Complications after Surgical Correction of Anorectal Malformations
CATELOG
Complications after Surgical Correction of Anorectal Malformations
T Hosokawa1, Y Yamada2, Y Tanami1, Y Sato1, Y Tanaka3, H Kawashima4, E Oguma1
1 Department of Radiology, Saitama Children’s Medical Center, Saitama, Japan
2 Department of Radiology, Keio University School of Medicine, Tokyo, Japan
3 Department of Pediatric Surgery, Nagoya University Graduate School of Medicine, Saitama, Japan
4 Department of Surgery, Saitama Children’s Medical Center, Saitama, Japan
Correspondence: Dr Takahiro Hosokawa, Department of Radiology, Saitama Children’s Medical Center, Saitama, Japan. Email: snowglobe@infoseek.jp
Submitted: 5 Nov 2018; Accepted: 3 Dec 2018.
Contributors: TH, YY and YTanami contributed to the design of the study. YTanami, YS and YTanaka acquired the data. TH, YY, YTanami,
YS and EO performed analysis or interpretation of data. TH and YY wrote the article. HK and EO carried out critical revision for important
intellectual content. All authors had full access to the data, contributed to the study, approved the final version for publication, and take
responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethics Approval: This study is in accordance with the tenets of the Declaration of Helsinki and was approved by the ethics committee of our
institution. Informed consent was waived.
Abstract
Radiologists are often unfamiliar with anorectal malformations and have limited knowledge of the surgical procedures
for their repair. In this article, we provide a comprehensible description of the surgical procedures for radiologists,
review previous literature, and summarise the incidence of the complications. Moreover, we detail major postoperative
complications consequent to the use of various imaging techniques, including anorectal prolapse, anal stenosis,
urethral injury, posterior urethral diverticulum, neurogenic bladder, adhesion of reconstructed vagina, leakage
from suture lines, and trocar site hernia. Knowledge of these complications and surgical procedures is important to
radiologists for diagnosis and determination of a treatment strategy.
Key Words: Anorectal malformations; Anus, imperforate
中文摘要
肛門直腸畸形矯正術後的併發症
T Hosokawa、Y Yamada、Y Tanami、Y Sato、Y Tanaka、H Kawashima、E Oguma
放射科醫師通常不熟悉肛門直腸畸形,並且對其修復的手術程序認識有限。本文為放射科醫生提供
全面的手術方法說明、回顧文獻並總結併發症的發生率。此外,我們詳細介紹由於使用各種成像技
術顯示主要術後併發症,包括肛門直腸脫垂、肛門狹窄、尿道損傷、後尿道憩室、神經源性膀胱、
重建陰道粘連、縫合線滲漏以及套管針疝。這些併發症和手術程序的知識對於放射科醫生診斷和確
定治療策略很重要。
INTRODUCTION
Congenital anorectal malformations (ARMs), also
known as imperforate anus, affect approximately 1 in
5000 newborns.[1] These ARMs are classified as low,
intermediate, or high types,1 with treatment based on
this classification.[2] Although a variety of treatments
are available for imperforate anus, almost all cases of
low-type imperforate anus are managed with a one-step
anoplasty immediately after birth.[2] [3] In contrast, although
primary anorectal repair without a diverting enterostomy
is performed in some patients with intermediate- or
high-type imperforate anus,[3] [4] [5] almost all patients with
these types are treated first with a diverting colostomy,
then anorectoplasty.[3] [4] [5] Patients with ARMs are treated
with anorectoplasty for complete repair of the ARMs,
regardless of type. There are several other approaches
similar to anorectoplasty for complete surgical repair of
ARM.[4] [6] [7] [8] Currently, many surgical procedures, such as
perineal anorectoplasty, sacroperineal anorectoplasty,
abdominosacroperineal anorectoplasty, posterior
sagittal anorectoplasty (PSARP),[6] anterior sagittal
anorectoplasty (ASARP),[8] and laparoscopically assisted
anorectoplasty (LAARP)[4] are performed for complete
surgical repair of ARM. Despite advances in surgical
procedures, there are possibilities of postoperative
complications.
Reports on postoperative complications of surgical
repair of ARMs have documented the involvement
of pelvic organs (such as anus, rectum, urethra, and
vagina) as well as cutaneous structures.[9] [10] [11] [12] [13] [14] [15] [16] [17] [18] [19] [20] [21] [22] [23] [24] [25] [26] [27] [28] Various
imaging techniques, such as plain radiography,
colonography, voiding cystourethrography,
ultrasonography, computed tomography, and
magnetic resonance imaging (MRI) can be used for
diagnosis.[9] [11] [16] [18] [19] [21] [29] [30] Unlike surgeons, radiologists
are often unfamiliar with ARMs and have little
knowledge about the surgical procedures for their
repair; to date, only one review article related to
radiography has been published.[30]
The aim of this article was to familiarise radiologists
with common complications of specific surgical
approaches and ARM types, which would be useful in
diagnosis and in assisting surgeons with the management
of these complications. In this article, we provide a
comprehensible description of the surgical procedures for
radiologists, review previous literature, and summarise
the incidence of complications. Moreover, we describe
and discuss eight major postoperative complications
specific to ARM, including anorectal prolapse, anal stenosis, urethral injury, posterior urethral diverticulum,
adhesion of reconstructed vagina, leakage from suture
lines, neurogenic bladder, and trocar site hernia.
SURGICAL PROCEDURES
Several surgical procedures are performed to repair
ARMs. Innovative approaches such as PSARP by Peña
and Devries[6] and LAARP by Georgeson et al[4] have been
reported. The anterior or posterior perineal approach is
selected according to fistula location and ARM type.
The anterior perineal approach is usually selected in low-type
or anovestibular ARM, and the posterior perineal
approach is usually selected for intermediate-type ARM
(Figure 1).
