Current Strategies and Recent Advances in Gynaecological Oncology Imaging
REVIEW ARTICLE CME
Current Strategies and Recent Advances in Gynaecological
Oncology Imaging
EMF Wong1, AYT Lai1, EYP Lee2
1 Department of Radiology, Pamela Youde Nethersole Eastern Hospital, Hong Kong
2 Department of Diagnostic Radiology, The University of Hong Kong, Hong Kong
Correspondence: Dr EMF Wong, Department of Radiology, Pamela Youde Nethersole Eastern Hospital, Hong Kong. Email: esthermfwong@gmail.com
Submitted: 27 Jan 2019; Accepted: 13 Mar 2019.
Contributors: All authors designed the study, acquired the data, analysed the data, drafted the manuscript, and critically revised the manuscript
for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for publication, and
take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics Approval: Patients were treated in accordance with the Declaration of Helsinki. All patients provided informed consent for all treatments
and procedures.
Acknowledgement: We would like to thank Dr Amy TY Chang and Dr Rebecca MW Yeung from the Department of Clinical Oncology, Pamela
Youde Nethersole Eastern hospital for contributing to the figures of magnetic resonance imaging–guided brachytherapy.
Abstract
Imaging is now a crucial tool in the management of gynaecological cancers to optimise clinical outcomes. This review
provides an update on the current role and future trends of imaging in cervical, endometrial, and ovarian cancers.
Modern imaging protocols, post-processing techniques, functional imaging modalities and reporting systems are
discussed in the setting of staging and guiding of treatment decisions.
Key Words: Endometrial neoplasms; Genital neoplasms, female; Ovarian neoplasms; Uterine cervical neoplasm
中文摘要
婦科腫瘤影像學的當前策略和最新進展
黃文鳳、黎爾德、李燕蘋
影像學是現時婦科腫瘤治療優化臨床轉歸的關鍵工具。本文描述當前子宮頸癌、子宮內膜癌和卵巢
癌影像學的角色和未來趨勢。從腫瘤分期及指導治療決策的角度討論現代影像學的掃描方案、後處
理技術、功能性影像學方法和報告系統。
INTRODUCTION
Imaging in gynaecological oncology has been
revolutionised due to the advances in magnetic resonance
imaging (MRI) and functional imaging in the past
decades. Imaging methodologies have been integrated
into disease diagnosis, staging, and treatment. The aim
of this review is to provide an update on the current role
and future trends of imaging in cervical, endometrial
and ovarian cancers. Modern imaging protocols, post-processing
techniques, functional imaging modalities
and reporting systems are discussed in the setting of
staging and guiding of treatment decisions.
CERVICAL CANCER
Cervical cancer is the seventh most common cancer in
Hong Kong with about 500 new diagnoses every year. As
there is no territory-wide screening programme in Hong
Kong and the free human papillomavirus vaccination
programme started only in 2019, the prevalence of
cervical cancer is not expected to fall until a decade
later. More than half of the patients present with disease
stage II or above.[1] Precise staging at diagnosis is essential
in order to optimise treatment. Early cervical cancer
(International Federation of Gynecology and Obstetrics
[FIGO] stage IIA or below) can be treated with radical
hysterectomy with or without pelvic lymphadenectomy.
For selected cases of early cancer, fertility-sparing
surgery can be offered to patients who have not yet
completed their families.[2] [3] Locally advanced disease is
treated by chemoradiotherapy.[4] [5]
The 2018 FIGO revised staging incorporated imaging
findings into the staging system for the first time. Prior
to the revision, FIGO staging for cervical cancer was
entirely based on clinical and surgical findings. The
revised system stated that imaging and pathology findings
can be used to supplement tumour size and extent at all
stages. In addition, there was a newly introduced “stage
IIIC” for lymph node involvement, which is further
subdivided to IIIC1 (pelvic lymph node) and IIIC2
(para-aortic lymph node). A small letter “r” for imaging
and a “p” for pathology is used as a suffix to the stage to
denote the method of lymph node detection.[6]
MRI is the imaging modality of choice in evaluating local
disease extent given its exquisite soft tissue resolution.[6]
The presence of parametrial invasion, which upstages
disease to at least FIGO IIB and classifies the disease
as locally advanced, is best identified by MRI, with
sensitivity and specificity of 73% and 93%, respectively.[7]
T1-weighted images (T1WI) can be useful to visualise haematometra, lymphadenopathy, and bone metastases,
and should be incorporated in the MRI protocol
(Table 1).[8]
Table 1. Sample scanning protocol for common gynaecological malignancy.
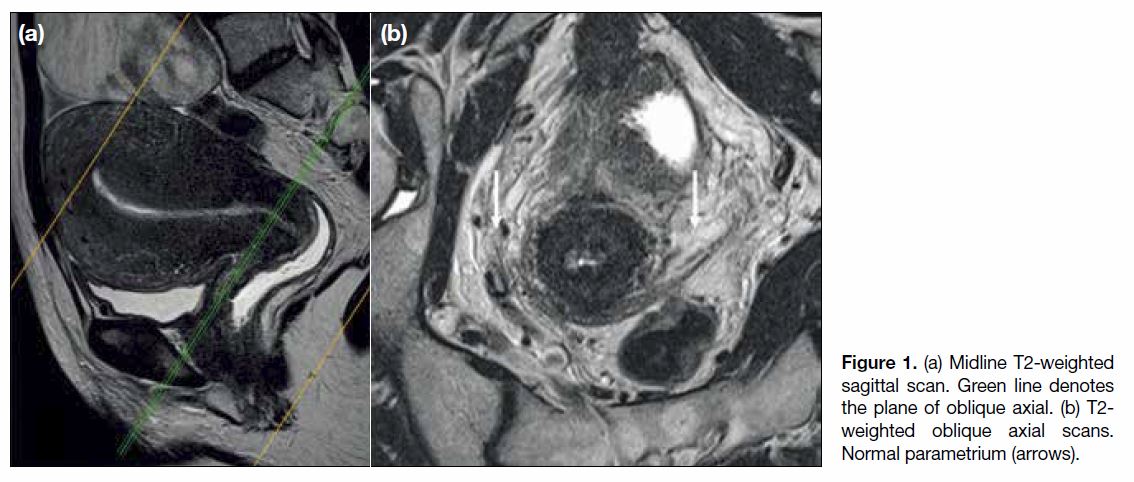
To optimise the assessment of the parametrium, oblique
axial images perpendicular to the long axis of the cervix
are essential (Figure 1).[8] An intact hypointense fibrous
stromal ring on T2-weighted images (T2WI) has high
negative predictive value for parametrial invasion. Signs
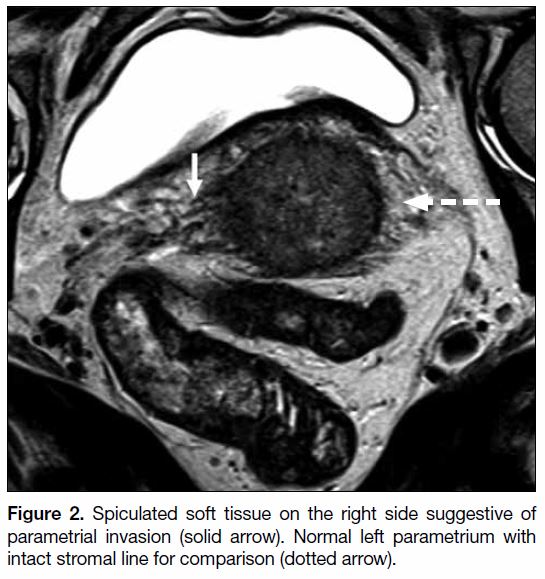
of parametrial invasion include T2 intermediate signal in
the parametrium with spiculated borders and encasement
of periuterine vessels (Figure 2).[9] [10] Local invasion
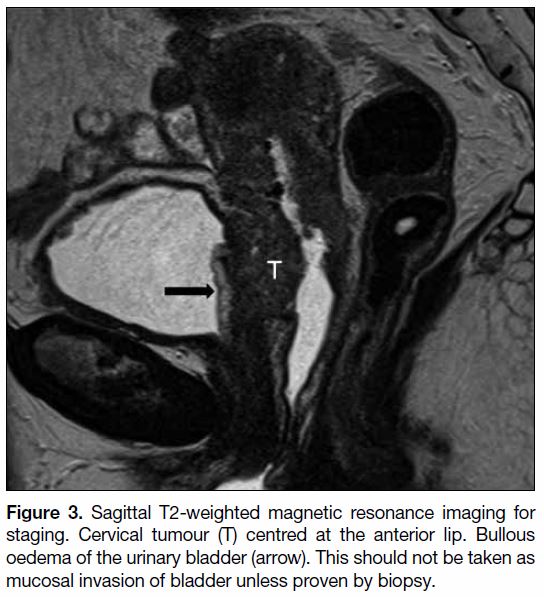
is well depicted on MRI. Abnormality of the urinary
bladder on MRI is not uncommon.[11] Bullous oedema of
the urinary bladder, which is seen on T2WI as markedly
hyperintense thickening of the urinary mucosa, cannot
be distinguished from mucosal involvement (Figure 3).[12]
Cystoscopy and biopsy are needed to confirm mucosal
involvement.
