Significance of Coronary Artery Anomalies and Variants Found on Coronary Computed Tomography Angiography
ORIGINAL ARTICLE
Significance of Coronary Artery Anomalies and Variants Found on Coronary Computed Tomography Angiography
G Yarlagadda, U Kumar K, L Kumar T, Nagaraj BR, A Prasath P
Department of Radiology, Mahatma Gandhi Medical College and Research Institute, Pondicherry, India
Correspondence: Dr U Kumar K, Department of Radiology, Mahatma Gandhi Medical College and Research Institute, Pondicherry,
India. Email: rdudhay@gmail.com
Submitted: 23 Jan 2018; Accepted: 2 Oct 2018.
Contributors: GY, UKK and APP designed the study. GY, UKK and NBR were responsible for acquisition of data. GY and UKK analysed
the data. GY, UKK and LKT wrote the manuscript. All authors had critical revision of the manuscript for important intellectual content. All
authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and
integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics Approval: This study was approved by the Mahatma Gandhi Medical College and Research Institute Institutional Human Ethics
Committee (Ref ECR/451/Inst/PY/2013). This was a retrospective study of computed tomographic images and all identifiable patient information
was removed from the displayed images. Thus, the requirement for patient consent was waived by the ethics board.
Abstract
Objective
We sought to assess the significance of coronary artery anatomical variants and anomalies found in
patients referred for coronary computed tomographic angiography (CTA).
Methods
Imaging was done on a 128-detector computed tomography machine followed by three-dimensional
reconstruction. Pre- and post-contrast angiographic images of the coronary arteries of 108 patients from January
2013 to December 2016 were retrospectively analysed for coronary artery anomalies and variants.
Results
A total of 74 anomalies and variants were noted in 58 of 108 patients. Among variants, co-dominance, left
dominant circulation, ramus intermedius, early origin of posterior descending artery (PDA), and double PDA were
noted. Among anomalies, right coronary artery (RCA) origin from the ascending aorta and left coronary sinus, left
circumflex artery (LCX) arising from the RCA, malignant course of RCA, retrograde aortic course of LCX, myocardial
bridging, and coronary hypoplasia were noted.
Conclusion
Prior assessment for coronary anomalies and variants can guide the interventionalist and surgeon
before coronary angioplasty and coronary artery bypass grafting, thereby reducing procedural complications.
Key Words: Aorta; Coronary artery disease; Coronary sinus; Heart defects, congenital; Myocardial bridging;
Tomography, X-ray computed
中文摘要
冠狀動脈斷層掃描血管造影術中發現的冠狀動脈異常和變異的意義
G Yarlagadda, U Kumar K, L Kumar T, Nagaraj BR, A Prasath P
目的
評估在接受冠狀動脈斷層掃描血管造影術患者中發現的冠狀動脈解剖異常和變異的意義。
方法
利用128層探頭斷層掃描儀進行成像,然後進行三維重建。回顧分析2013年1月至2016年12月
期間108例患者的冠狀動脈造影前後血管造影圖像的冠狀動脈異常和變異情況。
結果
108例患者中,58例發現74個動脈異常和變異。動脈變異包括左右側共主導血供、左側主導血
供、中間支、後降支動脈的早期起源和雙後降支動脈。動脈異常包括源於升主動脈和左冠狀竇的右
冠狀動脈、源於右冠狀動脈的左旋支動脈、右冠狀動脈的彎曲走向、左旋支動脈的逆行走向、心肌
橋,以及冠狀動脈發育不全。
結論
進行冠狀動脈成形術和冠狀動脈搭橋術前,事先評估冠狀動脈異常和變異可為介入醫師和外科醫生作出指引,減少手術併發症的機會。
INTRODUCION
Coronary artery anomalies and variants are in the
differential diagnosis of patients with suspected
coronary artery disease. A meticulous evaluation of the
coronary tree to assess the arteries’ origins and course
by electrocardiogram (ECG)-gated coronary computed
tomography angiography (CTA) allows accurate
analysis with the added advantages of being non-invasive
and quick to acquire, with exquisite delineation
of cardiac and vascular anatomy. Appropriate depiction
of incidentally discovered coronary artery anomalies
and variants in individuals with coronary artery disease
symptoms is important to prevent unexpected adverse
cardiac events.
METHODS
This study was approved by the institutional review board
and ethics committee of our institution. This study was
conducted at our hospital. This is a retrospective study of
the CTA images of patients referred to our hospital for
coronary computed tomography (CT) between January
2013 and December 2016. Exclusion criteria were a
serum creatinine >1.5 mg/dL, any absolute contra-indications
to CT or intravenous contrast agents, and
suboptimal images.
Procedure
The patients took ivabradine 5 mg BID for 2 days before
the scheduled CTA to control and regularise the heart
rate for better ECG gating and undistorted images.
In selected patients, a slow intravenous injection of
metoprolol was given before scanning to maintain the
heart rate <70 bpm with minimum beat-to-beat variation.
The CTAs were performed with a 128-detector
scanner (GE 660 Optima, General Electric Company,
Tokyo, Japan). The technical parameters were:
tube current = 400 mAs, tube voltage = 120 kv, tube rotation time = 400 ms, and section thickness = 0.625 mm.
Post-image acquisition reformatting and calcium score
calculation were performed at a GE 660 ADWOPTIMA
workstation.
Frontal and lateral topograms of the chest were acquired
to plan the study. Initially, unenhanced CT images were
acquired for calcium scoring from the level of the carina
to 2.5 cm below the diaphragm with prospective ECG
gating. After administration of iohexol (Magnapaque;
Magnus Health Management Pvt. Ltd., Mumbai, India)
containing 350 mg iodine per mL, 1.5 mL/kg of body
weight at a rate of 5.5 mL/s with a double-barrel
pressure injector, enhanced coronary angiographic
images were acquired with retrospective ECG gating,
using the bolus tracking technique.
The images were meticulously analysed with semiautomated
commercial software (SmartScore 4.0; GE
Healthcare, Chicago [IL], United States) which allows
the observer to individually select the calcium foci and
label the affected artery, after which the total Agatston
calcium score is automatically generated.