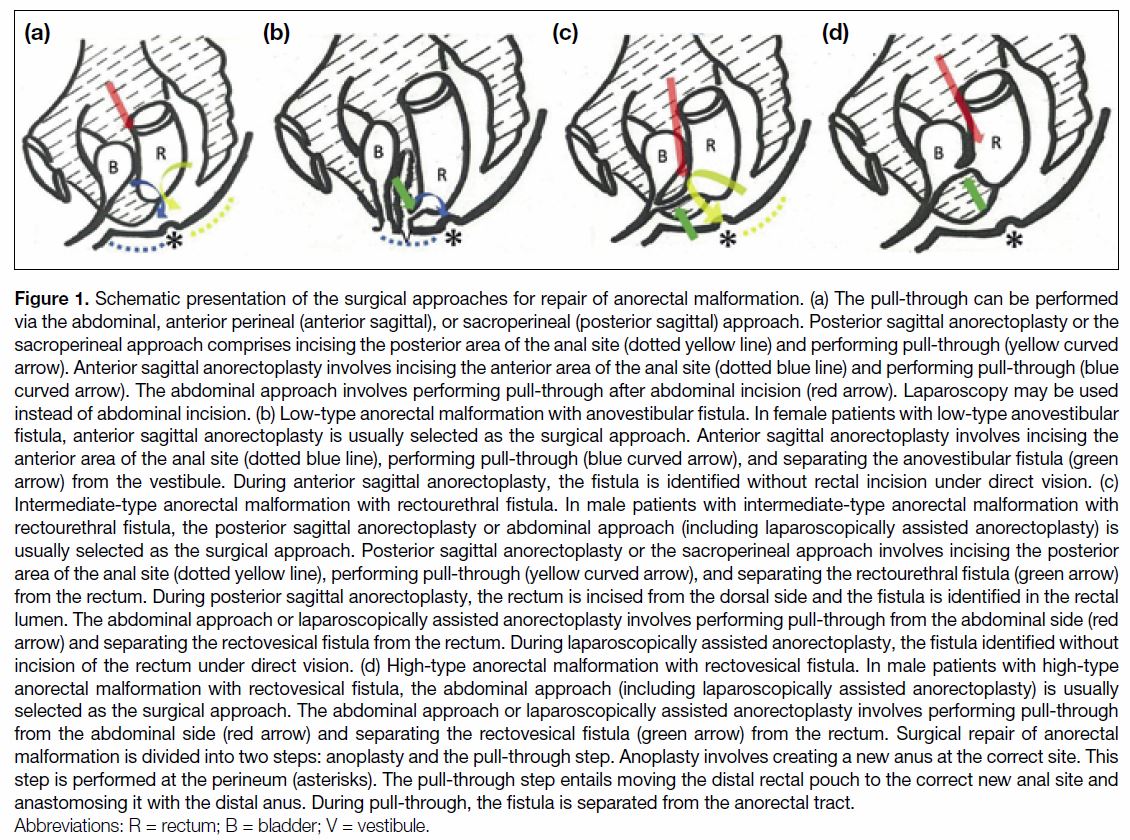
Figure 1. Schematic presentation of the surgical approaches for repair of anorectal malformation. (a) The pull-through can be performed
via the abdominal, anterior perineal (anterior sagittal), or sacroperineal (posterior sagittal) approach. Posterior sagittal anorectoplasty or the
sacroperineal approach comprises incising the posterior area of the anal site (dotted yellow line) and performing pull-through (yellow curved
arrow). Anterior sagittal anorectoplasty involves incising the anterior area of the anal site (dotted blue line) and performing pull-through (blue
curved arrow). The abdominal approach involves performing pull-through after abdominal incision (red arrow). Laparoscopy may be used
instead of abdominal incision. (b) Low-type anorectal malformation with anovestibular fistula. In female patients with low-type anovestibular
fistula, anterior sagittal anorectoplasty is usually selected as the surgical approach. Anterior sagittal anorectoplasty involves incising the
anterior area of the anal site (dotted blue line), performing pull-through (blue curved arrow), and separating the anovestibular fistula (green
arrow) from the vestibule. During anterior sagittal anorectoplasty, the fistula is identified without rectal incision under direct vision. (c)
Intermediate-type anorectal malformation with rectourethral fistula. In male patients with intermediate-type anorectal malformation with
rectourethral fistula, the posterior sagittal anorectoplasty or abdominal approach (including laparoscopically assisted anorectoplasty) is
usually selected as the surgical approach. Posterior sagittal anorectoplasty or the sacroperineal approach involves incising the posterior
area of the anal site (dotted yellow line), performing pull-through (yellow curved arrow), and separating the rectourethral fistula (green arrow)
from the rectum. During posterior sagittal anorectoplasty, the rectum is incised from the dorsal side and the fistula is identified in the rectal
lumen. The abdominal approach or laparoscopically assisted anorectoplasty involves performing pull-through from the abdominal side (red
arrow) and separating the rectovesical fistula from the rectum. During laparoscopically assisted anorectoplasty, the fistula identified without
incision of the rectum under direct vision. (d) High-type anorectal malformation with rectovesical fistula. In male patients with high-type
anorectal malformation with rectovesical fistula, the abdominal approach (including laparoscopically assisted anorectoplasty) is usually
selected as the surgical approach. The abdominal approach or laparoscopically assisted anorectoplasty involves performing pull-through
from the abdominal side (red arrow) and separating the rectovesical fistula (green arrow) from the rectum. Surgical repair of anorectal
malformation is divided into two steps: anoplasty and the pull-through step. Anoplasty involves creating a new anus at the correct site. This
step is performed at the perineum (asterisks). The pull-through step entails moving the distal rectal pouch to the correct new anal site and
anastomosing it with the distal anus. During pull-through, the fistula is separated from the anorectal tract.
INCIDENCE AND DESCRIPTION OF
COMPLICATIONS
There are several approaches for surgical repair of ARMs,
and there are numerous reports on related complications.
We reviewed previous reports on complications after
surgery for ARM by the abdominal pull-through
approach (Table 1 [17] [27]), PSARP (Table 2 [9] [12] [13] [15] [25] [26] [27] [31] [32] [33]),
anterior sagittal anorectoplasty (Table 3 [20] [23]), and LAARP
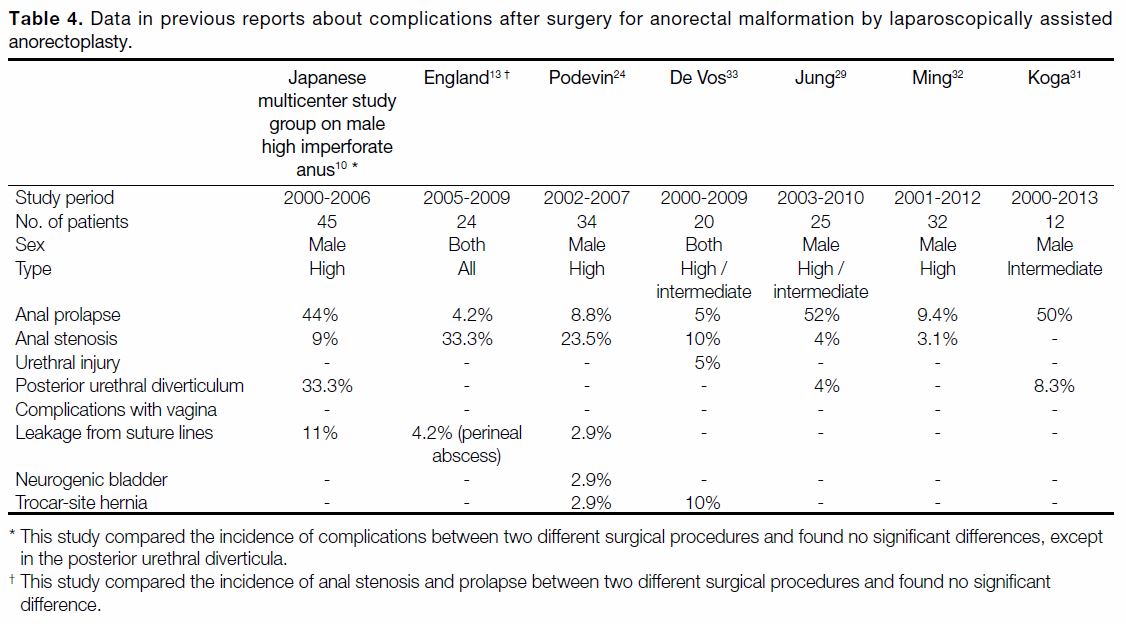
(Table 4 [10] [13] [24] [29] [31] [32] [33]). Previous reports that included
multiple surgical approaches are excluded. The reports
exhibit differences with respect to patient sex and ARM
type. Therefore, the prevalence of each complication
shows variations. Furthermore, while the incidence of
the complications decreases with the improvement in
surgical techniques and skills,[10] [15] [25] some complications
still occur when the techniques are applied by highly
skilled surgeons.
