Nasopharyngeal Carcinoma Recurrence with Orbital Metastasis through the Nasolacrimal Duct: Uncommon Presentation of a Common Phenomenon
CASE REPORT
Nasopharyngeal Carcinoma Recurrence with Orbital Metastasis through the Nasolacrimal Duct: Uncommon Presentation of
a Common Phenomenon
KTF Ng1, WH Luk1, WT Ngai2
1 Department of Radiology, Princess Margaret Hospital, Laichikok, Hong Kong
2 Department of Nuclear Medicine, Pamela Youde Nethersole Eastern Hospital, Chai Wan, Hong Kong
Correspondence: Dr KTF Ng, Department of Radiology, Princess Margaret Hospital, Laichikok, Hong Kong. Email: h0440257@gmail.com
Submitted: 5 Jun 2019; Accepted: 9 Aug 2019.
Contributors: All authors contributed to the concept and design of study, acquisition of data, interpretation of data, drafting of the manuscript,
and critical revision of the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved
the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: The authors have no conflicts of interest to disclose.
Funding/Support: This case report received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics Approval: The patient was treated in accordance with the Declaration of Helsinki. Verbal consent was obtained from the patient.
INTRODUCTION
Nasopharyngeal carcinoma (NPC) is one of the
most common malignancies with the tenth highest
mortality of all malignancies in Hong Kong in 2016.[1]
Despite the emergence of more advanced treatments
such as intensity-modulated radiotherapy, tumour
recurrence is still encountered. The presentation of
NPC recurrence is known to be highly variable. We
report an uncommon presentation with metastasis
through the nasolacrimal duct detected on positron
emission tomography–computed tomography
(PET-CT).
HISTORY
A 44-year-old man was diagnosed with undifferentiated
NPC in July 2015 (Figure 1). He first presented with
nasal obstruction, postnasal dripping with blood stained
saliva, and self-palpated neck mass for 2 months.
Nasoendoscopy revealed a left nasopharyngeal mass and
biopsy confirmed an undifferentiated NPC. Ultrasound
of the neck revealed multiple cervical lymph nodes.
PET-CT and magnetic resonance imaging scans showed
a corresponding hypermetabolic tumour at the left nasopharynx with involvement of the left parapharyngeal
space, pterygoid base, and prevertebral muscles. Multiple
hypermetabolic left cervical and supraclavicular lymph
nodes were also detected. Initial tumour staging was
cT3N2.
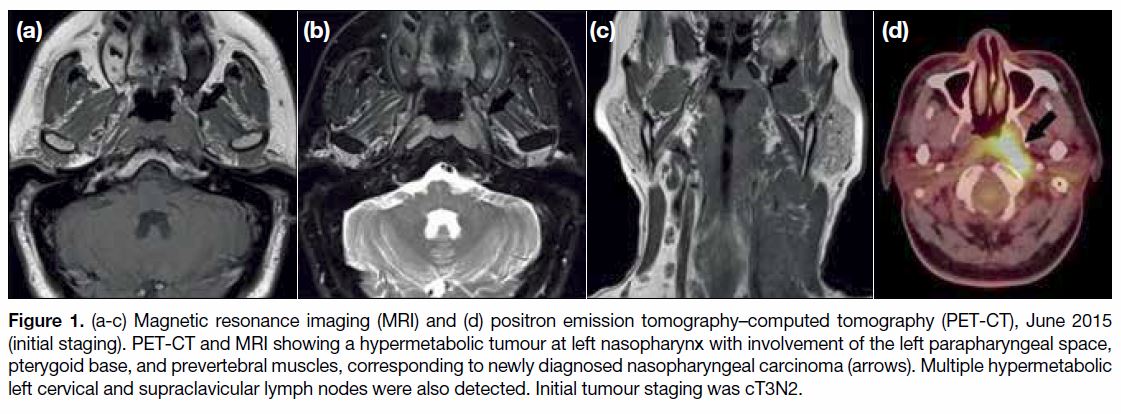
Figure 1. (a-c) Magnetic resonance imaging (MRI) and (d) positron emission tomography–computed tomography (PET-CT), June 2015
(initial staging). PET-CT and MRI showing a hypermetabolic tumour at left nasopharynx with involvement of the left parapharyngeal space,
pterygoid base, and prevertebral muscles, corresponding to newly diagnosed nasopharyngeal carcinoma (arrows). Multiple hypermetabolic
left cervical and supraclavicular lymph nodes were also detected. Initial tumour staging was cT3N2.