Five phases during diastole (50%, 55%, 60%, 65%, and
70%) underwent angiographic analysis after formatting
including multiplanar reconstruction, curved multiplanar
reconstruction, and three-dimensional volume rendering.
RESULTS
In total, CTA images of 108 patients were identified. We
classified the anomalies based on the widely accepted
Angelini classification.[1] A total of 74 anomalies and
variants were identified in 58 of 108 patients; the
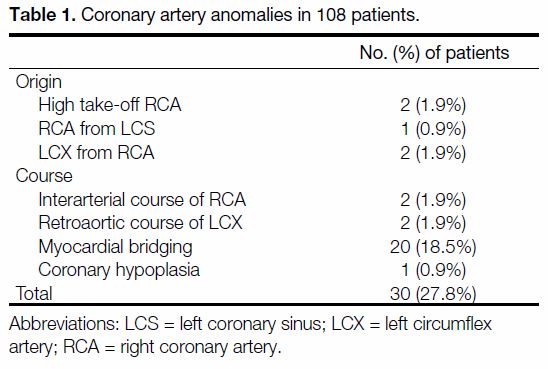
distribution is shown in Tables 1 and 2. Among origin
anomalies (Table 1), a high take-off of the right coronary
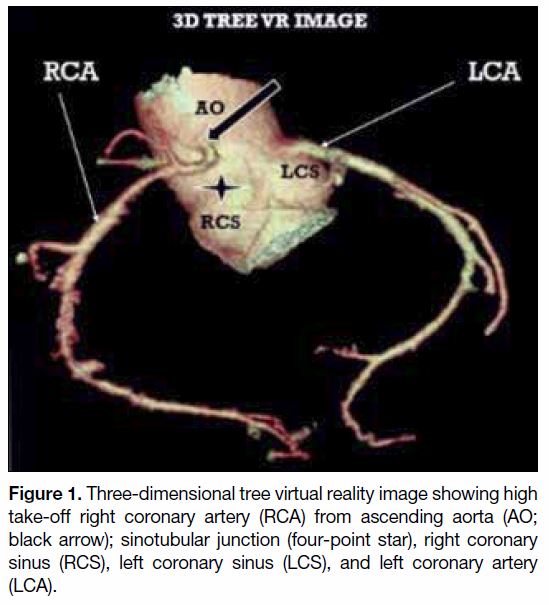
artery (RCA) from the ascending aorta (n = 2; Figure 1),
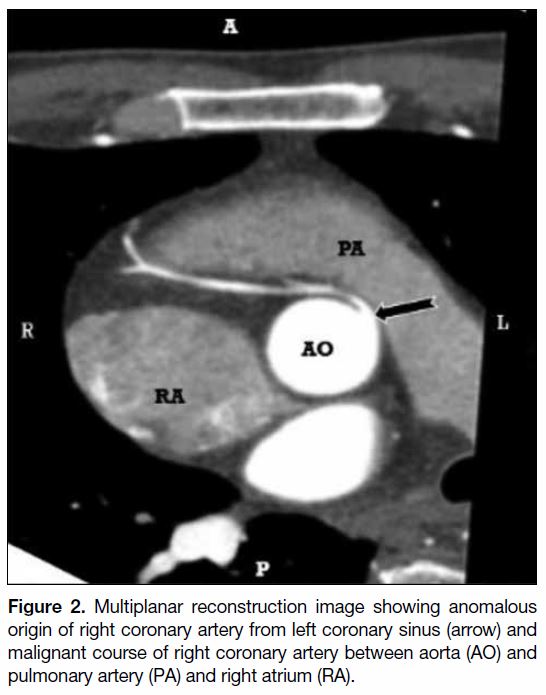
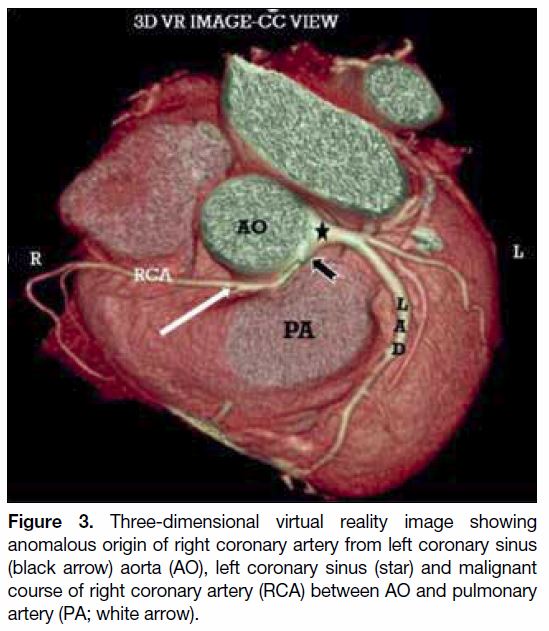
an RCA originating from the left coronary sinus (n = 1; Figures 2 and 3), and a left circumflex artery
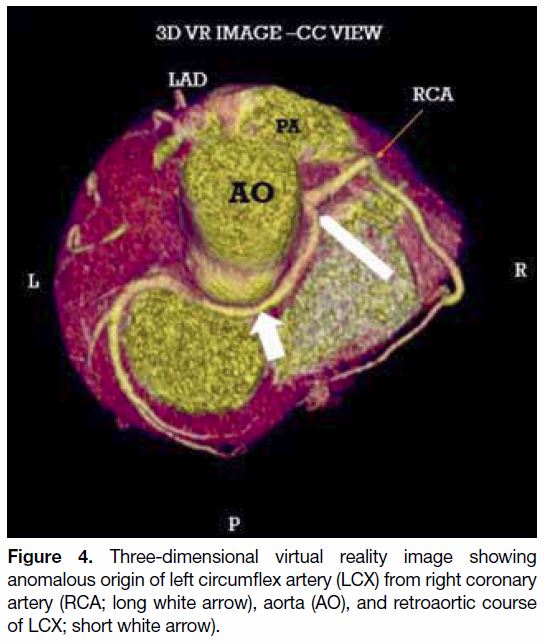
(LCX) arising from the RCA (n = 2; Figure 4) were
noted.
Table 1. Coronary artery anomalies in 108 patients.