Figure 1. (a) Midline T2-weighted
sagittal scan. Green line denotes
the plane of oblique axial. (b) T2-weighted oblique axial scans.
Normal parametrium (arrows).
Figure 2. Spiculated soft tissue on the right side suggestive of
parametrial invasion (solid arrow). Normal left parametrium with
intact stromal line for comparison (dotted arrow).
Figure 3. Sagittal T2-weighted magnetic resonance imaging for
staging. Cervical tumour (T) centred at the anterior lip. Bullous
oedema of the urinary bladder (arrow). This should not be taken as
mucosal invasion of bladder unless proven by biopsy.
Endovaginal ultrasound is an inexpensive method for
visualisation of the vaginal wall and outer contour of
the cervix.[13] [14] Intravenous contrast gives no significant
improvement in diagnostic accuracy.[15]
The presence of metastatic pelvic lymphadenopathy
is an important adverse prognostic indicator[16] and has
been revised in the latest FIGO 2018 staging system.[6]
Compared to the 2014 staging system, the presence of
pelvic and paraaortic lymphadenopathy now upgrades
the disease to stage IIIC1 and IIIC2, respectively.
Conventionally, size and shape criteria were used to
differentiate metastatic from benign nodes. Morphology
indicators such as lobulated or spiculated borders are
highly specific but not sensitive. Size criteria vary
in accuracy, sensitivity and specificity depending on
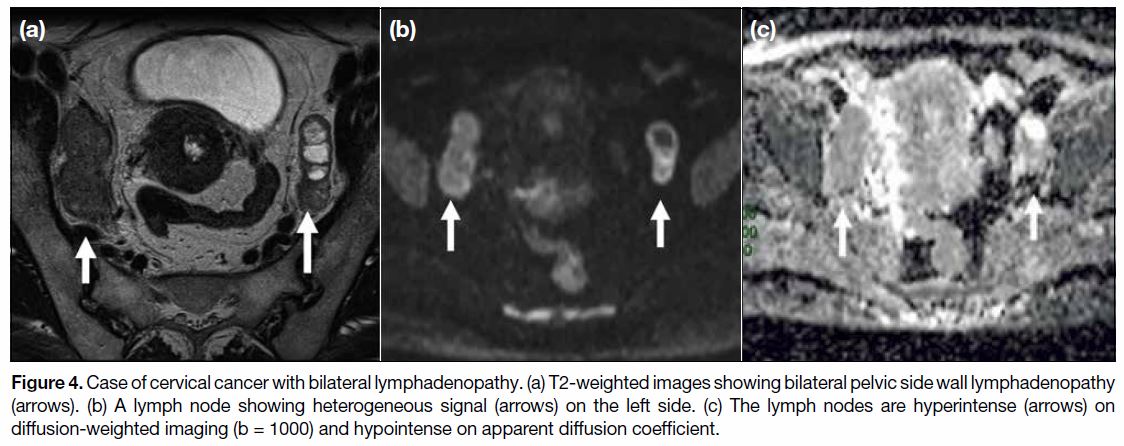
the cut-off thresholds.[17] Diffusion-weighted imaging
(DWI) in conjunction with T2WI increases the ability
of MRI to differentiate benign from malignant nodes
(Figure 4). A meta-analysis by Shen et al[18] involving
15 studies found a pooled sensitivity and specificity
of 85% and 84% for DWI. The analysed studies
were, however, heterogeneous due to a lack of
optimal standardised DWI protocol among studies.
Metabolic imaging based on 18-fluorodeoxyglucose
positron emission tomography/computed tomography
(FDG-PET/CT) adds to the diagnostic accuracy of nodal
involvement. Meta-analysis showed that FDG-PET/CT
had a pooled sensitivity and specificity of 82% and
95% in determining pelvic lymph node involvement,
compared to 56% and 91% respectively by MRI.[19] With these, FDG-PET/CT offers higher specificity while
DWI-MRI is more sensitive in identifying nodal
involvement in cervical cancer.[20]
Figure 4. Case of cervical cancer with bilateral lymphadenopathy. (a) T2-weighted images showing bilateral pelvic side wall lymphadenopathy
(arrows). (b) A lymph node showing heterogeneous signal (arrows) on the left side. (c) The lymph nodes are hyperintense (arrows) on
diffusion-weighted imaging (b = 1000) and hypointense on apparent diffusion coefficient.
Fertility Preservation
Fertility sparing treatment such as conisation and
trachelectomy can be alternatives to radical surgery
in disease of FIGO 1B1 or below. Selection of cases
requires a multidisciplinary approach and MRI plays a
key role in this.[21] [22]
In addition to parametrial assessment, preoperative
MRI gives accurate delineation of the craniocaudal
extent of tumour, especially with endocervical cancer
and its relationship to the internal os. Measurement on
MRI correlates well with pathological measurement.
A distance of 5 mm to 10 mm between the tumour and
the internal os puts the patient at high risk for local
recurrence after surgery.[23] [24] Other factors to consider
include a maximum tumour size of <2 cm with sufficient
cervical length after resection (at least 1 cm), absence
of deep cervical stromal invasion, and absence of lymph
node involvement.[25]
Image-guided Brachytherapy
The GEC-ESTRO (The Groupe Européen de
Curiethérapie and the European SocieTy for
Radiotherapy & Oncology) guidelines recommend
MRI-guided brachytherapy as a component of the
radiotherapy in locally advanced cervical cancer treated
with chemoradiotherapy (FIGO IB-IVA).[26] Studies
showed that it improved local control and overall survival as compared with two-dimensional radiation planning of
previous generation[27] [28] Brachytherapy was historically
planned using orthogonal radiographs. From then it
evolved to CT-based three-dimensional planning in the
1990’s. Compared with traditional CT/X-ray–guided
approaches, MRI gives superior contrast delineation
and thus makes better tumour delineation from normal
tissue.[29] [30] Three-dimensional contouring allows dose
escalation to residual disease while sparing the organs
at risk, hence improves local control and reduces
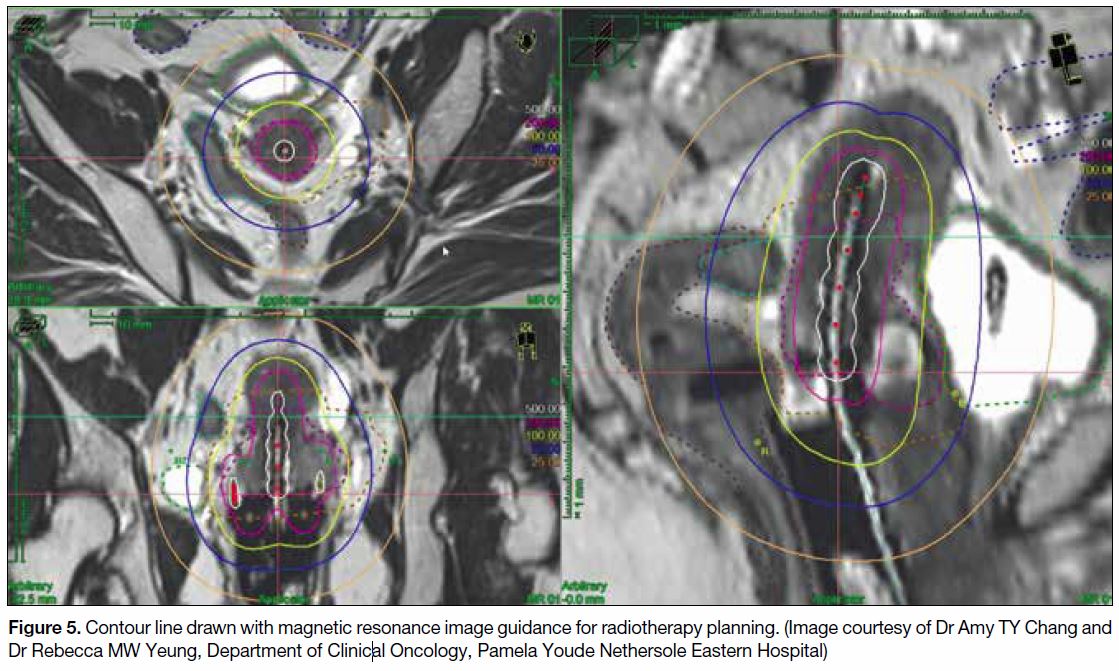
complication rate (Figure 5).