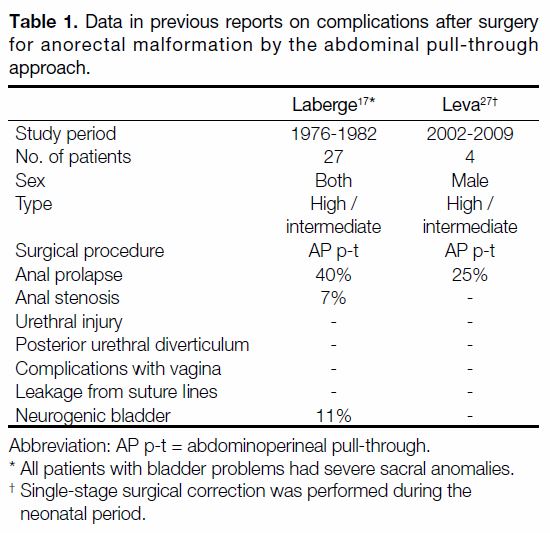
Table 1. Data in previous reports on complications after surgery
for anorectal malformation by the abdominal pull-through
approach.
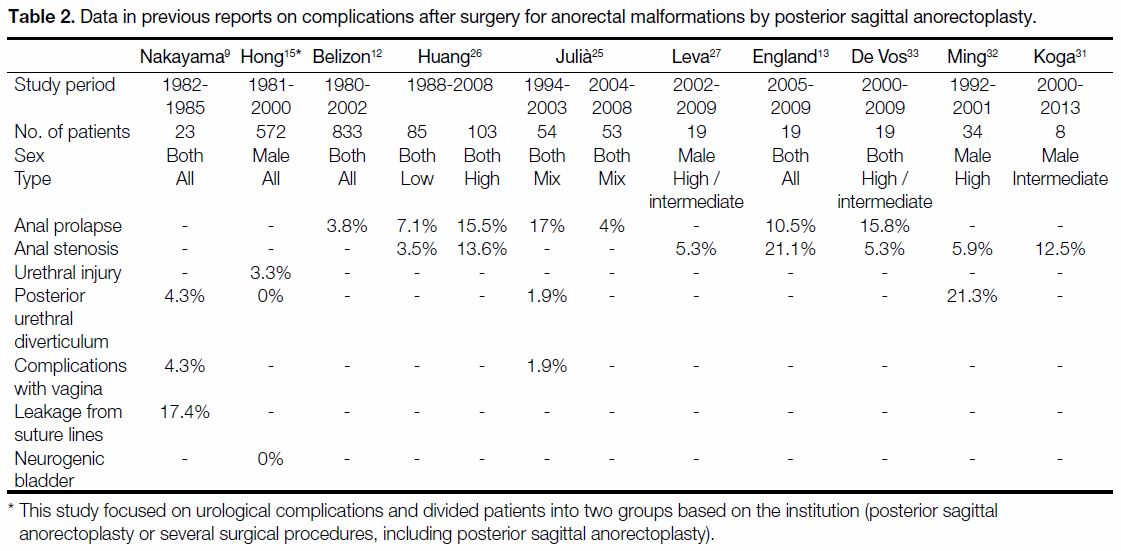
Table 2. Data in previous reports on complications after surgery for anorectal malformations by posterior sagittal anorectoplasty.
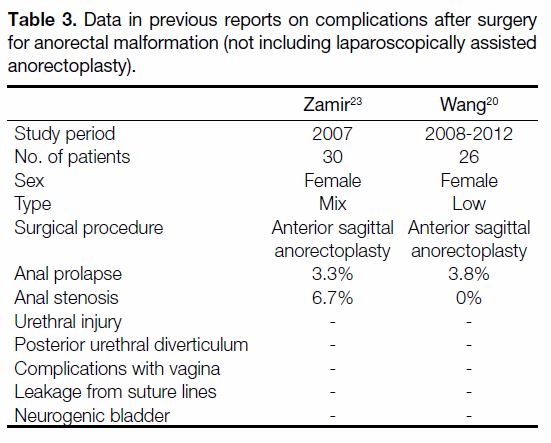
Table 3. Data in previous reports on complications after surgery
for anorectal malformation (not including laparoscopically assisted
anorectoplasty).
Table 4. Data in previous reports about complications after surgery for anorectal malformation by laparoscopically assisted
anorectoplasty.
Anal Prolapse
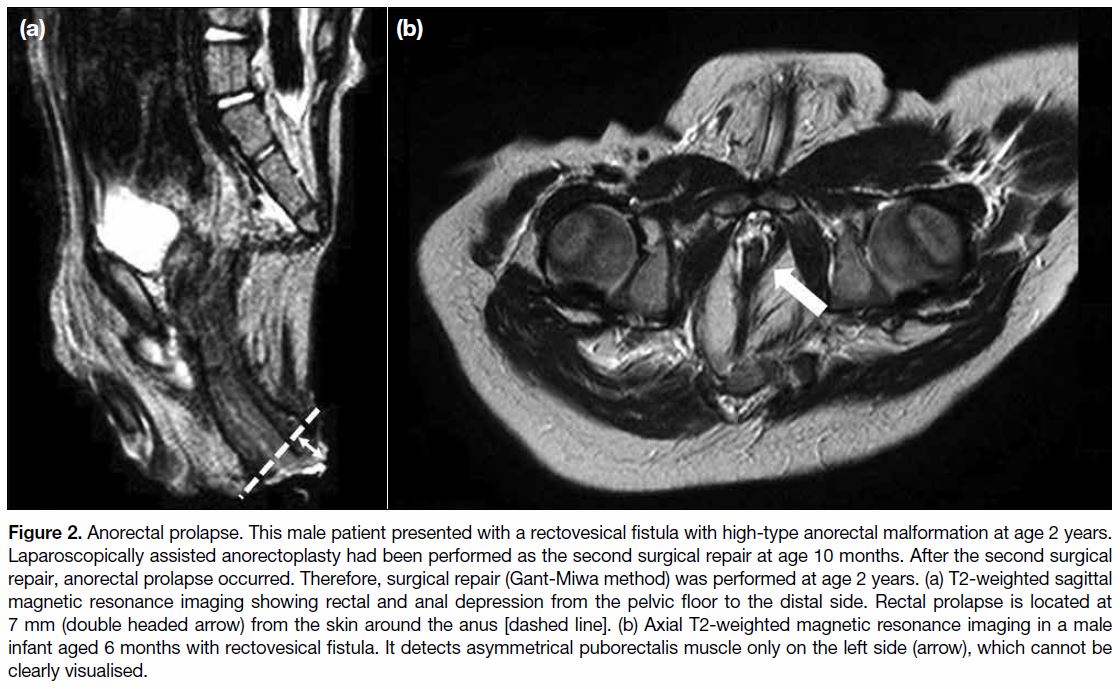
Anorectal prolapse (Figure 2) is defined as anal prolapse
>5 mm.[12] There have been no radiographic reports on
anorectal prolapse, as this complication is clinically
diagnosed. Anal prolapse has a significantly higher
incidence in patients with a low quality of the levator
ani muscle and in those with vertebral anomalies,[12] [22] and
the frequency of this complication is also reported to be
associated with surgical approaches as LAARP.[29] [31] [32] [34]
High-type ARM is characterised by poor muscle
quality, which may render anal prolapse an inevitable
complication, with a higher likelihood of recurrence than in low-type ARM.[26] [35] It may be accidentally detected on
an MRI requested to evaluate the levator ani muscle.[31] [36]
Figure 2. Anorectal prolapse. This male patient presented with a rectovesical fistula with high-type anorectal malformation at age 2 years.