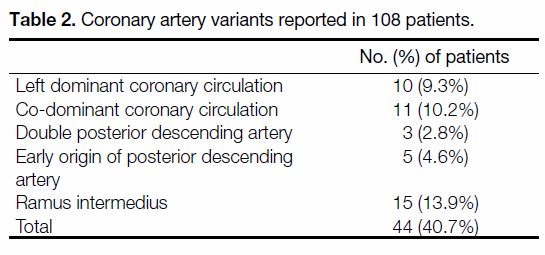
Table 2. Coronary artery variants reported in 108 patients.
Figure 1. Three-dimensional tree virtual reality image showing high
take-off right coronary artery (RCA) from ascending aorta (AO;
black arrow); sinotubular junction (four-point star), right coronary
sinus (RCS), left coronary sinus (LCS), and left coronary artery
(LCA).
Figure 2. Multiplanar reconstruction image showing anomalous
origin of right coronary artery from left coronary sinus (arrow) and
malignant course of right coronary artery between aorta (AO) and
pulmonary artery (PA) and right atrium (RA).
Figure 3. Three-dimensional virtual reality image showing
anomalous origin of right coronary artery from left coronary sinus
(black arrow) aorta (AO), left coronary sinus (star) and malignant
course of right coronary artery (RCA) between AO and pulmonary
artery (PA; white arrow).
Figure 4. Three-dimensional virtual reality image showing
anomalous origin of left circumflex artery (LCX) from right coronary
artery (RCA; long white arrow), aorta (AO), and retroaortic course
of LCX; short white arrow).
The course anomalies (Table 1) included malignant
course of the RCA between the aorta and the pulmonary
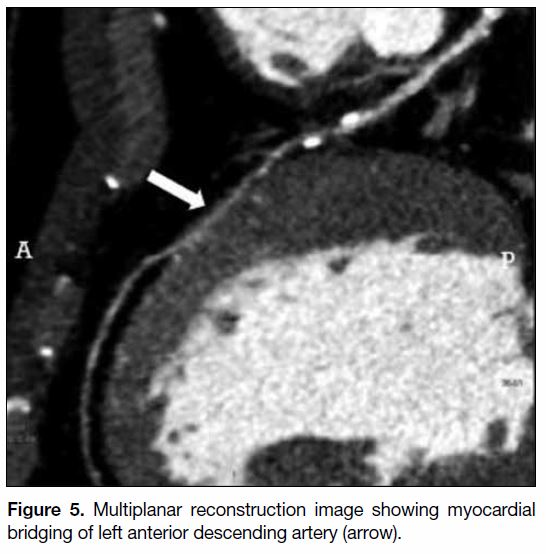
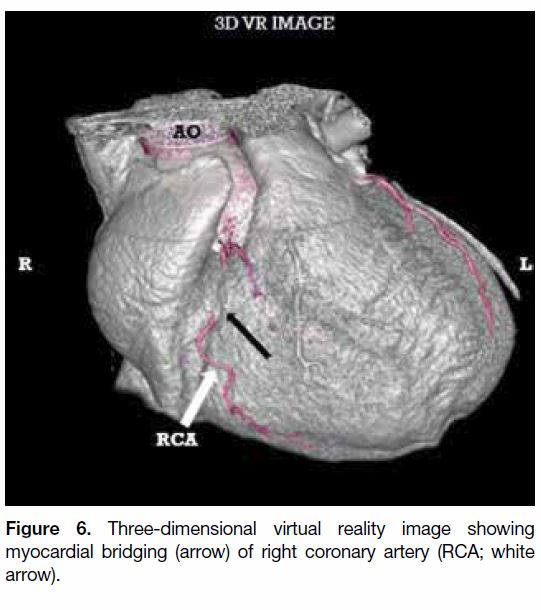
trunk (n = 2; Figures 2 and 3), a retroaortic course of the LCX (n = 2; Figure 4), and myocardial bridging
(n = 20) affecting the left anterior descending artery
(LAD; Figure 5) and RCA (Figure 6).
Figure 5. Multiplanar reconstruction image showing myocardial
bridging of left anterior descending artery (arrow).
Figure 6. Three-dimensional virtual reality image showing
myocardial bridging (arrow) of right coronary artery (RCA; white
arrow).
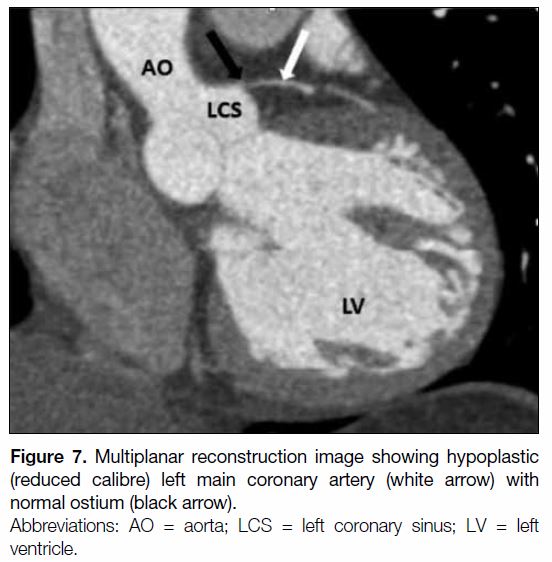
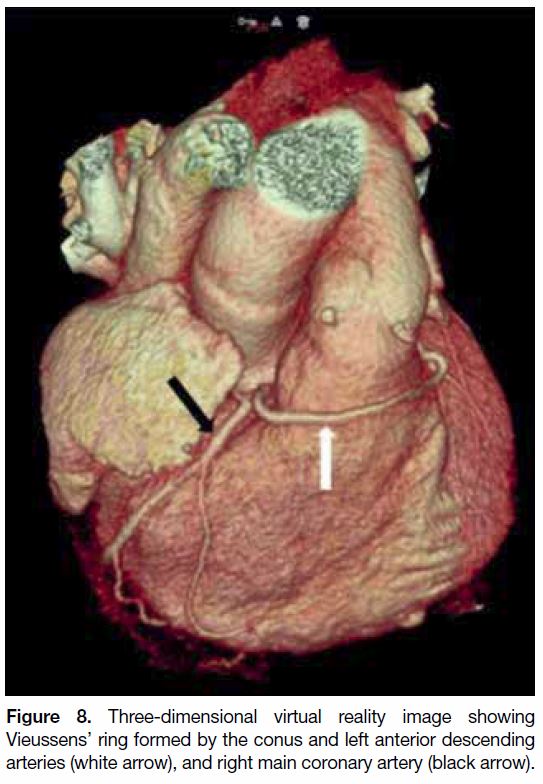
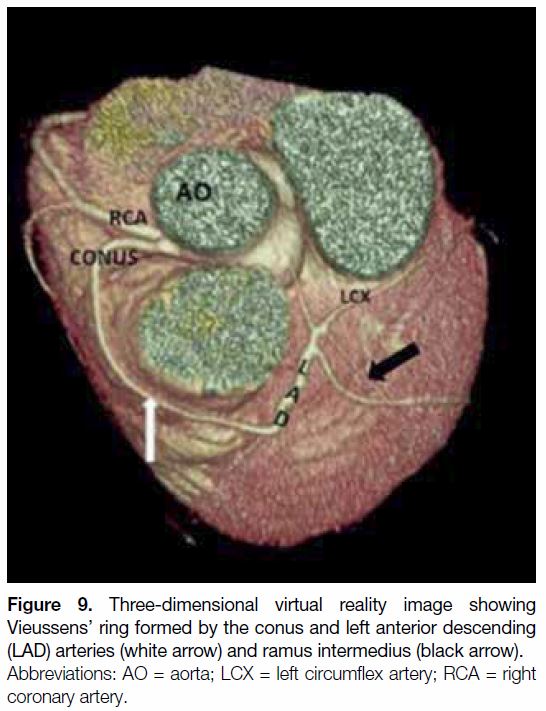
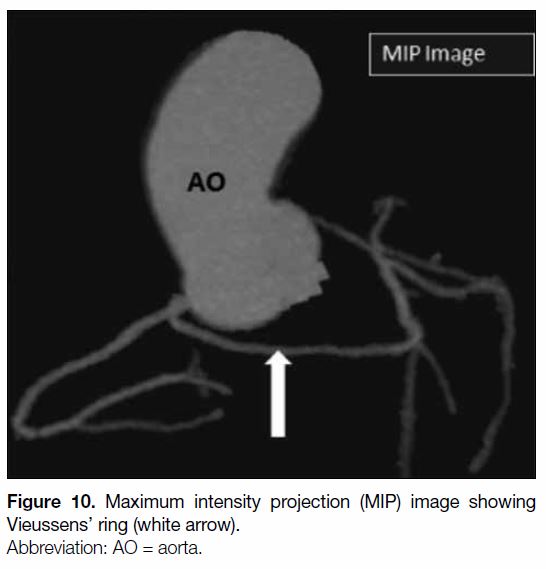