Figure 5. Contour line drawn with magnetic resonance image guidance for radiotherapy planning. (Image courtesy of Dr Amy TY Chang and
Dr Rebecca MW Yeung, Department of Clinical Oncology, Pamela Youde Nethersole Eastern Hospital)
Brachytherapy is performed following whole pelvis
irradiation. The regimen of brachytherapy varies.
Planning MRI for brachytherapy is performed
immediately after applicator insertion. Logistics on how
to minimise transfer time between operating theatre
and MRI suite, and to the radiation suite have to be
worked out, in addition to the appointment booking and
coordination among different units.[31]
Scanning time is an important factor to consider with
the applicator in situ. Shorter scanning time minimises patient discomfort and facilitates appointment booking
in a busy radiology unit. According to GEC-ESTRO
recommendations, mandatory sequences with an
applicator are T2WI acquired in axial, coronal and
sagittal planes through the cervix (Figure 6) to delineate
the urinary bladder, uterus, and rectum.[29] T2WI
orthogonal to the MRI table can be added if required for
treatment planning.[32] DWI and post-contrast sequences
are non-essential for this purpose.
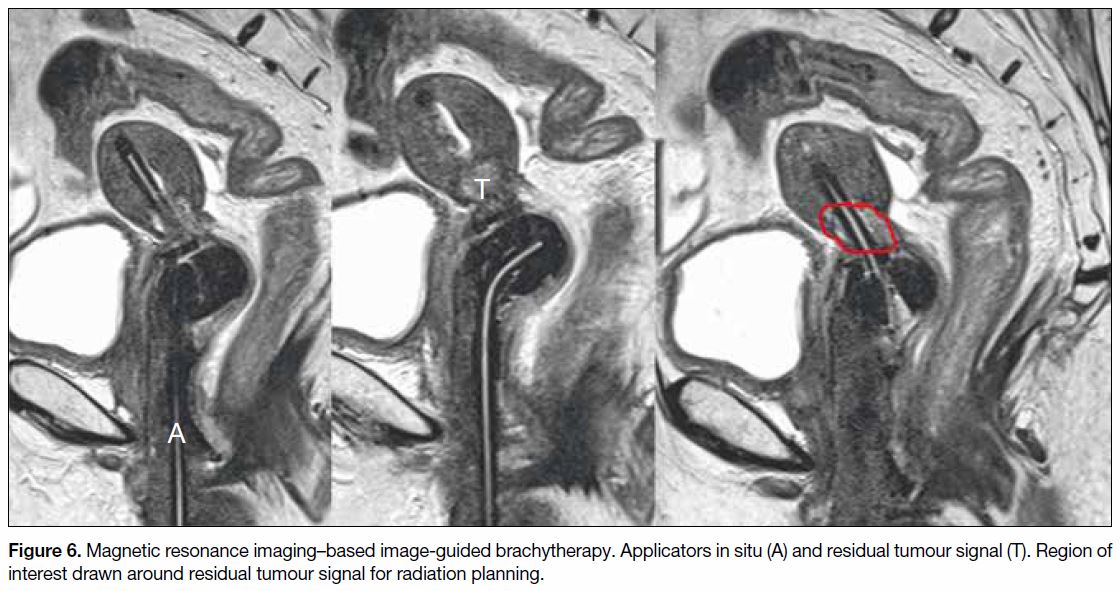
Figure 6. Magnetic resonance imaging–based image-guided brachytherapy. Applicators in situ (A) and residual tumour signal (T). Region of
interest drawn around residual tumour signal for radiation planning.
ENDOMETRIAL CANCER
The global incidence of endometrial cancer is on the rise,
with a postulated association with increased exogenous
hormones use, endogenous hormone exposure, and
obesity.[33]
The presence of deep myometrial invasion, defined by
tumour invasion beyond half of the myometrial thickness,
is positively correlated with pelvic lymphadenopathy and adverse disease prognosis.[34] [35] The FIGO staging divides
stage I disease into IA and IB, for superficial (<50%
thickness of myometrium) and deep (>50% thickness of
myometrium) invasion, respectively.[36]
Lymphadenectomy in early endometrial cancer (stage I)
is controversial and may bear no survival benefit.[37] [38]
However, intermediate- and high-risk groups may benefit
from pelvic and paraaortic lymphadenectomy.[39] The
presence of deep myometrial invasion or unfavourable
histology (non-endometrioid adenocarcinoma) results
in an upgrade from low risk to intermediate/high risk.
Cervical stromal invasion is associated with increased
likelihood of pelvic lymphadenopathy[40] [41] and an adverse
prognosis.[42] [43]
Scanning Protocol and Standard of
Measurement
Most modern protocols incorporate T2, DWI, and post-gadolinium
images, either by multiphase or dynamic
contrast-enhanced (DCE) MRI. Intravenous contrast
aids tumour visualisation through increased contrast of
the tumour with normal myometrium. The endometrial
tumour shows less enhancement than normal
myometrium. Depiction of myometrial invasion is at
equilibrium phase (2 min 30 s after contrast injection).
The cervical stroma enhances later than myometrium.
Thus, invasion of cervical stroma is best assessed in
delayed phase (3-5 min after contrast injection).[15] [44]
DWI has also been used to depict deep myometrial
invasion. Evidence suggested that DWI was at least
equivalent to DCE, in detecting deep myometrial invasion
(Figure 7).[45] [46] DWI has the potential to be an alternative
to DCE in assessment of myometrial invasion, especially
when intravenous contrast injection is contraindicated.
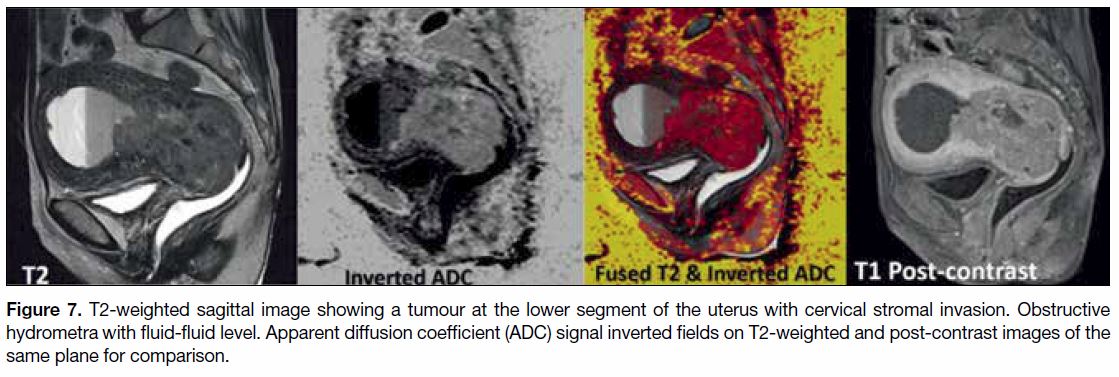
Figure 7. T2-weighted sagittal image showing a tumour at the lower segment of the uterus with cervical stromal invasion. Obstructive
hydrometra with fluid-fluid level. Apparent diffusion coefficient (ADC) signal inverted fields on T2-weighted and post-contrast images of the
same plane for comparison.