Laparoscopically assisted anorectoplasty had been performed as the second surgical repair at age 10 months. After the second surgical
repair, anorectal prolapse occurred. Therefore, surgical repair (Gant-Miwa method) was performed at age 2 years. (a) T2-weighted sagittal
magnetic resonance imaging showing rectal and anal depression from the pelvic floor to the distal side. Rectal prolapse is located at
7 mm (double headed arrow) from the skin around the anus [dashed line]. (b) Axial T2-weighted magnetic resonance imaging in a male
infant aged 6 months with rectovesical fistula. It detects asymmetrical puborectalis muscle only on the left side (arrow), which cannot be
clearly visualised.
Anal Stenosis
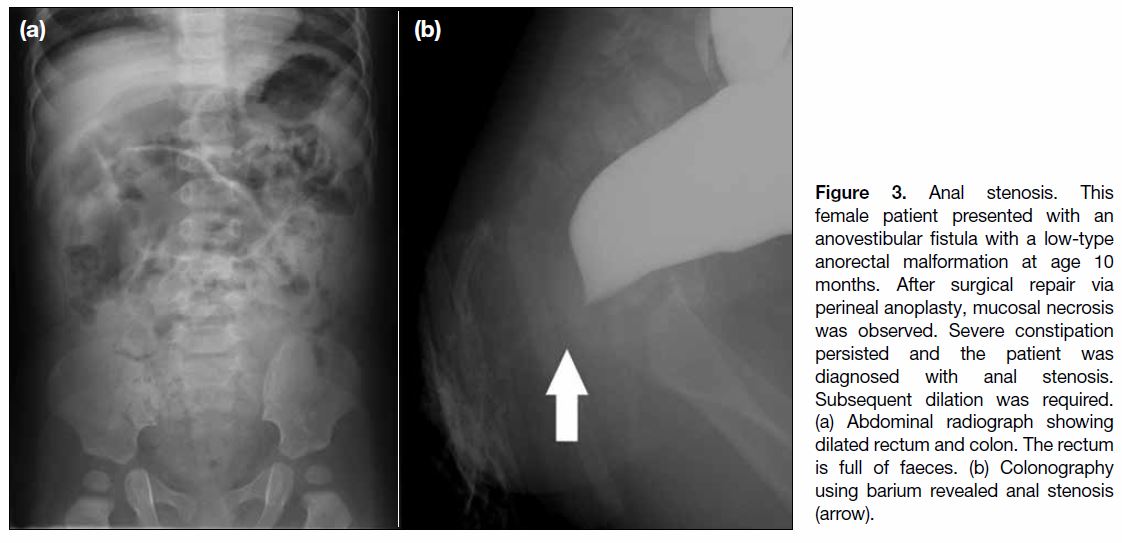
Anal stenosis (Figure 3) may occur with all surgical
procedures and ARM types, and it may be caused by
ischaemia or inadequate dilation of the anus.[7] Ischaemic
necrosis of the pull-through bowel is a technical problem
caused by a reduction in vascular supply to the border
after colon mobilisation.[17] In abdominal radiography
after surgical repair of ARM, constipation rather than
poor levator ani muscle function may be observed, but
anal stenosis must still be considered.[36] [37]
Figure 3. Anal stenosis. This
female patient presented with an
anovestibular fistula with a low-type
anorectal malformation at age 10
months. After surgical repair via
perineal anoplasty, mucosal necrosis
was observed. Severe constipation
persisted and the patient was
diagnosed with anal stenosis.
Subsequent dilation was required.
(a) Abdominal radiograph showing
dilated rectum and colon. The rectum
is full of faeces. (b) Colonography
using barium revealed anal stenosis
(arrow).
Urethral Injury
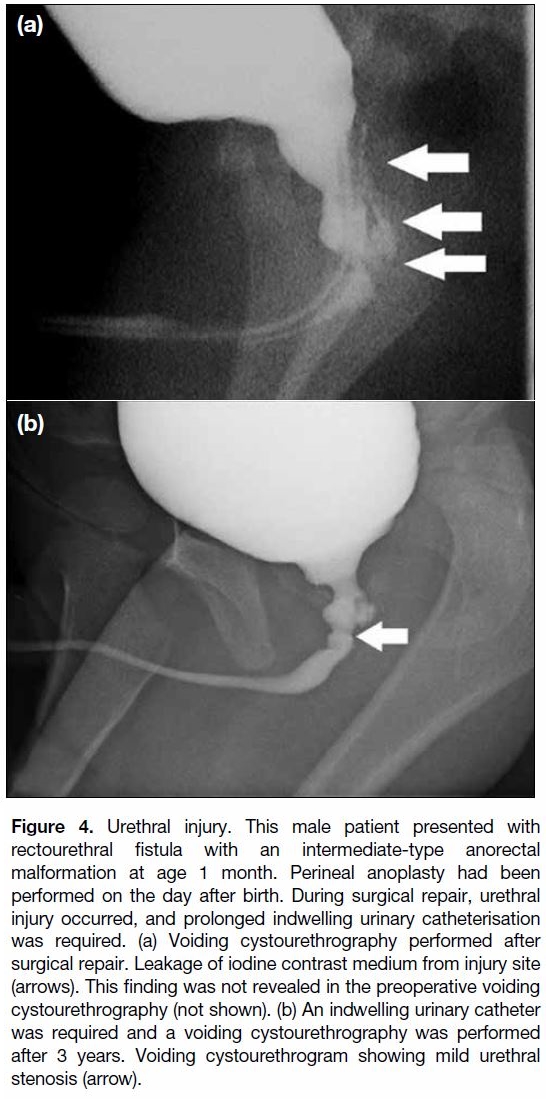
Urethral injury (Figure 4) during surgery has been found
to occur more often in male patients with intermediate- or
high-type ARM.[15] [38] To repair a rectourethral fistula,
separation of the urinary tract from the rectum is required.