One case of coronary hypoplasia was identified
(Figures 7 8 9 10). The left main coronary artery (LMCA)
was reduced in its calibre (1.1 mm; normal range, 4.5±0.5 mm in men, 4.0±0.5 mm in women)[2] along its
entire length (1.5 cm) before it trifurcated into the LAD,
a ramus intermedius artery, and the LCX. The conal
artery measured 3 mm in calibre and coursed right to
left anterior to the pulmonary conus to anastomose with
the LAD distal to the D1 branch forming a Vieussens’
ring (Figures 8 9 10). During conventional angiography, nonselective injection into the left coronary sinus
showed a hypoplastic LMCA and hypoplastic mid and
distal LAD. The treatment of choice, coronary artery
bypass grafting (CABG), was performed, with two grafts
placed, one to the left internal mammary artery for the
D1 branch and the other, a saphenous vein graft of the
obtuse marginal artery.
Figure 7. Multiplanar reconstruction image showing hypoplastic
(reduced calibre) left main coronary artery (white arrow) with
normal ostium (black arrow).
Figure 8. Three-dimensional virtual reality image showing
Vieussens’ ring formed by the conus and left anterior descending
arteries (white arrow), and right main coronary artery (black arrow).
Figure 9. Three-dimensional virtual reality image showing
Vieussens’ ring formed by the conus and left anterior descending
(LAD) arteries (white arrow) and ramus intermedius (black arrow).
Figure 10. Maximum intensity projection (MIP) image showing
Vieussens’ ring (white arrow).
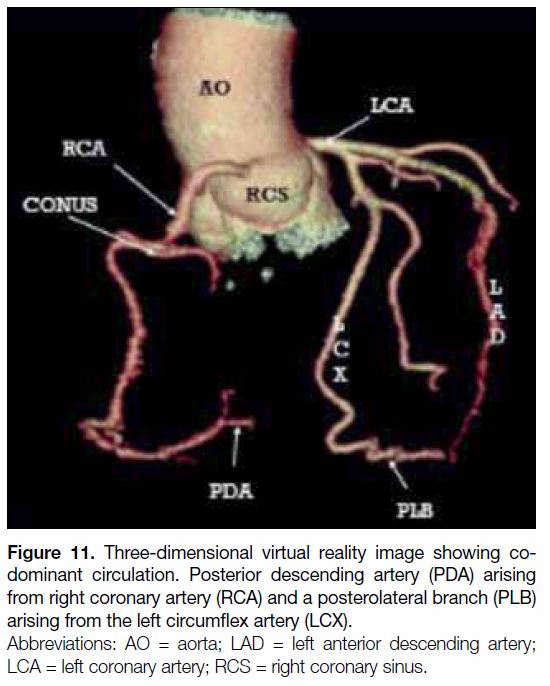
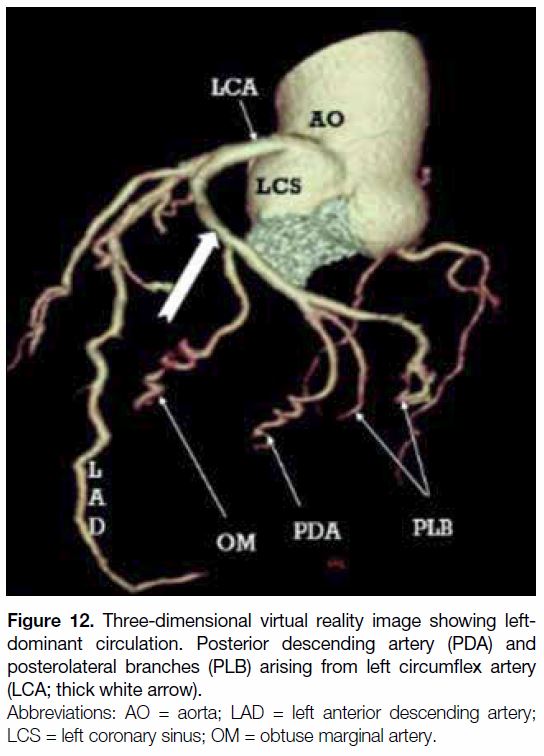
Among other variants (Table 2), co-dominant circulation
(n = 11; Figure 11), left dominant coronary circulation
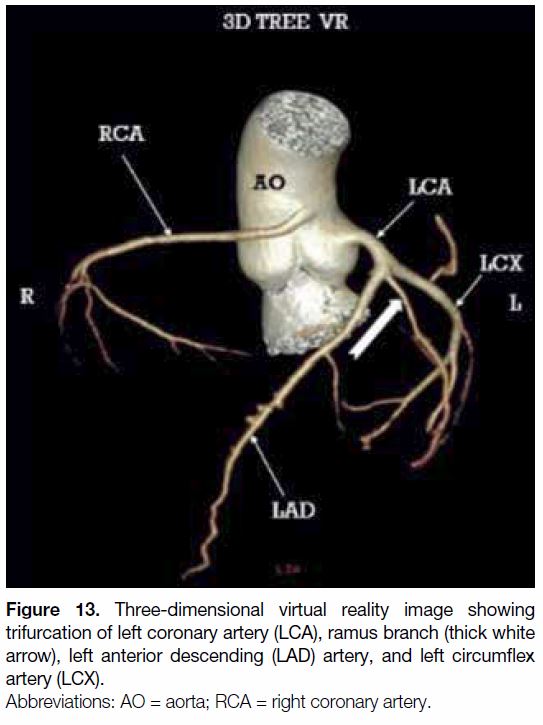
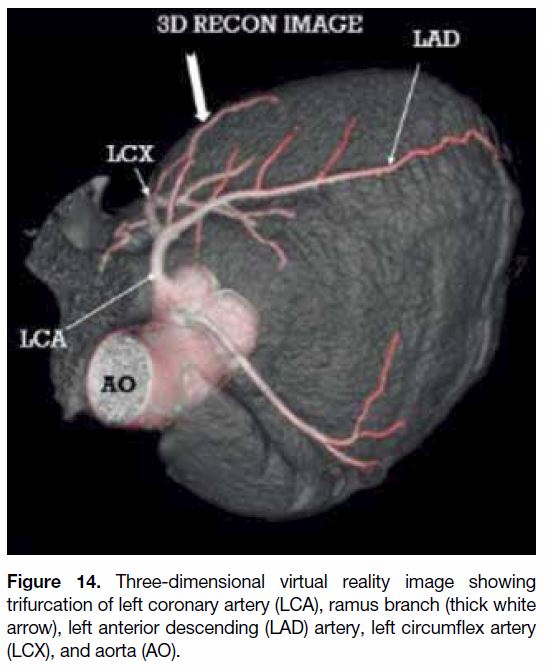
(n = 10; Figure 12), ramus intermedius (n = 15;
Figures 9, 13 and 14), early origin of posterior descending