Methods of measuring depth of myometrial invasion
vary, both in radiology and histology. This is further
confounded when endometrial contour is distorted, as
commonly occurs in the presence of benign pathology
such as fibroids and adenomyosis causing irregular
endometrial-myometrial junction, and in the presence
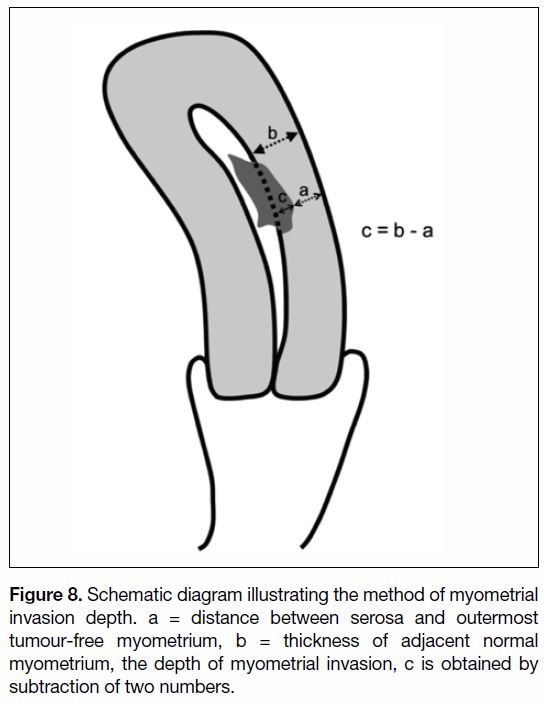
of exophytic tumour.[47] [48] Measurement by subtraction
might be a more reliable method.[49] The thickness of
adjacent uninvolved myometrium is first obtained. Then
the distance between the serosa and outermost tumour-free
myometrium is obtained. The depth of invasion is
obtained by subtraction of the two numbers (Figure 8).
This method attenuates the effect of endometrial
distortion from irregular endometrial-myometrial
junction and excludes exophytic areas from calculation.
Figure 8. Schematic diagram illustrating the method of myometrial
invasion depth. a = distance between serosa and outermost
tumour-free myometrium, b = thickness of adjacent normal
myometrium, the depth of myometrial invasion, c is obtained by
subtraction of two numbers.
Nodal Staging
Surgical staging remains the gold standard in determining
nodal status in endometrial cancer.[50] [51] MRI with
DWI showed higher sensitivity but lower specificity
than FDG-PET/CT (83% vs. 39% and 51% vs. 96%,
respectively).[52] In a meta-analysis of seven studies, the sensitivity and specificity of FDG-PET/CT in detecting
pelvic and/or paraaortic nodal metastasis were 63%
and 95%, respectively, with overall accuracy of 90%.[53]
The authors concluded that FDG-PET/CT was highly
specific but only moderately sensitive, and thus cannot
replace lymphadenectomy. Surgical staging remains
important and the decision to perform lymphadenectomy
or nodal sampling should be determined by pathological
risk factors.
OVARIAN CANCER
Adnexal Mass Characterisation
Endovaginal ultrasonography (USG) is usually the first
line of investigation for pelvic masses. Terminology
and measurements on endovaginal USG have been
standardised by the International Ovarian Tumor Analysis
group.[54] The ROMA (Risk of Ovarian Malignancy
Algorithm) score that incorporates ultrasound findings,
CA125 and HE4 levels, is useful for prediction of the
likelihood of malignancy of an adnexal mass.[55]
Adnexal lesions with benign USG features, for example,
simple anechoic cysts <5 cm in premenopausal women,
can be safely dismissed. Depending on the USG features,
some cases are safe to be followed up.[56] However, with
frankly malignant adnexal mass or a patient with a high
ROMA score, CT can be performed for disease staging
and assessment of extrapelvic spread. The vast diversity
of ovarian masses, and the wide overlap of benign and
malignant imaging features, make specific radiological
diagnosis difficult. Methods to risk stratify adnexal
lesions with quantitative and qualitative measures have
been put forward.[57] [58] MRI is useful in indeterminate
adnexal masses for further characterisation.[59] T1WI
(with and without fat saturation) is useful to detect fatty
components, mucin, and haemorrhage. Post-contrast
T1WI is important in further lesion characterisation.
T2WI detects cystic components and detailed anatomical
characteristics. Morphological features favouring
malignancy include diameter >4 cm, a complex cystic
mass with thick internal septations, thickness of the wall
>3 mm, lobulated contour, tiny amorphic calcifications,
necrosis, papillary projections, and tumour vascularity
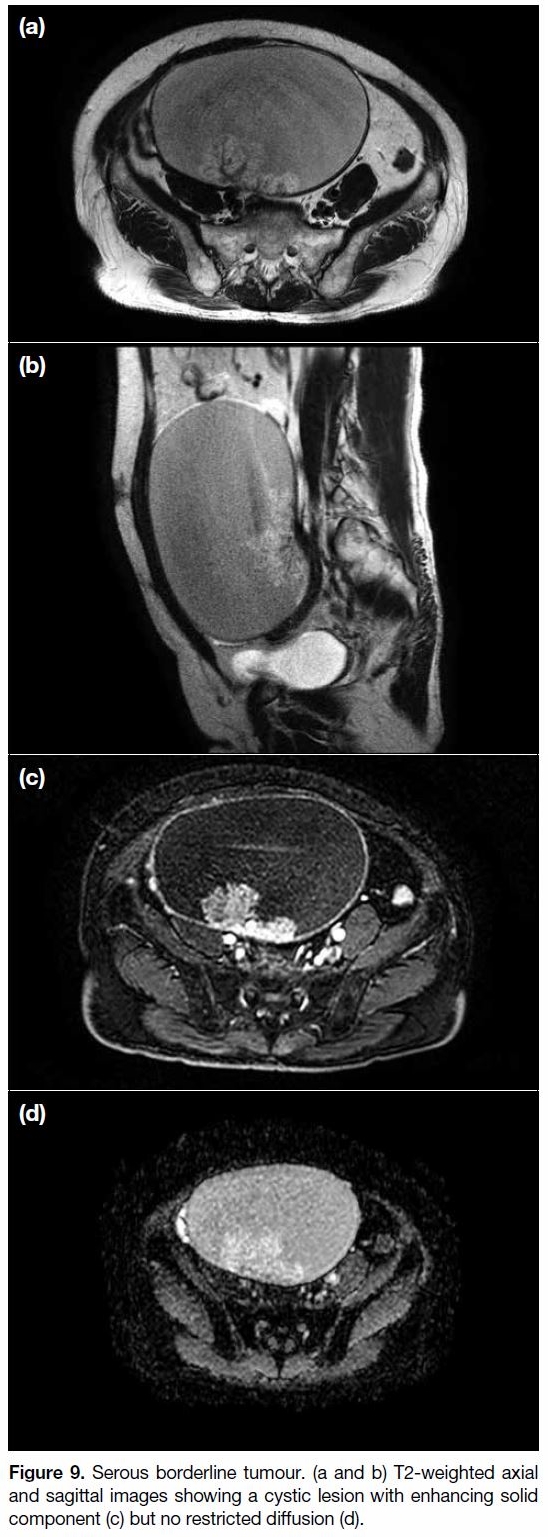
(Figure 9).[60] [61]
Figure 9. Serous borderline tumour. (a and b) T2-weighted axial
and sagittal images showing a cystic lesion with enhancing solid
component (c) but no restricted diffusion (d).
The apparent diffusion coefficient (ADC) value of the
solid portion of an adnexal mass is lower in malignant
than in benign lesions. With an ADC cut-off threshold
value, malignant lesions can be excluded with high
confidence. The ADC value could be a tool to streamline
management strategies; however, inter-vendor and
intersystem variability of ADC measurements render
cross-centre validation difficult and thus limit the
applicability of the method.[62]
In a retrospective analysis of 37 pre-operative DCE-MRI
performed for ovarian epithelial tumour, a Type 3
curve, defined by an initial rise in signal in the solid
portion of an ovarian mass steeper than the myometrium,
was present in malignant lesions and not in benign or
borderline lesions.[60] Other semi-quantitative DCE
parameters offer useful information in that the absolute
and relative maximum contrast enhancement could
identify malignant lesions with 100% sensitivity and
specificity, the but studied cohort was small (n = 26).[63]
A subsequent large-scale study with 102 patients also
suggested the usefulness of DCE-MRI in differentiating
benign from borderline and malignant tumours.[64]
Contrast enhancement can thus be considered a tool to
identify lesions that are safe to follow-up.