The patient was commenced on chemoradiotherapy. A
total of six cycles of cisplatin and radiation therapy at a
dose of 61.48 Gy in 29 fractions were given. Subsequent
nasopharyngoscopy showed no residual tumour and
biopsy was negative for malignancy. Subsequent
adjuvant chemotherapy of three cycles of cisplatin-fluorouracil
was completed in December 2015.
During a follow-up visit in July 2017, multiple enlarged
cervical lymph nodes were detected. Magnetic resonance
imaging and PET-CT scans of the neck and nasopharynx
in July 2017 (Figure 2) showed prominent soft tissue
lesions with hypermetabolism over the anterior nasal
cavity, left nasopharynx, left tonsil, and bilateral cervical
lymph nodes. Subsequent upper endoscopy detected
corresponding friable soft tissue masses in the left nasal
cavity, as well as a left nasopharyngeal mass involving the left roof, lateral wall, and left choana. Biopsy of both
masses confirmed recurrent undifferentiated NPC.
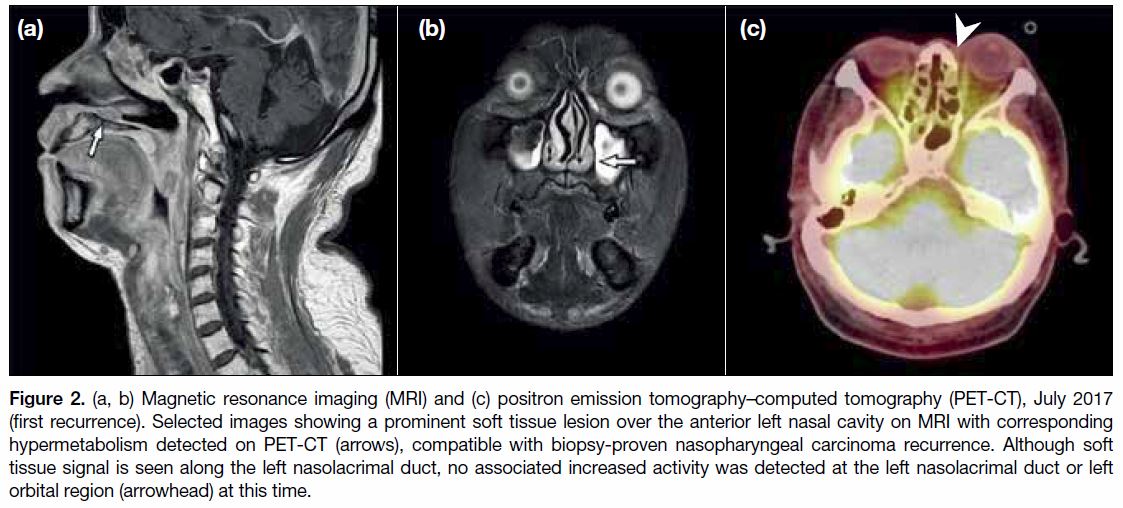
Figure 2. (a, b) Magnetic resonance imaging (MRI) and (c) positron emission tomography–computed tomography (PET-CT), July 2017
(first recurrence). Selected images showing a prominent soft tissue lesion over the anterior left nasal cavity on MRI with corresponding
hypermetabolism detected on PET-CT (arrows), compatible with biopsy-proven nasopharyngeal carcinoma recurrence. Although soft
tissue signal is seen along the left nasolacrimal duct, no associated increased activity was detected at the left nasolacrimal duct or left
orbital region (arrowhead) at this time.
The patient declined surgery or repeat radiotherapy so
second-line chemotherapy was offered. A total of five
cycles of gemcitabine-cisplatin were given, switched
to gemcitabine-carboplatin for two more cycles due
to hearing loss. The patient showed an initial partial
response. Serologically, Epstein-Barr virus (EBV) DNA
titre in February 2018 had reduced to an undetectable
level from a pretreatment baseline of 765 copies/mL.
Follow-up CT in March 2018 showed a less conspicuous
soft tissue thickening at the left nasopharynx and
oropharynx with interval resolution of the nasal floor
soft tissue lesion. Bilateral cervical lymphadenopathy
also showed interval shrinkage.