artery (PDA, n = 5; Figure 15), and double PDA (n = 3)
were noted.
Figure 11. Three-dimensional virtual reality image showing codominant
circulation. Posterior descending artery (PDA) arising
from right coronary artery (RCA) and a posterolateral branch (PLB)
arising from the left circumflex artery (LCX).
Figure 12. Three-dimensional virtual reality image showing leftdominant
circulation. Posterior descending artery (PDA) and
posterolateral branches (PLB) arising from left circumflex artery
(LCA; thick white arrow).
Figure 13. Three-dimensional virtual reality image showing
trifurcation of left coronary artery (LCA), ramus branch (thick white
arrow), left anterior descending (LAD) artery, and left circumflex
artery (LCX).
Figure 14. Three-dimensional virtual reality image showing
trifurcation of left coronary artery (LCA), ramus branch (thick white
arrow), left anterior descending (LAD) artery, and left circumflex
artery (LCX), and aorta (AO).
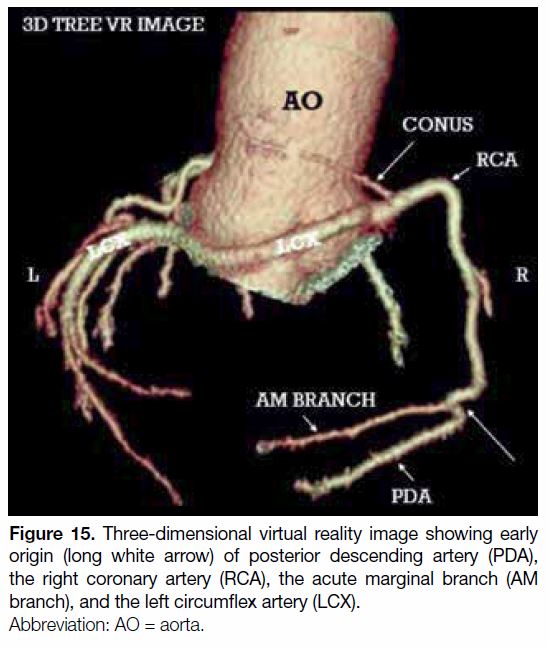
Figure 15. Three-dimensional virtual reality image showing early
origin (long white arrow) of posterior descending artery (PDA),
the right coronary artery (RCA), the acute marginal branch (AM
branch), and the left circumflex artery (LCX).
DISCUSSION
Cardiac catheterisation has remained the gold standard
imaging modality to visualise coronary anatomy.
However, owing to the potentially complex three-dimensional
nature of these anomalies, conventional
coronary angiography incompletely delineates the
anatomical course of the coronary arteries.[3] The threedimensional
nature of multidetector CT coronary
angiography datasets allows proper analysis of anomalous
coronary arteries. We have presented a series of patients
whose routine CTAs showed a wide range of anomalies
in great detail, which could help the interventionalist and
cardiac surgeon in the preoperative planning.