The role of FDG-PET/CT in ovarian lesion
characterisation is not clearly established. It has
been suggested that FDG-PET/CT could assist in
differentiating malignant from benign ovarian masses
when DCE-MRI is indeterminate.[65]
Peritoneal Disease
Ovarian malignancies often present late with
disseminated peritoneal disease. Cytoreductive surgery
followed by systemic chemotherapy is the treatment
of choice for advanced disease. The ability to achieve
complete cytoreduction is related to improved survival
rate.[66] [67] [68]
The volume of residual disease after cytoreductive surgery
is one of the most important prognostic indicators.[66] [69]
Optimal cytoreduction is defined as the largest residual
disease of <1 cm.[70] In recent years, there has been a shift
in the surgical paradigm in pursuit of a cytoreductive goal
of no gross residual disease, which has been shown to be
associated with improved progression-free and overall
survival.[71] Extensive surgical procedures are often
required to achieve complete cytoreduction or minimal
residual disease, and these can be technically challenging
in patients with disseminated tumours. Neoadjuvant chemotherapy followed by debulking surgery is an
alternative to primary cytoreductive surgery, yielding
similar outcomes.[72] Resectability criteria differ across
centres. In general, disease in the upper abdomen might
require more complex surgical procedures, including
splenectomy and diaphragmatic resection, and likely
involvement of more than one surgical specialty. The
European Society of Urogenital Radiology guidelines
suggest several negative prognostic factors for complete
cytoreduction, including deposits >2 cm in the upper
abdomen, parenchymal or subcapsular involvement
of the liver and spleen, involvement of small bowel
mesentery, and lymph node involvement above the renal
hila.[73]
Contrast-enhanced CT of the abdomen and pelvis is
currently the first-line radiological investigation for
detection of peritoneal disease. Its reported sensitivity in
detecting peritoneal metastasis in ovarian cancer ranges from 85% to 93%, but this substantially drops to 25% to
50% for subcentimetre peritoneal implants.[74]
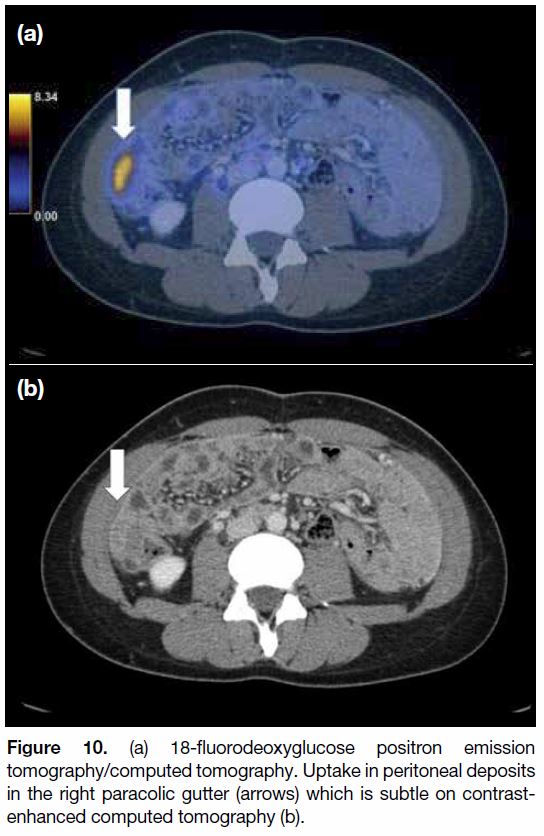
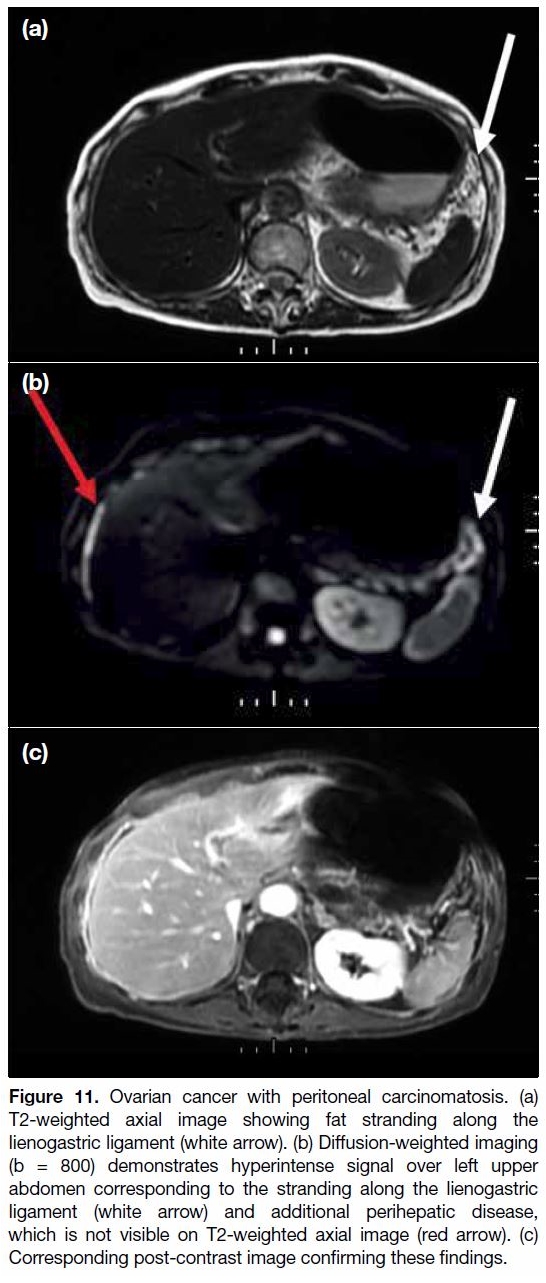
Techniques such as FDG-PET/CT and DWI enhance the
visibility of peritoneal metastases (Figures 10 and 11).[75] [76]
Figure 10. (a) 18-fluorodeoxyglucose positron emission
tomography/computed tomography. Uptake in peritoneal deposits
in the right paracolic gutter (arrows) which is subtle on contrastenhanced
computed tomography (b).
Figure 11. Ovarian cancer with peritoneal carcinomatosis. (a)
T2-weighted axial image showing fat stranding along the
lienogastric ligament (white arrow). (b) Diffusion-weighted imaging
(b = 800) demonstrates hyperintense signal over left upper
abdomen corresponding to the stranding along the lienogastric
ligament (white arrow) and additional perihepatic disease,
which is not visible on T2-weighted axial image (red arrow). (c)
Corresponding post-contrast image confirming these findings.
DWI can detect peritoneal deposits with the additional
advantage of no intravenous contrast administration. It
has reported sensitivity and specificity of up to 95% and
95%, respectively.[77] False positives from bowel content
can be reduced by using high b-value images such as
800 s/mm2. The high signal of peritoneal deposits on
high b-value DWI-MRI adds to lesion conspicuity.