However, the EBV DNA titre showed a rebound level of
52 copies/mL in November 2018, suspicious of disease
progression. The patient also complained of a new
1.5-cm firm mass at the left nasal bridge just medial to
the left orbit and enlarging over the last month. The
patient was referred to our centre for PET-CT to
determine disease progress.
Follow-up PET-CT in December 2018 (Figure 3)
revealed a markedly hypermetabolic tumour centred at
the anterior floor of the left nasal cavity with involvement
of the nasal septum and right anterior nasal cavity. The
hypermetabolism showed further superior extension
along the left nasolacrimal duct that was mildly expanded
by soft tissue, and reached a soft tissue prominence at
the left side of the nasal bridge, compatible with the clinically detected periorbital mass. This was suggestive
of the biopsy-proven nasal cavity NPC showing direct
extension into the left orbit through the inferior nasal
meatus and nasolacrimal duct. Hypermetabolic nodal lesions were also noted at bilateral parotid, submental,
and upper cervical regions, likely representing nodal
recurrence. Overall imaging features were compatible
with disease progression.
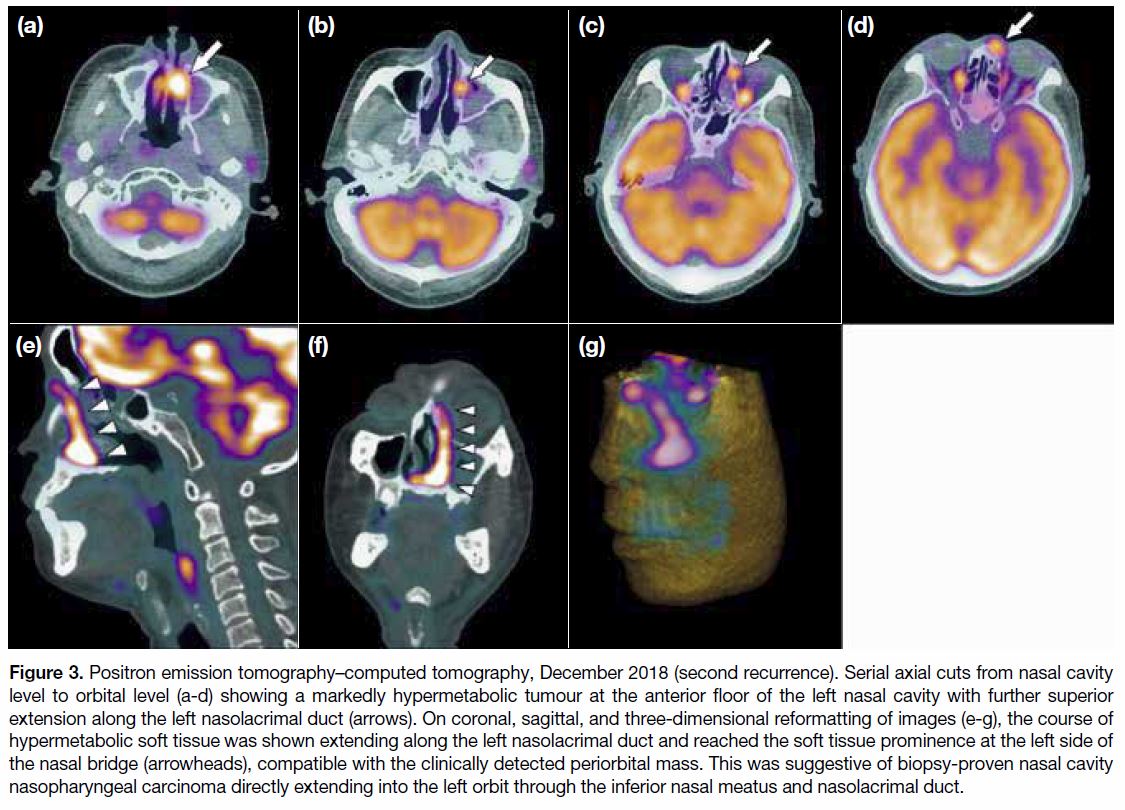
Figure 3. Positron emission tomography–computed tomography, December 2018 (second recurrence). Serial axial cuts from nasal cavity
level to orbital level (a-d) showing a markedly hypermetabolic tumour at the anterior floor of the left nasal cavity with further superior
extension along the left nasolacrimal duct (arrows). On coronal, sagittal, and three-dimensional reformatting of images (e-g), the course of
hypermetabolic soft tissue was shown extending along the left nasolacrimal duct and reached the soft tissue prominence at the left side of
the nasal bridge (arrowheads), compatible with the clinically detected periorbital mass. This was suggestive of biopsy-proven nasal cavity
nasopharyngeal carcinoma directly extending into the left orbit through the inferior nasal meatus and nasolacrimal duct.
