 2014, a new classification
system was introduced by the multidisciplinary
ESMO-ESGO-ESTRO consensus panel,[19] in which a
group of high-intermediate-risk patients was defined
and the prognostic importance of grade 3 and LVSI was recognised. Additionally, the panel provided evidence-based
recommendations on adjuvant treatment strategies
tailored for each risk subgroup.
The best definition of risk groups has always been
evolving based on the latest evidence. Consequently, the
factors entering into the decision to administer adjuvant
therapies remain fluid.
Bendifallah et al[24] compared the ESMO risk
classification with PORTEC-1, GOG-99, SEPAL, and
the ESMO-modified RSS and reported limited diagnostic
accuracy in all five RSSs in stratifying patients with
regard to the risk of recurrence and nodal involvement
in early-stage endometrial cancers. A recent analysis
by the FRANCOGYN study group according to the
ESMO-ESGO-ESTRO classification showed promise in its ability to reflect outcome, demonstrating a higher
incidence of locoregional failure in patients with highand
high-intermediate-risk endometrial cancers, while
patients at high risk experienced more distant recurrences
compared with other risk groups.[25] To our knowledge, this
is the first study to evaluate the ESMO-ESGO-ESTRO
risk classification system compared with other RSSs in
terms of discriminative ability.
Our data revalidated the high prognostic value and
applicability of the joint 2010 FIGO/AJCC staging system,
which had a C-index of 0.75 for prediction of recurrence.
For each classification system, the discriminative power
in terms of C-index was higher when they were further
divided into subgroups. In terms of prediction of cancer-specific
survival, the ESMO-ESGO-ESTRO subgroup
classification had the highest C-index of 0.80.
Although classification based on FIGO staging remains
robust and widely used, it is insufficient to rely solely
upon it to determine the optimal adjuvant treatment in
clinical practice. FIGO staging cannot guide adjuvant
therapy precisely with the current treatment algorithms.
Patients of the same FIGO stage may have indications for different adjuvant therapies in clinical practice depending
upon different prognostic factors that are disregarded,
e.g. LVSI, tumour grade, and histological subtypes.
Compared with FIGO, the ESMO-ESGO-ESTRO
classification is more exacting by taking into account
the aforementioned prognostic factors in the risk grouping. Thus, the same stage I patients in FIGO can
be stratified into the four risk groups (low, intermediate,
high-intermediate, high) by LVSI, tumour grade and
histologic subtypes. The choice of adjuvant therapy is
suggested for each risk group by the level of evidence.
Risk stratification for endometrial carcinoma is
continually evolving to help clinicians to assign the
appropriate treatments to different risk groups. At
present, there are still unresolved questions on the
choice of adjuvant treatment. For instance, the benefit
of combining radiotherapy and chemotherapy in high-risk
groups is controversial.[14] [19] In addition to providing
prognostic value and guiding treatment, a good risk
stratification system also allows the identification
of patients for studies in developing new treatment
strategies to improve the outcomes of high-risk cases
while minimising overtreatment of low-risk groups.
The strength of this study is that the present cohort had
a relatively high proportion of cases that underwent
pelvic lymphadenectomy (78.9%), compared with other
large studies evaluating FIGO staging.[22] [26] However,
there are some limitations, including the relatively small
sample size and a low number of events, as well as
the potential selection bias inherent in its retrospective
design. Moreover, during the relatively long study
period, there had been modifications in surgical and
adjuvant treatment. Cases that did not receive adjuvant
oncological treatment were excluded from the study.
This was due to concern about inaccurate assessment of
long-term outcome resulting from early loss to follow-up
of this group of cases, as they might not have been
referred to our unit postoperatively, or in cases where
they were referred to us, they would receive subsequent
follow-up at their mother units only. However, this also
means that our results might not have been the same if all
cases of endometrial cancer had been included.
Moving forward, a major obstacle in improving care for
patients with endometrial carcinomas is the presence of
interobserver variation among pathologists in evaluating
key pathological variables that current RSSs heavily
rely on,[27] [28] implying risk group misassignment thus
over- and under-treatment. This inspired the search for
complementary tools such as immunohistochemical
markers (p53, oestrogen receptors, etc.) and mutational
profiles for more precise risk quantification. Data from
The Cancer Genome Atlas studies support classification
of endometrial carcinomas into four prognostically
distinct subgroups based on genomic architecture, but this genomic approach is not in routine clinical use due
to cost, logistics and lack of applicability to biopsies and
curettings, implying that patients can only be stratified
after surgical staging.[29] Talhouk et al[30] presented a
clinically practical method for molecular classification
of endometrial cancers using formalin-fixed paraffin-embedded
samples, which could replicate the Cancer
Genome Atlasʼ genomic-based classification without
the need for labour-intensive and cost-prohibitive
genomic methodology; additionally, it can be applied
to biopsy specimens, enabling earlier planning of
the optimal course of treatment and consideration
of fertility-sparing options in selected cases. When
integrated with clinicopathological factors or risk group
classifications, the molecular classifier provided the
highest level of discrimination of survival outcomes.