Therefore, there is a risk of urethral injury while repairing
such an ARM, which should be avoided by paediatric
surgeons.[16] [19] To prevent injury to the urinary tract, an
augmented-pressure distal colostogram before surgical
repair is recommended.[15] [38]
Figure 4. Urethral injury. This male patient presented with
rectourethral fistula with an intermediate-type anorectal
malformation at age 1 month. Perineal anoplasty had been
performed on the day after birth. During surgical repair, urethral
injury occurred, and prolonged indwelling urinary catheterisation
was required. (a) Voiding cystourethrography performed after
surgical repair. Leakage of iodine contrast medium from injury site
(arrows). This finding was not revealed in the preoperative voiding
cystourethrography (not shown). (b) An indwelling urinary catheter
was required and a voiding cystourethrography was performed
after 3 years. Voiding cystourethrogram showing mild urethral
stenosis (arrow).
Urethral Injury
Urethral injury (Figure 4) during surgery has been found
to occur more often in male patients with intermediateor
high-type ARM.[15] [38] To repair a rectourethral fistula,
separation of the urinary tract from the rectum is required.
Therefore, there is a risk of urethral injury while repairing
such an ARM, which should be avoided by paediatric
surgeons.[16] [19] To prevent injury to the urinary tract, an
augmented-pressure distal colostogram before surgical
repair is recommended.[15] [38]
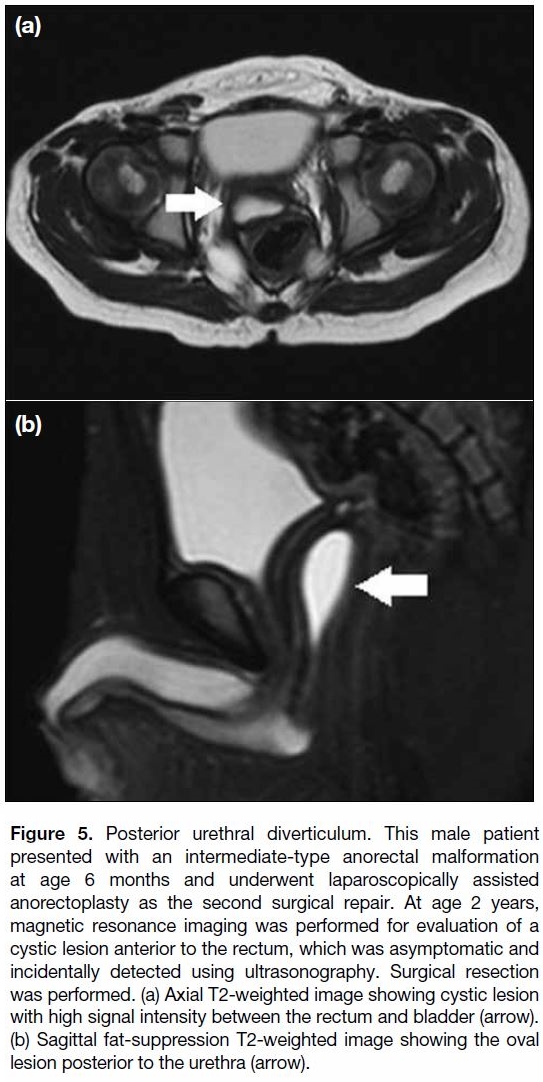
Posterior Urethral Diverticulum
Posterior urethral diverticulum (Figure 5) is more
likely to occur in LAARP than in the other types of
surgery.[10] This is important because it may result in dysuria, formation of urinary stones, infection, and
malignancy.[10] [16] [18] [19] Meanwhile, some patients with
posterior urethral diverticula may not exhibit any
symptoms.[10] [16] [18] [19] Therefore, it may be accidentally
detected on an MRI performed to evaluate the levator ani
muscle.[31] [36] There have been some radiographic reports about posterior urethral diverticulum, and in some cases,
posterior urethral diverticula could not be revealed using
voiding cystourethrography, being detectable only using
MRI.[10] [11] [16] [18] Histopathology of the excised mucosa of
the cyst showed colonic mucosa and confirmed that cyst
was indeed an enlarged residual rectourethral fistula.[16] To prevent posterior urethral diverticula, novel surgical
approaches and enhanced surgical skills are required.[16] [19] [39]
Figure 5. Posterior urethral diverticulum. This male patient
presented with an intermediate-type anorectal malformation
at age 6 months and underwent laparoscopically assisted
anorectoplasty as the second surgical repair. At age 2 years,
magnetic resonance imaging was performed for evaluation of a
cystic lesion anterior to the rectum, which was asymptomatic and
incidentally detected using ultrasonography. Surgical resection
was performed. (a) Axial T2-weighted image showing cystic lesion
with high signal intensity between the rectum and bladder (arrow).
(b) Sagittal fat-suppression T2-weighted image showing the oval
lesion posterior to the urethra (arrow).
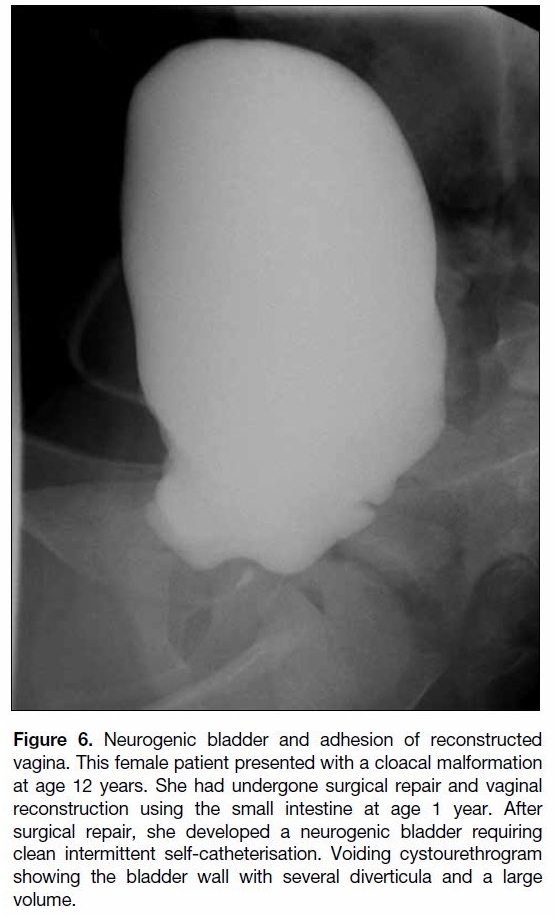
Neurogenic Bladder
Although neurogenic bladder (Figure 6) is a
complication of ARM repair,[15] [21] spinal anomalies
commonly accompany ARMs.[35] Laberge et al[17] reported
that three patients had prolonged poor bladder emptying
and that these patients had severe sacral anomalies.