High Take-off of Right Coronary Artery
The positions of the coronary orifices are described in
terms of their relationship to the sinotubular junction.
We have reported a relatively less frequent variation
known as “high take-off” RCA.
High origin of coronary artery is defined as origin of a
coronary artery >1 cm above the sinotubular junction.[4] [5]
According to studies based on autopsy examination,
high take-off anomaly with acute downward angulation
of the proximal RCA and acute downward angulation
of the LMCA led to sudden death.[6] A few studies
identified infants with a high take-off of the RCA who
had coexisting ventricular septal defect and bicuspid
aortic valve. The importance of screening patients with
a bicuspid aortic valve for a high-origin RCA has also
been emphasised in the surgery literature.
The process of catheterisation of a coronary artery with
a high origin may be difficult. In patients with high
take-off of the RCA from anterior or especially left
anterior part of the ascending aorta, the right radial
approach is preferred over the femoral approach to
save time.[7] Preoperative identification of a high-origin
coronary artery and its course are important in patients
undergoing aortotomy as part of aortic valve surgery or
ascending aortic replacement.
Cross-clamping of the aorta below a high-origin
coronary artery may result in unsuccessful induction of
cardioplegia. If the proximal part of the RCA is cut off,
it may lead to preoperative myocardial ischaemia. High
take-off RCA may also traverse the site of the planned
proximal saphenous vein graft anastomosis in CABG
surgery.
We documented two cases of high take-off RCA, of
which one was originating 11 mm above the sinotubular
junction, sharing its origin with the left coronary artery.
In one patient, the RCA also travelled an anomalous
interarterial course between the pulmonary artery and
aorta. Another high take-off RCA that has been identified
in our study originated from the ascending aorta above
the right coronary sinus showing normal course and
insertion.
Anomalous Right Coronary Ostium with
Interarterial Course
The anomalous origin of the RCA from the left coronary
sinus can be subdivided into two types based on the
location of the RCA ostium. In the high interarterial
course, the RCA ostium is located between the aorta
and the pulmonary artery. In this condition the proximal
segment of the anomalous RCA courses between the
aorta and pulmonary artery. This pattern of course is
called “malignant” as it is associated with sudden death
of the individual.[8] [9] In the low interarterial course, the
RCA ostium is located between the aorta and the right
ventricular outflow tract (RVOT). In this scenario, the
ostium is below the level of the pulmonary valve, with
no segment between the aorta and the RVOT.[5]
It is also known that a coronary artery arising from the
opposite sinus can take any of four common courses:
interarterial (between the aorta and the pulmonary artery),
retroaortic, prepulmonic, or subpulmonic (septal).
The possible mechanisms of restricted coronary blood
flow seen in interarterial courses are suggested to
be the acute take-off angle, a slit-like ostium, and
compression of the intramural segment by the aortic
valve commissure.[5] [10] [11] The mechanism which may lead
to cardiac events in individuals with the high interarterial
subtype is that an ostium that is located above the
pulmonary valve would be compressed, due to the
blood that is forced into the aorta and pulmonary artery
during systole. This may also lead to compression of the
interarterial segment between them. Cases of sudden
death during exercise were reported. In cases with coronary artery anomalies, the risk of death is higher in
patients <30 years and the risk lower in aged people.[3]
However, in the low interarterial course variant, the
RVOT contracts during systole, so an RCA ostium
below the pulmonary valve would be less compressed
between the aorta and RVOT. These coronary anomalies
if detected in early stages of life and followed by proper
treatment could prevent sudden death.
The choice of treatment according to most of the literature
is surgical revascularisation in all cases. The available
options are CABG and reimplantation of the coronary
ostia and unroofing of the coronary artery. Unroofing
of the coronary artery is being considered as a better
treatment option if anatomically feasible.[12] [13] In Japan,
the treatment for this condition is conservative with the
patient being treated medically with beta blockers.[12] [14] In
our study we report a case of anomalous origin of the
RCA from the left coronary sinus showing an interarterial
and malignant course between the pulmonary artery and
aorta.
Left Circumflex Artery from Right Coronary
Artery with Retroaortic Course
The ectopic origin of the LCX is considered the
most common coronary anomaly and can be found
in approximately 0.37% to 0.7% of all patients. The
anomalous LCX most commonly arises from a separate
ostium within the right coronary sinus, or as a proximal
branch of the RCA.[4] Although this anomaly was
considered benign and asymptomatic, a few cases of
sudden death, myocardial infarction, and angina pectoris
in the absence of coronary artery disease have been
reported.[5]
An anomalous origin of LCX from right coronary sinus
is divided into three types: separate ostia for RCA
and LCX (Type I), common ostia in the right sinus
(Type II), or LCX arising as a branch of the proximal RCA
(Type III).[15]
On selective conventional coronary angiography in
the left anterior oblique and right anterior oblique
projections the exact anatomical course is typically
identified.[16] Visualisation of the “dot” sign on the left
ventricular angiogram in the right anterior oblique view
(just posterior and to the left of the posterior aortic
margin) is used as a clue by the coronary interventionalist
for identification of an LCX arising from RCA with a
retroaortic course.[16] In our study we reported two cases of LCX originating from the right coronary sinus and
showing a retroaortic course. Balloon angioplasty seems
to be a favourable approach for revascularisation in these
vessels.[17] [18] [19] Appropriate selection of a guiding catheter is
important.[18]
Coronary Hypoplasia
Hypoplastic coronary artery disease was first reported
in 1970. It is underdevelopment of one or more major
branches of the coronary arteries characterised by a
narrowed lumen or shorter course.[20] [21] [22] [23] [24] [25] [26] [27] Its incidence is
0.02% of the general population and 2.2% of all the
congenital coronary artery anomalies, however, its
aetiology is still unknown. It was postulated to result
from, for example, stenosis of the coronary artery orifice,
an aberrant course between the pulmonary artery and
aorta, a coronary artery ostium in ectopic position, or
stenosis of the coronary ostium.[9] [23] [24] [27] [28] [29] [30] [31]
The condition is mostly asymptomatic. However, some
present with chest pain and palpitations. It bears a high
risk of sudden cardiac death as a result of sudden and total
occlusion of the artery. Mechanisms involved in cardiac
events are coronary artery spasm reflecting abnormal
vasodilator mechanisms and endothelial dysfunction
leading to myocardial ischaemia. We reported a case of
hypoplastic LMCA and LAD in a 33-year-old female
as discussed in our results. This unusual clinical entity
has rarely been diagnosed in living individuals. Most of
them are asymptomatic and a high proportion experience
sudden cardiac death. Diagnosis of this condition is often
made at autopsy.