Another future direction for personalised medicine is
the development of risk scoring models. For example,
AlHilli et al[31] [32] created nomograms for individualised
prediction of lymphatic dissemination and OS. Further
clinical validation is certainly needed to determine the
best way of incorporating these molecular classifiers and
risk scoring models into clinical care and evaluating their
impact on outcome.
CONCLUSION
We demonstrated the discriminative abilities of the
joint 2010 FIGO/AJCC staging system, the ESMO
classification, and the ESMO-ESGO-ESTRO
classification in predicting DFS, CSS, and OS using
Harrell’s C-index. The ESMO-ESGO-ESTRO
classification has potential in guiding clinical decision
making and patients’ risk assignment in studies.
Integration of molecular classification may represent the
way forward in classifying endometrial carcinoma and
instituting personalised treatment algorithms.
REFERENCES
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A.
Global cancer statistics 2018: GLOBOCAN estimates of incidence
and mortality worldwide for 36 cancers in 185 countries. CA Cancer
J Clin. 2018;68:394-424. Crossref
2. The Hong Kong Cancer Registry, Hospital Authority, Hong Kong
SAR Government. Overview of Hong Kong Cancer statistics
of 2015. Available from: http://www3.ha.org.hk/cancereg/pdf/overview/Summary%20of%20CanStat%20201.... Accessed
28 Mar 2018.
3. The Hong Kong Cancer Registry, Hospital Authority, Hong Kong
SAR Government. Corpus cancer in 2015. Available from: http://www3.ha.org.hk/cancereg/pdf/factsheet/2015/corpus_2015.pdf.
Accessed 28 Mar 2018.
4. Creasman WT, Odicino F, Maisonneuve P, Quinn MA, Beller U,
Benedet JL, et al. Carcinoma of the corpus uteri. FIGO 26th Annual
Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006;95(Suppl 1):S105-43. Crossref
5. Creasman WT, Morrow CP, Bundy BN, Homesley HD, Graham JE,
Heller PB. Surgical pathologic spread patterns of endometrial
cancer. A Gynecologic Oncology Group Study. Cancer. 1987;60(8
Suppl):2035-41. Crossref
6. Gadducci A, Cosio S, Fabrini MG, Fanucchi A, Barsotti C,
Cristofani R, et al. Patterns of failure in endometrial cancer:
clinicopathological variables predictive of the risk of local, distant
and retroperitoneal failure. Anticancer Res. 2011;31:3483-8.