FDG-PET/CT has been suggested to be more sensitive
and specific in predicting disease resectability as
compared with conventional contrast-enhanced CT
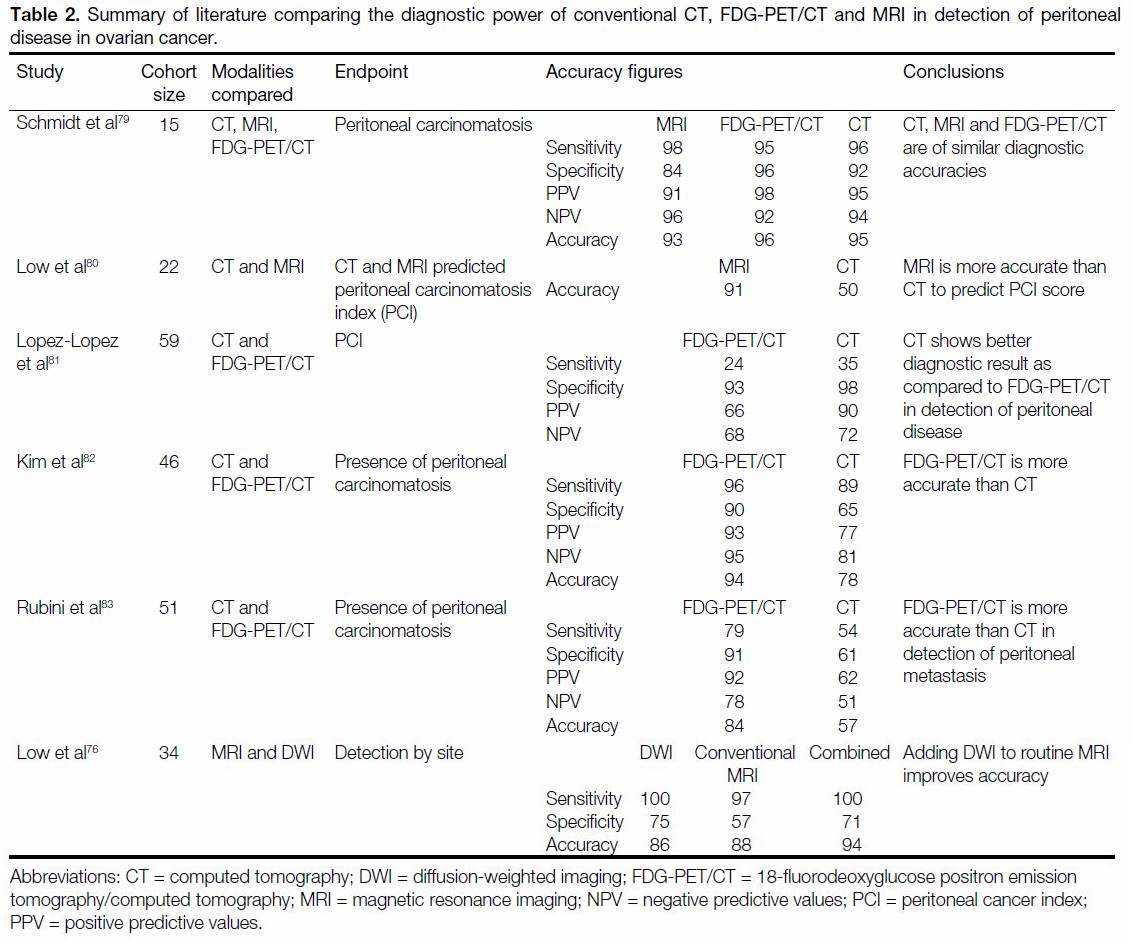
alone.[78] Table 2[76] [79] [80] [81] [82] [83] summarises current evidence of the
use of FDG-PET/CT and MRI in detection of peritoneal
disease in ovarian cancer.
Table 2. Summary of literature comparing the diagnostic power of conventional CT, FDG-PET/CT and MRI in detection of peritoneal
disease in ovarian cancer.
CONCLUSION
Imaging plays a crucial role in gynaecological oncology, from diagnosis to treatment stratification. The revised
2018 FIGO incorporates radiological findings in cervical
cancer staging. Use of MRI planning in image-guided
brachytherapy for cervical cancer improves treatment
outcome. MRI is highly accurate in depicting myometrial
invasion and cervical stromal invasion in endometrial
cancer. Functional imaging is effective for detecting
peritoneal carcinomatosis.
REFERENCES
1. Hong Kong Cancer Registry. Cervical cancer in 2015. Available
from: http://www3.ha.org.hk/cancereg/pdf/factsheet/2015/cx_2015.pdf. Accessed 1 Nov 2018.
2. Machida H, Iwata T, Okugawa K, Matsuo K, Saito T, Tanaka K,
et al. Fertility-sparing trachelectomy for early-stage cervical cancer:
A proposal of an ideal candidate. Gynecol Oncol. 2020;156:341-8. Crossref
3. Bogani G, Chiappa V, Vinti D, Somigliana E, Filippi F, Murru G,
et al. Long-term results of fertility-sparing treatment for early-stage
cervical cancer. Gynecol Oncol. 2019;154:89-94. Crossref
4. Morris M, Eifel PJ, Lu J, Grigsby PW, Levenback C, Stevens RE,
et al. Pelvic radiation with concurrent chemotherapy compared
with pelvic and para-aortic radiation for high-risk cervical cancer.
N Engl J Med. 1999;340:1137-43. Crossref
5. Rose PG, Bundy BN, Watkins EB, Thigpen JT, Deppe G,
Maiman MA, et al. Concurrent cisplatin-based radiotherapy and
chemotherapy for locally advanced cervical cancer. N Engl J Med.
1999;340:1144-53. Crossref
6. Bhatla N, Aoki D, Sharma DN, Sankaranarayanan R. Cancer of
the cervix uteri. Int J Gynaecol Obstet. 2018;143 Suppl 2:22-36. Crossref
7. Woo S, Suh CH, Kim SY, Cho JY, Kim SH. Magnetic resonance
imaging for detection of parametrial invasion in cervical cancer:
An updated systematic review and meta-analysis of the literature
between 2012 and 2016. Eur Radiol. 2018;28:530-41. Crossref
8. Balleyguier C, Sala E, Da Cunha T, Bergman A, Brkljacic B, Danza F,
et al. Staging of uterine cervical cancer with MRI: guidelines
of the European Society of Urogenital Radiology. Eur Radiol.
2011;21:1102-10. Crossref
9. Miccò M, Sala E, Lakhman Y, Hricak H, Vargas HA. Role of
imaging in the pretreatment evaluation of common gynecological
cancers. Womens Health (Lond). 2014;10:299-321. Crossref
10. Sala E, Micco M, Burger IA, Yaker D, Kollmeier MA, Goldman DA,
et al. Complementary prognostic value of pelvic magnetic
resonance imaging and whole-body fluorodeoxyglucose positron
emission tomography/computed tomography in the pretreatment
assessment of patients with cervical cancer. Int J Gynecol Cancer.
2015;25:1461-7. Crossref
11. Nam H, Huh SJ, Park W, Bae DS, Kim BG, Lee JH, et al. Prognostic
significance of MRI-detected bladder muscle and/or serosal
invasion in patients with cervical cancer treated with radiotherapy.
Br J Radiol. 2010;83:868-73. Crossref
12. Patel S, Liyanage SH, Sahdev A, Rockall AG, Reznek RH. Imaging
of endometrial and cervical cancer. Insights Imaging. 2010;1:309-28. Crossref
13. Engelaere C, Poncelet E, Durot C, Dohan A, Rousset P, Hoeffel C.
Pelvic MRI: Is endovaginal or rectal filling needed? Korean J
Radiol. 2018;19:397-409. Crossref
14. Brown MA, Mattrey RF, Stamato S, Sirlin CB. MRI of the female
pelvis using vaginal gel. AJR Am J Roentgenol. 2005;185:1221-7. Crossref
15. Sala E, Wakely S, Senior E, Lomas D. MRI of malignant
neoplasms of the uterine corpus and cervix. AJR Am J Roentgenol.
2007;188:1577-87. Crossref
16. Kwon J, Eom KY, Kim YS, Park W, Chun M, Lee J, et al. The
prognostic impact of the number of metastatic lymph nodes and a
new prognostic scoring system for recurrence in early-stage cervical
cancer with high risk factors: a multicenter cohort study (KROG
15-04). Cancer Res Treat. 2018;50:964-74. Crossref
17. Choi HJ, Kim SH, Seo SS, Kang S, Lee S, Kim JY, et al. MRI for
pretreatment lymph node staging in uterine cervical cancer. AJR
Am J Roentgenol. 2006;187:W538-43. Crossref
18. Shen G, Zhou H, Jia Z, Deng H. Diagnostic performance of
diffusion-weighted MRI for detection of pelvic metastatic lymph
nodes in patients with cervical cancer: a systematic review and
meta-analysis. Br J Radiol. 2015;88:20150063. Crossref
19. Choi HJ, Ju W, Myung SK, Kim Y. Diagnostic performance of
computer tomography, magnetic resonance imaging, and positron
emission tomography or positron emission tomography/computer
tomography for detection of metastatic lymph nodes in patients
with cervical cancer: meta-analysis. Cancer Sci. 2010;101:1471-9. Crossref
20. Liu B, Gao S, Li S. A comprehensive comparison of CT, MRI,
positron emission tomography or positron emission tomography/
CT, and diffusion weighted imaging-MRI for detecting the lymph
nodes metastases in patients with cervical cancer: a meta-analysis based on 67 studies. Gynecol Obstet Invest. 2017;82:209-22. Crossref
21. Stein EB, Hansen JM, Maturen KE. Fertility-sparing approaches
in gynecologic oncology: role of imaging in treatment planning.