In view of disease progression, the patient was offered
palliative chemotherapy with capecitabine. The medial
orbital mass showed progressive flattening on subsequent
clinical follow-ups and showed interval resolution on
follow-up CT after 3 months (Figure 4).
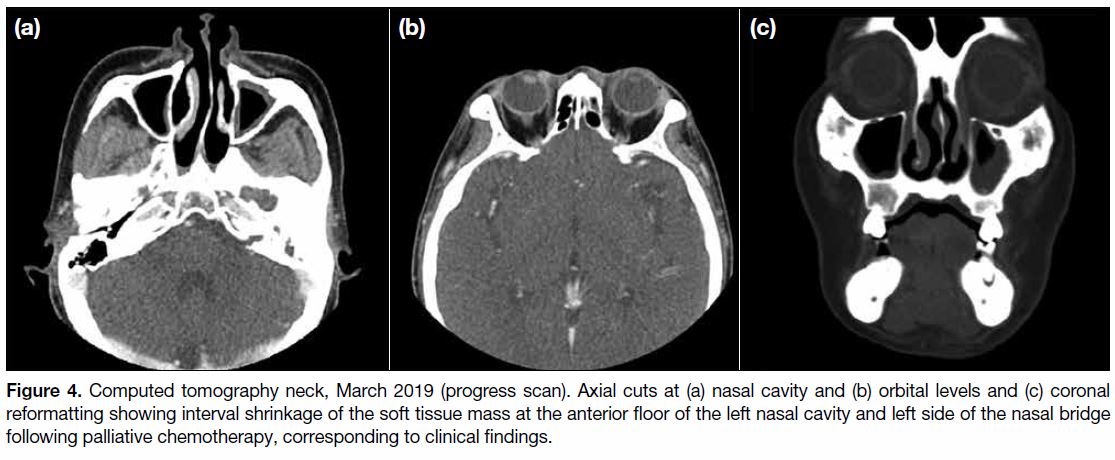
Figure 4. Computed tomography neck, March 2019 (progress scan). Axial cuts at (a) nasal cavity and (b) orbital levels and (c) coronal
reformatting showing interval shrinkage of the soft tissue mass at the anterior floor of the left nasal cavity and left side of the nasal bridge
following palliative chemotherapy, corresponding to clinical findings.
DISCUSSION
Although skull base infiltration of NPC through the
neuroforamen or potential spaces is a well-known
phenomenon, orbital infiltration is uncommon. It has
been reported by Luo et al[2] and Colaco et al[3] that the most
common pathway for NPC infiltration into the orbit is
via the pterygopalatine fossa and inferior orbital fissure,
followed by via ethmoid sinus and sphenoid sinus,
reaching the orbital apex. Common orbital metastatic
tumours often present with a rather abrupt onset of
diplopia, blurred vision, pain, and occasionally a visible
lump. Examination may disclose proptosis, displacement
of the globe, blepharoptosis, and a visible or palpable
mass.[4] NPC infiltration of the anteromedial corner of the
orbit, probably through the nasolacrimal duct, is a rare
phenomenon with only a few reported cases.
In our patient, known anterior nasal cavity tumour
recurrence was biopsy-proven. On three-dimensional
reformatting of images, hypermetabolic soft tissue was
clearly shown extending along the nasolacrimal duct,
connecting the hypermetabolic anterior nasal cavity
mass and medial orbital mass. This is evidence of the
possible NPC invasion route to the anterior orbit through
the nasolacrimal duct. A similar case was reported by
Amrith5 of a 59-year-old woman with NPC recurrence
at the anterior nasal cavity who reported left orbital
swelling with bloody tears. On follow-up CT, a soft
tissue lesion was seen infiltrating bilateral nasolacrimal
ducts from nasal cavity masses, connected superiorly
to the anteromedial mass in the left orbit. Biopsy of
the orbital mass was compatible with NPC recurrence.