7. Guntupalli SR, Zighelboim I, Kizer NT, Zhang Q, Powell MA,
Thaker PH, et al. Lymphovascular space invasion is an independent
risk factor for nodal disease and poor outcomes in endometrioid
endometrial cancer. Gynecol Oncol. 2012;124:31-5. Crossref
8. Morrow CP, Bundy BN, Kurman RJ, Creasman WT, Heller P,
Homesley HD, et al. Relationship between surgical-pathological
risk factors and outcome in clinical stage I and II carcinoma of the
endometrium: A Gynecologic Oncology Group Study. Gynecol
Oncol. 1991;40:55-65. Crossref
9. Keys HM, Roberts JA, Brunetto VL, Zaino RJ, Spirtos NM,
Bloss JD, et al. A phase III trial of surgery with or without
adjunctive external pelvic radiation therapy in intermediate risk
endometrial adenocarcinoma: a Gynecologic Oncology Group
study. Gynecol Oncol. 2004;92:744-51. Crossref
10. Creutzberg CL, van Putten WL, Koper PC, Lybeert ML, Jobsen JJ,
Wárlám-Rodenhuis CC, et al. Surgery and postoperative
radiotherapy versus surgery alone for patients with stage-1
endometrial carcinoma: multicentre randomised trial. PORTEC
Study Group. Post Operative Radiation Therapy in Endometrial
Carcinoma. Lancet. 2000;355:1404-11. Crossref
11. ASTEC/EN.5 Study Group, Blake P, Swart AM, Orton J, Kitchener H,
Whelan T, et al. Adjuvant external beam radiotherapy in the
treatment of endometrial cancer (MRC ASTEC and NCIC CTG
EN.5 randomised trials): pooled trial results, systematic review,
and meta-analysis. Lancet. 2009;373:137-46. Crossref
12. Nout RA, Smit VT, Putter H, Jürgenliemk-Schulz IM, Jobsen JJ,
Lutgens LC, et al. Vaginal brachytherapy versus pelvic external
beam radiotherapy for patients with endometrial cancer of high-intermediate
risk (PORTEC-2): an open-label, non-inferiority,
randomised trial. Lancet. 2010;375:816-23. Crossref
13. Randall ME, Filiaci VL, Muss H, Spirtos NM, Mannel RS, Fowloer J,
et al. Randomized phase III trial of whole-abdominal irradiation
versus doxorubicin and cisplatin chemotherapy in advanced
endometrial carcinoma: a Gynecologic Oncology Group Study. J
Clin Oncol. 2006;24:36-44. Crossref
14. Hogberg T, Signorelli M, de Oliveira CF, Fossati R, Lissoni AA,
Sorbe B, et al. Sequential adjuvant chemotherapy and radiotherapy
in endometrial cancer — results from two randomised studies. Eur
J Cancer. 2010;46:2422-31. Crossref
15. de Boer SM, Powell ME, Mileshkin L, Katsaros D, Bessette P,
Haie-Meder C, et al. Adjuvant chemoradiotherapy versus
radiotherapy alone for women with high-risk endometrial cancer
(PORTEC-3): final results of an international, open-label,
multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19:295-309. Crossref
16. Creasman W. Revised FIGO staging carcinoma of the endometrium.
Int J Gynaecol Obstet. 2009;105:109. Crossref
17. American Joint Committee on Cancer. Corpus uteri. In: AJCC
Staging Manual. 7th ed. New York (NY): Springer; 2010: p 403. Crossref
18. Colombo N, Preti E, Landoni F, Carinelli S, Colombo A, Marini C,
et al. Endometrial cancer: ESMO Clinical Practice Guidelines for
diagnosis, treatment and follow-up. Ann Oncol. 2013;24 Suppl 6:vi33-8. Crossref
19. Colombo N, Creutzberg C, Amant F, Bosse T, González-Martín A,
Ledermann J, et al. ESMO-ESGO-ESTRO consensus conference
on endometrial cancer: diagnosis, treatment and follow-up. Int J
Gynecol Cancer. 2016;26:2-30. Crossref
20. Uno H, Cai T, Pencina MJ, D’Agostino RB, Wei LJ. On the
C-statistics for evaluating overall adequacy of risk prediction
procedures with censored survival data. Stat Med. 2011;30:1105-17. Crossref
21. Austin PC, Steyerberg EW. Interpreting the concordance statistic of
a logistic regression model: relation to the variance and odds ratio
of a continuous explanatory variable. BMC Med Res Methodol.
2012;12:82. Crossref
22. Werner HM, Trovik J, Marcickiewicz J, Tingulstad S, Staff AC,
Amant F, et al. Revision of FIGO surgical staging in 2009 for
endometrial cancer validates to improve risk stratification. Gynecol
Oncol. 2012;125:103-8. Crossref
23. Cirisano Jr FD, Robboy SJ, Dodge RK, Bentley RC, Krigman HR,
Synan IS, et al. The outcome of stage I-II clinically and surgically
staged papillary serous and clear cell endometrial cancers
when compared with endometrioid carcinoma. Gynecol Oncol.
2000;77:55-65. Crossref
24. Bendifallah S, Canlorbe G, Collinet P, Arsène E, Huguet F, Coutant C,
et al. Just how accurate are the major risk stratification systems
for early-stage endometrial cancer? Br J Cancer. 2015;112:793-801. Crossref
25. Bendifallah S, Ouldamer L, Lavoue V, Canlorbe G, Raimond E,
Coutant C, et al. Patterns of recurrence and outcomes in surgically
treated women with endometrial cancer according to ESMO-ESGO-
ESTRO Consensus Conference risk groups: results from the
FRANCOGYN Study Group. Gynecol Oncol. 2017;144:107-12. Crossref
26. Page BR, Pappas L, Cooke EW, Gaffney DK. Does the FIGO
2009 endometrial cancer staging system more accurately correlate
with clinical outcome in different histologies? Revised staging,
endometrial cancer, histology. Int J Gynecol Cancer. 2012;22:593-8. Crossref
27. Han G, Sidhu D, Duggan MA, Arseneau J, Cesari M, Clement PB,
et al. Reproducibility of histological cell type in high-grade
endometrial carcinoma. Mod Pathol. 2013;26:1594-604. Crossref
28. Guan H, Semaan A, Bandyopadhyay S, Arabi H, Feng J, Fathallah L,
et al. Prognosis and reproducibility of new and existing binary
grading systems for endometrial carcinoma compared to FIGO
grading in hysterectomy specimens. Int J Gynecol Cancer.
2011;21:654-60. Crossref
29. Goebel EA, Vidal A, Matias-Guiu X, Blake Gilks C. The evolution
of endometrial carcinoma classification through application of
immunohistochemistry and molecular diagnostics: past, present
and future. Virchows Arch. 2018;472:885-96. Crossref
30. Talhouk A, McConechy MK, Leung S, Li-Chang HH, Kwon JS,
Melnyk N, et al. A clinically applicable molecular-based
classification for endometrial cancers. Br J Cancer. 2015;113:299-310. Crossref
31. AlHilli MM, Mariani A, Bakkum-Gamez JN, Dowdy SC,
Weaver AL, Peethambaram PP, et al. Risk-scoring models for
individualized prediction of overall survival in low-grade and
high-grade endometrial cancer. Gynecol Oncol. 2014;133:485-93. Crossref
32. AlHilli MM, Podratz KC, Dowdy SC, Bakkum-Gamez JN,
Weaver AL, McGree ME, et al. Risk-scoring system for
the individualized prediction of lymphatic dissemination in
patients with endometrioid endometrial cancer. Gynecol Oncol.
2013;131:103-8. Crossref