Radiol Clin North Am. 2020;58:401-12. Crossref
22. Alvarez RM, Biliatis I, Rockall A, Papadakou E, Sohaib SA,
deSouza NM, et al. MRI measurement of residual cervical length
after radical trachelectomy for cervical cancer and the risk of
adverse pregnancy outcomes: a blinded imaging analysis. BJOG.
2018;125:1726-33. Crossref
23. Lakhman Y, Akin O, Park KJ, Sarasohn DM, Zheng J, Goldman DA,
et al. Stage IB1 cervical cancer: role of preoperative MR imaging
in selection of patients for fertility-sparing radical trachelectomy.
Radiology. 2013;269:149-58. Crossref
24. Noël P, Dubé M, Plante M, St-Laurent G. Early cervical carcinoma
and fertility-sparing treatment options: MR imaging as a tool
in patient selection and a follow-up modality. Radiographics.
2014;34:1099-119. Crossref
25. Rockall AG, Qureshi M, Papadopoulou I, Saso S, Butterfield N,
Thomassin-Naggara I, et al. Role of imaging in fertilitysparing
treatment of gynecologic malignancies. Radiographics.
2016;36:2214-33. Crossref
26. Mahantshetty U, Swamidas J, Khanna N, Engineer R, Merchant N,
Shrivastava S. Magnetic resonance image-based dose volume
parameters and clinical outcome with high dose rate brachytherapy
in cervical cancers—a validation of GYN GEC-ESTRO
brachytherapy recommendations. Clin Oncol (R Coll Radiol).
2011;23:376-7. Crossref
27. Derks K, Steenhuijsen JL, van den Berg HA, Houterman S,
Cnossen J, van Haaren P, et al. Impact of brachytherapy technique
(2D versus 3D) on outcome following radiotherapy of cervical
cancer. J Contemp Brachytherapy. 2018;10:17-25. Crossref
28. Potter R, Kirisits C, Fidarova EF, Dimopoulos JC, Berger D,
Tanderup K, et al. Present status and future of high-precision image
guided adaptive brachytherapy for cervix carcinoma. Acta Oncol.
2008;47:1325-36. Crossref
29. Dimopoulos JC, Petrow P, Tanderup K, Petric P, Berger D,
Kirisits C, et al. Recommendations from Gynaecological (GYN)
GEC-ESTRO Working Group (IV): Basic principles and parameters
for MR imaging within the frame of image based adaptive cervix
cancer brachytherapy. Radiother Oncol. 2012;103:113-22. Crossref
30. Cibula D, Pötter R, Planchamp F, Avall-Lundqvist E, Fischerova D,
Haie-Meder C, et al. The European Society of Gynaecological
Oncology/European Society for Radiotherapy and Oncology/European Society of Pathology guidelines for the management of
patients with cervical cancer. Virchows Arch. 2018;472:919-36. Crossref
31. Kim H, Houser CJ, Kalash R, Maceil CA, Palestra B, Malush D,
et al. Workflow and efficiency in MRI-based high-dose-rate
brachytherapy for cervical cancer in a high-volume brachytherapy
center. Brachytherapy. 2018;17:753-60. Crossref
32. Petric P, Dimopoulos J, Kirisits C, Berger D, Hudej R, Pötter R.
Inter- and intraobserver variation in HR-CTV contouring:
intercomparison of transverse and paratransverse image orientation
in 3D-MRI assisted cervix cancer brachytherapy. Radiother Oncol.
2008;89:164-71. Crossref
33. Lortet-Tieulent J, Ferlay J, Bray F, Jemal A. International patterns
and trends in endometrial cancer incidence, 1978-2013. J Natl
Cancer Inst. 2018;110:354-61. Crossref
34. Creasman WT, Morrow CP, Bundy BN, Homesley HD, Graham JE,
Heller PB. Surgical pathologic spread patterns of endometrial
cancer. A Gynecologic Oncology Group study. Cancer. 1987;60(8
Suppl):2035-41. Crossref
35. Larson DM, Connor GP, Broste SK, Krawisz BR, Johnson KK.
Prognostic significance of gross myometrial invasion with endometrial cancer. Obstet Gynecol. 1996;88:394-8. Crossref
36. Amant F, Mirza MR, Koskas M, Creutzberg CL. Cancer of the
corpus uteri. Int J Gynecol Obstet. 2018;143 Suppl 2:37-50. Crossref
37. Benedette Panici P, Basile S, Maneschi F, Alberto Lissoni A,
Signorelli M, Scambia G, et al. Systemic pelvic lymphadenectomy
versus no lymphadenectomy in early stage endometrial cancer: a
randomized clinical trial. J Nat Cancer Inst. 2008;100:1707-16. Crossref
38. ASTEC study group; Kitchener H, Swart AM, Qian Q, Amos C,
Parmar MK. Efficacy of systematic pelvic lymphadenectomy in
endometrial cancer (MRC ASTEC trial): a randomised study.
Lancet. 2009;373:125-36. Crossref
39. Todo Y, Kato H, Kaneuchi M, Watari H, Takeda M, Sakuragi N.
Survival effect of para-aortic lymphadenectomy in endometrial
cancer (SEPAL study): a retrospective cohort analysis. Lancet.
2010;375:1165-72. Crossref
40. Lin G, Huang YT, Chao A, Lin YC, Yang LY, Wu RC, et al.
Endometrial cancer with cervical stromal invasion: diagnostic
accuracy of diffusion-weighted and dynamic contrast enhanced
MR imaging at 3T. Eur Radiol. 2017;27:1867-76. Crossref
41. Solmaz U, Mat E, Dereli M, Turan V, Gungorduk K, Hasdemir P,
et al. Lymphovascular space invasion and cervical stromal invasion
are independent risk factors for nodal metastasis in endometrioid
endometrial cancer. Aust N Z J Obstet Gynaecol. 2015;55:81-6. Crossref
42. Taşkın S, Ortaç F, Kahraman K, Göç G, Öztuna D, Güngör M.
Cervical stromal involvement can predict survival in advanced
endometrial carcinoma: a review of 67 patients. Int J Clin Oncol.
2013;18:105-9. Crossref
43. Kwon JS, Qiu F, Saskin R, Carey MS. Are uterine risk factors more
important than nodal status in predicting survival in endometrial
cancer? Obstet Gynecol. 2009;114:736-43. Crossref
44. Nougaret S, Horta M, Sala E, Lakhman Y, Thomassin-Naggara I,
Kido A, et al. Endometrial cancer MRI staging: updated guidelines
of the European Society of Urogenital Radiology. Eur Radiol.
2019;29:792-805. Crossref
45. Beddy P, Moyle P, Kataoka M, Yamamoto AK, Joubert I, Lomas D,
et al. Evaluation of depth of myometrial invasion and overall
staging in endometrial cancer: comparison of diffusion-weighted
and dynamic contrast-enhanced MR imaging. Radiology.
2012;262:530-7. Crossref
46. Thieme SF, Collettini F, Sehouli J, Biocca L, Lella A, Wagner M,
et al. Preoperative evaluation of myometrial invasion in
endometrial carcinoma: prospective intra-individual comparison
of magnetic resonance volumetry, diffusion-weighted and dynamic
contrast-enhanced magnetic resonance imaging. Anticancer Res.
2018;38:4813-7. Crossref
47. Ali A, Black D, Soslow RA. Difficulties in assessing the depth
of myometrial invasion in endometrial carcinoma. Int J Gynecol
Pathol. 2007;26:115-23. Crossref
48. College of American Pathologists. Protocol for the Examination
of Specimens From Patients With Carcinoma and Carcinosarcoma
of the Endometrium. 2017. Available from: https://documents.cap.
org/protocols/cp-endometrium-2017-v4000.pdf. Accessed 1 Nov
2018.
49. van der Putten LJ, van de Vijver K, Bartosch C, Davidson B,
Gatius S, Matias-Guiu X, et al. Reproducibility of measurement
of myometrial invasion in endometrial carcinoma. Virchows Arch.
2017;470:63-8. Crossref
50. Rungruang B, Olawaiye AB. Comprehensive surgical staging for
endometrial cancer. Rev Obstet Gynecol. 2012;5:28-34.