Myocardial Bridging of Coronary Arteries
Myocardial bridging of a coronary artery is defined as a
band of myocardium overlying a segment of a coronary
artery.[32] [33] It is most commonly localised in the middle
segment of the LAD.
We documented myocardial bridging of LAD and
RCA in our study; LAD was more commonly involved.
Myocardial bridging in few cases might be responsible for
angina pectoris, myocardial infarction, life-threatening
arrhythmias, or even death. The typical “milking”
effect and a “step down–step up” phenomenon induced
by systolic compression of the tunnelled segment is
considered as a standard for diagnosing myocardial
bridging on conventional catheter angiography.[4] [33]
Multidetector-row CTA clearly shows the
intramyocardial location of the involved coronary arterial segment. The ECG-gated reconstruction
window used in standard multidetector-row CTA of
the coronary arteries is usually positioned within the
diastolic phase for maximal vasodilatation and minimal
motion artefacts. However, when there is suspicion for
myocardial bridging, it is recommended that ECG-gated
reconstruction be performed during the systolic phase as
well as the diastolic phase. Comparison of the images
obtained during the two phases will allow assessment of
luminal narrowing during the systolic phase.
Early Origin of Posterior Descending Artery
According to previous studies in RCA-dominant
individuals, 25% of them showed significant anatomical
variations in the origin of the PDA.
The variations associated with PDA are: partial supply
of the PDA territory by acute marginal branches, double
PDA, and early origin of the PDA proximal to the crux.
We reported five cases of early origin of PDA and three
cases of double PDA in our study.
Ramus Intermedius
The most common variation in left coronary artery
anatomy is the presence of a trifurcation of the LMCA. In
this instance, the LMCA trifurcates into the LAD, LCX,
and another artery called the ramus intermedius artery.
The ramus intermedius artery has variable branching. It
can be distributed as a diagonal branch if it supplies the
anterior wall or as an obtuse marginal branch when it
supplies the lateral wall.[4] [5]
Dominance
The artery which supplies both the posterior descending
branch and posterior left ventricular branch is
considered dominant. Usually RCA gives both PDA
and posterolateral branch.[34] In few individuals both the
branches arise from LCX, this is called left dominant
coronary circulation.
There is another scenario in which RCA gives the PDA
and LCX gives the posterolateral branch. This is stated
to be co-dominant circulation. In approximately 70% of
the population, the PDA originates from the RCA; it is
co-dominant in 20% and 10% are left dominant.
According to a study by the National Cardiovascular
Database Cath Percutaneous Coronary Intervention
Registry, left coronary dominance was associated with
1.19-fold increased odds of in-hospital mortality, and
co-dominance was associated with 1.16-fold increased odds of death, after percutaneous coronary intervention
for acute coronary syndrome after accounting for
23 demographic, clinical, and angiographic
characteristics.[35]
The detailed evaluation and reporting of the anomalies
and variants in the coronary tree is essential, primarily
as it might be the cause of the patient’s symptoms and
it guides the surgeon and/or interventionalist during
cardiac procedures, thereby reducing the intra- and postoperative
events.
CONCLUSION
Coronary artery anomalies occur in 1% to 5% of patients
undergoing coronary arteriography. Despite the large
number of reported anomalies, congenital anomalies of
coronary arteries are present in <3% of all congenital
heart diseases, and <1% among the general population.4
REFERENCES
1. Angelini P. Coronary artery anomalies — current clinical issues:
definitions, classification, incidence, clinical relevance, and
treatment guidelines. Tex Heart Inst J. 2002;29:271-8.
2. Dodge JT Jr, Brown BG, Bolson EL, Dodge HT. Lumen diameter
of normal human coronary arteries. Influence of age, sex, anatomic
variation, and left ventricular hypertrophy or dilation. Circulation.
1992;86:232-46. Crossref
3. Manghat NE, Morgan-Hughes GJ, Marshall AJ, Roobottom CA.
Multidetector row computed tomography: imaging congenital
coronary artery anomalies in adults. Heart. 2005;91:1515-22. Crossref
4. Kim SY, Seo JB, Do KH, Heo JN, Lee JS, Song JW, et al. Coronary
artery anomalies: classification and ECG-gated multi-detector
row CT findings with angiographic correlation. Radiographics.
2006;26:317-33. Crossref
5. Shriki JE, Shinbane JS, Rashid MA, Hindoyan A, Withey JG,
DeFrance A, et al. Identifying, characterizing, and classifying
congenital anomalies of the coronary arteries. Radiographics.
2012;32:453-68. Crossref
6. Eren B, Türkmen N, Gündoğmuş UN. Sudden death due to high take-off right coronary artery. Soud Lek. 2013;58:45-6.
7. Abbasov E, Bagirov I, Akhundova A. Radial approach is better
than the femoral one in anomalous high RCA take-off from the left-anterior
part of the ascending aorta. J Cardiol Cases. 2013;7:e126-8. Crossref
8. Hague C, Andrews G, Forster B. MDCT of a malignant anomalous right coronary artery. AJR Am J Roentgenol. 2004;182:617-8. Crossref
9. Dirksen MS, Langerak SE, de Roos A, Vliegen HW, Jukema JW,
Bax JJ, et al. Malignant right coronary artery anomaly detected
by magnetic resonance coronary angiography. Circulation.
2002;106:1881-2. Crossref
10. Lorenz EC, Mookadam F, Mookadam M, Moustafa S, Zehr KJ.
A systematic overview of anomalous coronary anatomy and an
examination of the association with sudden cardiac death. Rev
Cardiovasc Med. 2006;7:205-13.