However, determining whether the cause of neurogenic
bladder is iatrogenic may be difficult. Follow-up
regarding urological complications is important for management of patients with ARM.[40]
Figure 6. Neurogenic bladder and adhesion of reconstructed
vagina. This female patient presented with a cloacal malformation
at age 12 years. She had undergone surgical repair and vaginal
reconstruction using the small intestine at age 1 year. After
surgical repair, she developed a neurogenic bladder requiring
clean intermittent self-catheterisation. Voiding cystourethrogram
showing the bladder wall with several diverticula and a large
volume.
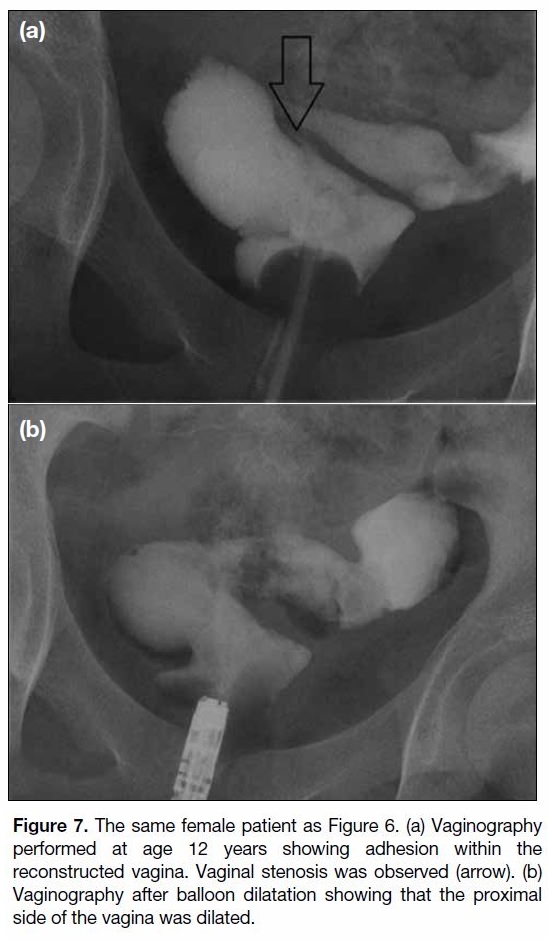
Adhesion of Reconstructed Vagina
Reconstruction of the vagina may be required in girls
with cloacal malformation, which is classified as high-type
ARM. Reconstruction of the vagina using the
intestine has been previously reported,[41] with some
patients requiring dilatation.[41] Partial adhesion (Figure 7)
of the reconstructed vagina or uterus must be diagnosed
early to reduce decline in quality of life of patients
with ARMs.[41] Furthermore, some patients may require
additional surgical repair.[42] [43] [44] [45]
Figure 7. The same female patient as Figure 6. (a) Vaginography
performed at age 12 years showing adhesion within the
reconstructed vagina. Vaginal stenosis was observed (arrow). (b)
Vaginography after balloon dilatation showing that the proximal
side of the vagina was dilated.
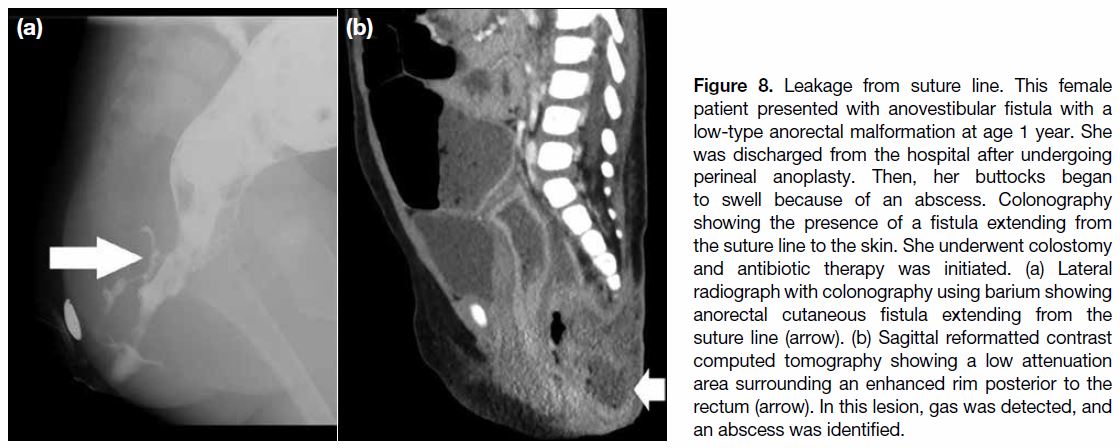
Leakage from Suture Lines
Leakage from suture lines (Figure 8), failed
anastomoses, and perineal abscesses has been
reported.[9] [10] [13] [14] Infection after operation is a common
complication,[9] [10] [13] [14] [20] and the diagnosis of leakage
from the suture line is important in determining the
best treatment option. Leaks may be detected with
colonography,[9] whereas only one report has included
radiographic images.[9] Fistula repair can be achieved via
colonostomy, antibiotic therapy, and spontaneous selfclosure.
[9] [13] [14]
Figure 8. Leakage from suture line. This female
patient presented with anovestibular fistula with a
low-type anorectal malformation at age 1 year. She
was discharged from the hospital after undergoing
perineal anoplasty. Then, her buttocks began
to swell because of an abscess. Colonography
showing the presence of a fistula extending from
the suture line to the skin. She underwent colostomy
and antibiotic therapy was initiated. (a) Lateral
radiograph with colonography using barium showing
anorectal cutaneous fistula extending from the
suture line (arrow). (b) Sagittal reformatted contrast
computed tomography showing a low attenuation
area surrounding an enhanced rim posterior to the
rectum (arrow). In this lesion, gas was detected, and
an abscess was identified.
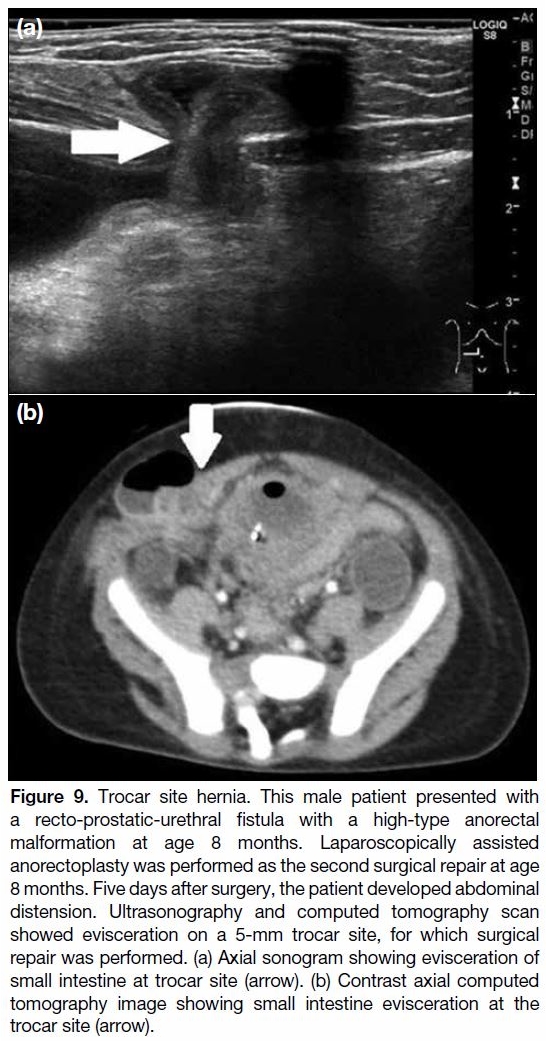
Trocar Site Hernia
LAARP is associated with trocar site hernia (Figure 9).