Amrith[5] also reported another case of recurrent NPC in
a 33-year-old man 4 years after initial radiotherapy. He
presented with a medial orbital mass and tearing. On
CT scan, bilateral lacrimal sac masses were seen with
extension into bilateral nasolacrimal ducts. Subsequent
biopsy of the orbital mass confirmed it to be recurrent
NPC.
Among few other reported cases of NPC recurrence
involving the nasolacrimal duct and lacrimal sac,
there have often been accompanying symptoms
related to tearing, including epiphora and bloody tears,
resembling a lacrimal sac tumour.[5] [6] [7] These symptoms may be due to obstruction of the nasolacrimal duct and
precede the occurrence of medial orbital mass. Prompt
investigation such as early progress PET-CT or EBV
DNA titre screening may help early detection of tumour
progression.[8] Early initiation of second-line or palliative
treatment may still offer symptomatic relief as in our
patient.
It has been shown by Li et al[9] that the probability of
NPC invasion into structures further away from the
nasopharynx, such as the paranasal sinuses or orbital
apex, is significantly higher in recurrent disease than in
primary disease. One possible explanation suggested by
the authors is that tumour cells of subclinical lesions in
low-dose radiotherapy areas receive mostly sublethal
damage and survive, then continue to split and lead to
tumour recurrence; another explanation is that patients
susceptible to NPC are prone to recurrence, but the
normal structure of the areas adjacent to the nasopharynx
have been destroyed by high-intensity rays during the
first treatment, with the local blood supply reduced and
unfavourable for tumour growth, so the tumour relocates
and occurs far away from the nasopharynx. Whichever
the case, close attention should be paid when examining
follow-up studies, not only at the primary tumour site
at the nasopharynx, but at marginal irradiation zones
peripheral to the nasopharynx where recurrence may
also occur.
CONCLUSION
We report a patient with NPC recurrence with an
uncommon presentation that involved orbital metastasis
from the nasal cavity through the nasolacrimal duct.
Radiological evidence was revealed on a PET-CT
scan. Careful correlation with early clinical symptoms
and radiological findings may allow early detection
of uncommon tumour recurrence. Subsequent early
initiation of second-line or palliative treatment may
improve treatment results and enhance patient care.
REFERENCES
1. Hong Kong Cancer Registry, Hospital Authority, Hong Kong SAR
Government. Available from: http://www3.ha.org.hk/cancereg/topten.html. Accessed 8 May 2019.
2. Luo CB, Teng MM, Chen SS, Lirng JF, Guo WY, Chang T. Orbital
invasion in nasopharyngeal carcinoma: evaluation with computed
tomography and magnetic resonance imaging. Zhonghua Yi Xue
Za Zhi (Taipei). 1998;61:382-8.
3. Colaco RJ, Betts G, Donne A, Swindell R, Yap BK, Sykes AJ,
et al. Nasopharyngeal carcinoma: a retrospective review of
demographics, treatment and patient outcome in a single centre.
Clin Oncol (R Coll Radiol) 2013;25:171-7. Crossref
4. Shin SC, Hong SL, Lee CH, Cho KS. Orbital metastasis as
the primary presentation of nasopharyngeal carcinoma. Braz J Otorhinolaryngol. 2016;82:614-7. Crossref
5. Amrith S. Antero-medial orbital masses associated with
nasopharyngeal carcinoma. Singapore Med J. 2002;43:97-9.
6. Huang TT, Chen PR, Hsu YH, Sheen TS, Chang YL, Hsu LP.
Nasopharyngeal carcinoma invading the lacrimal apparatus — a
case report. Tzu Chi Med J. 2005;17:349-52.
7. Sia KJ, Tang IP, Kong CK, Tan TY. Nasolacrimal relapse of
nasopharyngeal carcinoma. J Laryngol Otol. 2012;126:847-50. Crossref
8. Wang WY, Twu CW, Lin WY, Jiang RS, Liang KL, Chen KW,
et al. Plasma Epstein-Barr virus DNA screening followed by
18F-fluoro-2-deoxy-D-glucose positron emission tomography in
detecting posttreatment failures of nasopharyngeal carcinoma.
Cancer. 2011;117:4452-9. Crossref
9. Li JX, Lu TX, Huang Y, Han F. Clinical characteristics of
recurrent nasopharyngeal carcinoma in high-incidence area.
ScientificWorldJournal 2012;2012:719754. Crossref