11. Angelini P, Walmsley RP, Libreros A, Ott DA. Symptomatic
anomalous origination of the left coronary artery from the opposite
sinus of Valsalva. Clinical presentations, diagnosis, and surgical
repair. Tex Heart Inst J. 2006;33:171-9.
12. Satija B, Sanyal K, Katyayni K. Malignant anomalous right coronary artery detected by multidetector row computed tomography
coronary angiography. J Cardiovasc Dis Res. 2012;3:40-2. Crossref
13. Fedoruk LM, Kern JA, Peeler BB, Kron IL. Anomalous origin
of the right coronary artery: Right internal thoracic artery to right
coronary artery bypass is not the answer. J Thorac Cardiovasc Surg.
2007;133:456-60. Crossref
14. Ho JS, Strickman NE. Anomalous origin of the right coronary artery
from the left coronary sinus. Tex Heart Inst J. 2002;29:37-9.
15. Page HL Jr, Engel HJ, Campbell WB, Thomas CS Jr. Anomalous
origin of the left circumflex coronary artery. Recognition,
angiographic demonstration and clinical significance. Circulation.
1974;50:768-73. Crossref
16. Bhatia T, Kapoor A, Kumar S. Revisiting the angiographic “dot”
sign: a useful clue to diagnose anomalous origin of coronary
arteries. 8 Apr 2013. Available from: https://www.cathlabdigest.
com/Revisiting-Angiographic-%E2%80%9CDot%E2%80%9D-Sign-Useful-Clue-Diagnose-Anomalous-Origin-Coronary-Arteries. Accessed 23 Jan 2018.
17. Angelini P, Velasco JA, Ott D, Khoshnevis GR. Anomalous
coronary artery arising from the opposite sinus: descriptive features
and pathophysiologic mechanisms, as documented by intravascular
ultrasonography. J Invasive Cardiol. 2003;15:507-14.
18. Plastiras SC, Kampessi OS, Gotzamanidou M, Kastanis P.
Anomalous origin of the left circumflex artery from the right
coronary artery: a case report. Cases J. 2008;1:336. Crossref
19. Blanchard D, Ztot S, Boughalem K, Ledru F, Henry P, Battaglia
S, et al. Percutaneous transluminal angioplasty of the anomalous
circumflex artery. J Interv Cardiol. 2001;14:11-6. Crossref
20. Funabashi N, Kobayashi Y, Perlroth M, Rubin GD. Coronary
artery: quantitative evaluation of normal diameter determined with
electron-beam CT compared with cine coronary angiography initial
experience. Radiology. 2003;226:263-71. Crossref
21. Riede FN, Bulla S, Grundmann S, Werner M, Riede UN, Otto
C. Isolated hypoplastic circumflex coronary artery: a rare cause
of haemorrhagic myocardial infarction in a young athlete. Diagn
Pathol. 2013;8:91. Crossref
22. Rigatelli G, Rigatelli G. Congenital coronary artery anomalies in
the adult: a new practical viewpoint. Clin Cardiol. 2005;28:61-6. Crossref
23. De-Giorgio F, Arena V. Ostial plication: a rarely reported cause of
sudden death. Diagn Pathol. 2010;5:15. Crossref
24. De-Giorgio F, Grassi VM, Vetrugno G, Arena V. Sudden death in
a young female with an under-recognised coronary anomaly. Diagn
Pathol. 2013;8:41. Crossref
25. Menke DM, Waller BF, Bless JE. Hypoplastic coronary arteries
and high takeoff position of the right coronary ostium. A fetal
combination of congenital coronary artery anomalies in an amateur
athlete. Chest. 1985;88:299-301. Crossref
26. Tomimatu H, Sawada Y, Nakazawa M, Takao A, Hiroe M. Report
of a case of the hypoplastic left coronary artery with ostial stenosis
due to abnormal endothelial ridge. Ped Cardiol Cardiac Surg.
1987;2:329-35.
27. Fraisse A, Quilici J, Canavy I, Savin B, Aubert F, Bory M.
Myocardial infarction in children with hypoplastic coronary
arteries. Circulation. 2000;101:1219-22. Crossref
28. De Giorgio F, Abbate A, Stigliano E, Capelli A, Arena V.
Hypoplastic coronary artery disease causing sudden death. Report
of two cases and review of the literature. Cardiovasc Pathol.
2010;19:e107-11. Crossref
29. Mittal SR, Maheshwari M. Absent left circumflex artery and
unusual dominant right coronary artery. J Assoc Physicians India.
2008;56:711.
30. Kayalar N, Burkhart HM, Dearani JA, Cetta F, Schaff HV.
Congenital coronary anomalies and surgical treatment. Congenit
Heart Dis. 2009;4:239-51. Crossref
31. Liu Y, Lu X, Xiang FL, Poelmann RE, Gittenberger-de Groot AC,
Robbins J, et al. Nitric oxide synthase–3 deficiency results in
hypoplastic coronary arteries and postnatal myocardial infarction.
Eur Heart J. 2014;35:920-31. Crossref
32. Arjmand Shabestari A, Azma R, Nourmohammad A, Shakiba M.
Systolic compression of a myocardial bridged coronary artery and
its morphologic characteristics: a combination study of computed
tomography angiography and invasive angiography. Iran J Radiol.
2016;13:e31647. Crossref
33. Hazirolan T, Canyigit M, Karcaaltincaba M, Dagoglu MG,
Akata D, Aytemir K, et al. Myocardial bridging on MDCT. AJR
Am J Roentgenol. 2007;188:1074-80. Crossref
34. Kini S, Bis KG, Weaver L. Normal and variant coronary arterial
and venous anatomy on high-resolution CT angiography. AJR Am
J Roentgenol. 2007;188:1665-74. Crossref
35. Parikh NI, Honeycutt EF, Roe MT, Neely M, Rosenthal EJ,
Mittleman MA, et al. Left and codominant coronary artery
circulations are associated with higher in-hospital mortality among
patients undergoing percutaneous coronary intervention for acute
coronary syndromes: report from the National Cardiovascular
Database Cath Percutaneous Coronary Intervention (CathPCI)
Registry. Circ Cardiovasc Qual Outcomes. 2012;5:775-82. Crossref