Previous studies have shown that the incidence of this
complication ranges between 1% and 10%.[24] [33] [46] [47] More than 90% of trocar site hernias are within 10 mm,[46] [47]
and they have occurred in paediatric patients.[24] [33] Some
cases may need surgical repair because of small bowel
obstruction with strangulation caused by a port site
hernia.[48] If this complication is detected, radiologists
should evaluate the possibility of bowel strangulation.
To prevent this complication, laparoscopic port closure
is usually performed using different techniques.[49] [50] For
radiologists, knowledge of trocar site hernia is important
for early diagnosis.
Figure 9. Trocar site hernia. This male patient presented with
a recto-prostatic-urethral fistula with a high-type anorectal
malformation at age 8 months. Laparoscopically assisted
anorectoplasty was performed as the second surgical repair at age
8 months. Five days after surgery, the patient developed abdominal
distension. Ultrasonography and computed tomography scan
showed evisceration on a 5-mm trocar site, for which surgical
repair was performed. (a) Axial sonogram showing evisceration of
small intestine at trocar site (arrow). (b) Contrast axial computed
tomography image showing small intestine evisceration at the
trocar site (arrow).
CONCLUSION
We have described eight complications after surgery for
ARM. These complications involve the pelvic organs.
Various imaging techniques are used to diagnose these
complications. Although the incidence of these types of complications varies across reports, knowledge
of their manifestation and treatment is important for
radiologists.
REFERENCES
1. Santulli, TV, Kiesewetter WB, Bill AH Jr. Anorectal anomalies: a
suggested international classification. J Pediatr Surg. 1970;5:281-7. Crossref
2. Peña A. Management of anorectal malformations during the
newborn period. World J Surg. 1993;17:385-92. Crossref
3. Morandi A, Ure B, Leva E, Lacher M. Survey on the management
of anorectal malformations (ARM) in European pediatric surgical
centers of excellence. Pediatr Surg Int. 2015;31:543-50. Crossref
4. Georgeson KE, Inge TH, Albanese CT. Laparoscopically assisted
anorectal pull-through for high imperforate anus — a new
technique. J Pediatr Surg. 2000;35:927-30. Crossref
5. Vick LR, Gosche JR, Boulanger SC, Islam S. Primary laparoscopic
repair of high imperforate anus in neonatal males. J Pediatr Surg.
2007;42:1877-81. Crossref
6. Peña A, Devries PA. Posterior sagittal anorectoplasty: important
technical considerations and new applications. J Pediatr Surg.
1982;17:796-811. Crossref
7. Peña A. Atlas of Surgical Management of Anorectal Malformations.
New York (NY): Springer; 1989.
8. Okada A, Kamata S, Imura K, Fukuzawa M, Kubota A, Yagi
M, et al. Anterior sagittal anorectoplasty for rectovestibular and
anovestibular fistula. J Pediatr Surg. 1992;27:85-8. Crossref
9. Nakayama DK, Templeton JM Jr, Ziegler MM, O’Neill JA, Walker
AB. Complications of posterior sagittal anorectoplasty. J Pediatr
Surg. 1986;21:488-92. Crossref
10. Japanese multicenter study group on male high imperforate anus.
Multicenter retrospective comparative study of laparoscopically
assisted and conventional anorectoplasty for male infants with
rectoprostatic urethral fistula. J Pediatr Surg. 2013;48:2383-8. Crossref
11. Alam S, Lawal TA, Peña A, Sheldon C, Levitt MA. Acquired
posterior urethral diverticulum following surgery for anorectal
malformations. J Pediatr Surg. 2011;46:1231-5. Crossref
12. Belizon A, Levitt M, Shoshany G, Rodriguez G, Peña A. Rectal
prolapse following posterior sagittal anorectoplasty for anorectal
malformations. J Pediatr Surg. 2005;40:192-6. Crossref
13. England RJ, Warren SL, Bezuidenhout L, Numanoglu A, Millar AJ.
Laparoscopic repair of anorectal malformations at the Red Cross War Memorial Children’s Hospital: taking stock. J Pediatr Surg.
2012;47:565-70. Crossref
14. Freeman NV, Bulut M. “High” anorectal anomalies treated by early
(neonatal) operation. J Pediatr Surg. 1986;21:218-20. Crossref
15. Hong AR, Acuña MF, Peña A, Chaves L, Rodriguez G. Urologic
injuries associated with repair of anorectal malformations in male
patients. J Pediatr Surg. 2002;37:339-44. Crossref
16. Koga H, Okazaki T, Yamataka A, Kobayashi H, Yanai T, Lane GJ, et al.
Posterior urethral diverticulum after laparoscopic-assisted repair
of high-type anorectal malformation in a male patient: surgical
treatment and prevention. Pediatr Surg Int. 2005;21:58-60. Crossref
17. Laberge JM, Bosc O, Yazbeck S, Youssef S, Ducharme JC,
Guttman FM, et al. The anterior perineal approach for pull-through
operations in high imperforate anus. J Pediatr Surg. 1983;18:774-8. Crossref
18. Podberesky DJ, Weaver NC, Anton CG, Lawal T, Hamrick MC,
Alam S, et al. MRI of acquired posterior urethral diverticulum
following surgery for anorectal malformations. Pediatr Radiol.
2011;41:1139-45. Crossref
19. Vinnicombe SJ, Good CD, Hall CM. Posterior urethral diverticula:
a complication of surgery for high anorectal malformations. Pediatr
Radiol. 1996;26:120-6. Crossref
20. Wang C, Li L, Liu S, Chen Z, Diao M, Li X, et al. The management
of anorectal malformation with congenital vestibular fistula: a
single-stage modified anterior sagittal anorectoplasty. Pediatr Surg
Int. 2015;31:809-14. Crossref
21. Williams DI, Grant J. Urological complications of imperforate
anus. Br J Urol. 1969;41:660-5. Crossref
22. Zornoza M, Molina E, Cerdá J, Fanjul M, Corona C, Tardáguila AR, et al.
Postoperative anal prolapse in patients with anorectal malformations:
16 years of experience [in Spanish]. Cir Pediatr. 2012;25:140-4.
23. Zamir N, Mirza FM, Akhtar J, Ahmed S. Anterior sagittal approach
for anorectal malformations in female children: early results. J Coll
Physicians Surg Pak. 2008;18:763-7.
24. Podevin G, Petit T, Mure PY, Gelas T, Demarche M, Allal H, et al.
Minimally invasive surgery for anorectal malformation in boys:
a multicenter study. J Laparoendosc Adv Surg Tech A. 2009;19
Suppl 1:S233-5. Crossref
25. Julià V, Tarrado X, Prat J, Saura L, Montaner A, Castañón M,
et al. Fifteen years of experience in the treatment of anorectal
malformations. Pediatr Surg Int. 2010;26:145-9. Crossref
26. Huang CF, Lee HC, Yeung CY, Chan WT, Jiang CB, Sheu JC,
et al. Constipation is a major complication after posterior sagittal
anorectoplasty for anorectal malformations in children. Pediatr
Neonatol. 2012;53:252-6. Crossref
27. Leva E, Macchini F, Arnoldi R, Di Cesare A, Gentilino V,
Fumagalli M, et al. Single-stage surgical correction of anorectal
malformation associated with rectourinary fistula in male neonates.
J Neonatal Surg. 2013;2:3.
28. Ramasundaram M, Sundaram J, Agarwal P, Bagdi RK, Bharathi S,
Arora A. Institutional experience with laparoscopic-assisted
anorectal pull-through in a series of 17 cases: a retrospective
analysis. J Minim Access Surg. 2017;13:265-8. Crossref
29. Jung SM, Lee SK, Seo JM. Experience with laparoscopic-assisted
anorectal pull-through in 25 males with anorectal malformation and
rectourethral or rectovesical fistulae: postoperative complications
and functional results. J Pediatr Surg. 2013;48:591-6. Crossref
30. Eltomey MA, Donnelly LF, Emery KH, Levitt MA, Peña A.
Postoperative pelvic MRI of anorectal malformations. AJR Am J
Roentgenol. 2008;191:1469-76. Crossref
31. Koga H, Ochi T, Okawada M, Doi T, Lane GJ, Yamataka A.
Comparison of outcomes between laparoscopy-assisted and
posterior sagittal anorectoplasties for male imperforate anus with
recto-bulbar fistula. J Pediatr Surg. 2014;49:1815-7. Crossref
32. Ming AX, Li L, Diao M, Wang HB, Liu Y, Ye M, et al. Long term
outcomes of laparoscopic-assisted anorectoplasty: a comparison
study with posterior sagittal anorectoplasty. J Pediatr Surg.
2014;49:560-3. Crossref
33. De Vos C, Arnold M, Sidler D, Moore SW. A comparison of
laparoscopic-assisted (LAARP) and posterior sagittal (PSARP)
anorectoplasty in the outcome of intermediate and high anorectal
malformations. S Afr J Surg. 2011;49:39-43.
34. Yazaki Y, Koga H, Ochi T, Okawada M, Doi T, Lane GJ, et al.
Surgical management of recto-prostatic and recto-bulbar anorectal
malformations. Pediatr Surg Int. 2016;32:939-44. Crossref
35. Hosokawa T, Yamada Y, Tanami Y, Hattori S, Sato Y, Tanaka Y,
et al. Sonography for an imperforate anus: approach, timing of the
examination, and evaluation of the type of imperforate anus and
associated anomalies. J Ultrasound Med. 2017;36:1747-58. Crossref
36. Yong C, Ruo-yi W, Yuan Z, Shu-hui Z, Guang-Rui S. MRI findings
in patients with defecatory dysfunction after surgical correction of
anorectal malformation. Pediatr Radiol. 2013;43:964-70. Crossref
37. Gangopadhyay AN, Pandey V, Gupta DK, Sharma SP, Kumar V,
Verma A. Assessment and comparison of fecal continence in
children following primary posterior sagittal anorectoplasty and
abdominoperineal pull through for anorectal anomaly using clinical
scoring and MRI. J Pediatr Surg. 2016;51:430-4. Crossref
38. Kraus SJ, Levitt MA, Peña A. Augmented-pressure distal
colostogram: the most important diagnostic tool for planning
definitive surgical repair of anorectal malformations in boys. Pediatr
Radiol. 2018;48:258-69. Crossref
39. Yamataka A, Lane GJ, Koga H. Laparoscopy-assisted surgery for
male imperforate anus with rectourethral fistula. Pediatr Surg Int.
2013;29:1007-11. Crossref
40. Ralph DJ, Woodhouse CR, Ransley PG. The management of the
neuropathic bladder in adolescents with imperforate anus. J Urol.
1992;148(2 Pt 1):366-8. Crossref
41. O’Connor JL, DeMarco RT, Pope JC 4th, Adams MC, Brock JW 3rd.
Bowel vaginoplasty in children: a retrospective review. J Pediatr
Surg. 2004;39:1205-8. Crossref
42. Kyrklund, K, Taskinen S, Rintala RJ, Pakarinen MP. Sexual
function, fertility and quality of life after modern treatment of
anorectal malformations. J Urol. 2016;196:1741-6. Crossref
43. Grano C, Bucci S, Aminoff D, Lucidi F, Violani C. Quality of life
in children and adolescents with anorectal malformation. Pediatr
Surg Int. 2013;29:925-30. Crossref
44. Skerritt C, Vilanova Sanchez A, Lane VA, Wood RJ, Hewitt GD,
Breech LL, et al. Menstrual, sexual, and obstetrical outcomes after
vaginal replacement for vaginal atresia associated with anorectal
malformation. Eur J Pediatr Surg. 2017;27:495-502. Crossref
45. Vilanova-Sanchez A, Reck CA, McCracken KA, Lane VA, Gasior AC,
Wood RJ, et al. Gynecologic anatomic abnormalities following
anorectal malformations repair. J Pediatr Surg. 2018;53:698-703. Crossref
46. Di Lorenzo N, Coscarella G, Lirosi F, Pietrantuono M, Susanna F,
Gaspari A. Trocars and hernias: a simple, cheap remedy [in Italian].
Chir Ital. 2005;57:87-90.
47. Hussain A, Mahmood H, Singhal T, Balakrishnan S, Nicholls J,
El-Hasani S. Long-term study of port-site incisional hernia after
laparoscopic procedures. JSLS. 2009;13:346-9.
48. Rammohan A, Naidu RM. Laparoscopic port site Richter’s hernia—an important lesson learnt. Int J Surg Case Rep. 2011;2:9-11. Crossref
49. Di Lorenzo N, Coscarella G, Lirosi F, Gaspari A. Port-site closure:
a new problem, an old device. JSLS. 2002;6:181-3.
50. Singal R, Zaman M, Mittal A, Singal S, Sandhu K, Mittal A.
No need of fascia closure to reduce trocar site hernia rate in
laparoscopic surgery: a prospective study of 200 non-obese patients.
Gastroenterology Res. 2016;9:70-3. Crossref