51. Abu-Rustum NR. Sentinel lymph node mapping for endometrial
cancer: a modern approach to surgical staging. J Natl Compr Canc
Netw. 2014;12:288-97. Crossref
52. Kitajima K, Yamasaki E, Kaji Y, Murakami K, Sugimura K. Comparison of DWI and PET/CT in evaluation of lymph node
metastasis in uterine cancer. World J Radiol. 2012;4:207-14. Crossref
53. Chang MC, Chen JH, Liang JA, Yang KT, Cheng KY, Kao CH.
18F-FDG PET or PET/CT for detection of metastatic lymph nodes
in patients with endometrial cancer: a systematic review and metaanalysis.
Eur J Radiol. 2012;81:3511-7. Crossref
54. Timmerman D, Valentin L, Bourne TH, Collins WP, Verrelst H,
Vergote I, et al. Terms, definitions and measurements to describe
the sonographic features of adnexal tumors: a consensus opinion
from the International Ovarian Tumor Analysis (IOTA) Group.
Ultrasound Obstet Gynecol. 2000;16:500-5. Crossref
55. Anton C, Carvalho FM, Oliveira EI, Maciel GA, Baracat EC,
Carvalho JP. A comparison of CA125, HE4, risk ovarian
malignancy algorithm (ROMA), and risk malignancy index (RMI)
for the classification of ovarian masses. Clinics (Sao Paulo).
2012;67:437-41. Crossref
56. Levine D, Brown DL, Andreotti RF, Benacerraf B, Benson CB,
Brewster WR, et al. Management of asymptomatic ovarian
and other adnexal cysts imaged at US: Society of Radiologists
in Ultrasound Consensus Conference Statement. Radiology.
2010;256:943-54. Crossref
57. Thomassin-Naggara I, Poncelet E, Jalaguier-Coudray A, Guerra A,
Fournier LS, Stojanovic S, et al. Ovarian-Adnexal Reporting Data
System Magnetic Resonance Imaging (O-RADS MRI) score for
risk stratification of sonographically indeterminate adnexal masses.
JAMA Netw Open. 2020;3:e1919896. Crossref
58. Rockall A, Forstner R. Adnexal diseases. In: Hodler J, Kubik-Huch RA,
von Schulthess GK, editors. Diseases of the Abdomen and Pelvis
2018-2021: Diagnostic Imaging–IDKD Book. Cham (CH):
Springer; 2018. p 75-84. Crossref
59. Kinkel K, Lu Y, Mehdizade A, Pelte MF, Hricak H. Indeterminate
ovarian mass at US: incremental value of second imaging test for
characterization—meta-analysis and Bayesian analysis. Radiology.
2005;236:85-94. Crossref
60. Thomassin-Naggara I, Daraï E, Cuenod CA, Rouzier R, Callard P,
Bazot M. Dynamic contrast-enhanced magnetic resonance imaging:
A useful tool for characterizing ovarian epithelial tumors. J Magn
Reson Imaging. 2008;28:111-20. Crossref
61. Valentini AL, Gui B, Miccò M, Mingote MC, De Gaetano AM,
Ninivaggi V, et al. Benign and suspicious ovarian masses–MR
imaging criteria for characterization: Pictorial review. J Oncol.
2012;2012:481806. Crossref
62. Davarpanah AH, Kambadakone A, Holalkere NS, Guimaraes AR,
Hahn PF, Lee SI. Diffusion MRI of uterine and ovarian masses:
identifying the benign lesions. Abdom Radiol (NY). 2016;41:2466-75. Crossref
63. Dilks P, Narayanan P, Reznek R, Sahdev A, Rockall A. Can
quantitative dynamic contrast-enhanced MRI independently
characterize an ovarian mass? Eur Radiol. 2010;20:2176-83. Crossref
64. Li HM, Qiang JW, Ma FH, Zhao SH. The value of dynamic
contrast-enhanced MRI in characterizing complex ovarian tumors.
J Ovarian Res. 2017;10:4. Crossref
65. Tsuboyama T, Tatsumi M, Onishi H, Nakamoto A, Kim T, Hori M,
et al. Assessment of combination of contrast-enhanced magnetic
resonance imaging and positron emission tomography/computed
tomography for evaluation of ovarian masses. Invest Radiol.
2014;49:524-31. Crossref
66. Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ.
Survival effect of maximal cytoreductive surgery for advanced
ovarian carcinoma during the platinum era: a meta-analysis. J Clin
Oncol. 2002;20:1248-59. Crossref
67. Chang SJ, Hodeib M, Chang J, Bristow RE. Survival impact
of complete cytoreduction to no gross residual disease for advanced-stage ovarian cancer: a meta-analysis. Gynecol Oncol.
2013;130:493-8. Crossref
68. Balci S, Basturk O, Saka B, Bagci P, Postlewait LM, Tajiri T, et al.
Substaging nodal status in ampullary carcinomas has significant
prognostic value: proposed revised staging based on an analysis of
313 well-characterized cases. Ann Surg Oncol. 2015;22:4392-401. Crossref
69. Hacker NF, Berek JS, Lagasse LD, Nieberg RK, Elashoff RM.
Primary cytoreductive surgery for epithelial ovarian cancer. Obstet
Gynecol. 1983;61:413-20.
70. Whitney C, Spirtos N. Gynecologic Oncology Group
Surgical Procedures Manual. 2009. Available from:
https://www.semanticscholar.org/paper/Gynecologic-Oncology-Group-Surgica.... Accessed 2 Nov 2018.
71. Tseng JH, Cowan RA, Zhou Q, Iasonos A, Byrne M, Polcino T,
et al. Continuous improvement in primary debulking surgery for
advanced ovarian cancer: do increased complete gross resection
rates independently lead to increased progression-free and overall
survival? Gynecol Oncol. 2018;151:24-31. Crossref
72. Vergote I, Tropé CG, Amant F, Kristensen GB, Ehlen T, Johnson N,
et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC
or IV ovarian cancer. N Engl J Med. 2010;363:943-53. Crossref
73. Forstner R, Sala E, Kinkel K, Spencer JA, European Society of
Urogenital Radiology. ESUR guidelines: ovarian cancer staging
and follow-up. Eur Radiol. 2010;20:2773-80. Crossref
74. Coakley FV, Choi PH, Gougoutas CA, Pothuri B, Venkatraman E,
Chi D, et al. Peritoneal metastases: detection with spiral CT in
patients with ovarian cancer. Radiology. 2002;223:495-9. Crossref
75. Iafrate F, Ciolina M, Sammartino P, Baldassari P, Rengo M,
Lucchesi P, et al. Peritoneal carcinomatosis: imaging with 64-
MDCT and 3T MRI with diffusion-weighted imaging. Abdom
Imaging. 2012;37:616-27. Crossref
76. Low RN, Sebrechts CP, Barone RM, Muller W. Diffusion-weighted
MRI of peritoneal tumors: comparison with conventional MRI and
surgical and histopathologic findings—a feasibility study. AJR Am
J Roentgenol. 2009;193:461-70. Crossref
77. Fujii S, Matsusue E, Kanasaki Y, Kanamori Y, Nakanishi J,
Sugihara S, et al. Detection of peritoneal dissemination in
gynecological malignancy: evaluation by diffusion-weighted MR
imaging. Eur Radiol. 2008;18:18-23. Crossref
78. Roze JF, Hoogendam JP, van de Wetering FT, Spijker R, Verleye L,
Vlayen J, et al. Positron emission tomography (PET) and magnetic
resonance imaging (MRI) for assessing tumour resectability in
advanced epithelial ovarian/fallopian tube/primary peritoneal
cancer. Cochrane Database Syst Rev. 2018;10:CD012567. Crossref
79. Schmidt S, Meuli RA, Achtari C, Prior JO. Peritoneal carcinomatosis
in primary ovarian cancer staging: comparison between MDCT,
MRI, and 18F-FDG PET/CT. Clin Nucl Med. 2015;40:371-7. Crossref
80. Low RN, Barone RM, Lucero J. Comparison of MRI and CT for
predicting the Peritoneal Cancer Index (PCI) preoperatively in
patients being considered for cytoreductive surgical procedures.
Ann Surg Oncol. 2015;22:1708-15. Crossref
81. Lopez-Lopez V, Cascales-Campos P, Gil J, Frutos L, Andrade RJ,
Fuster-Quiñonero M, et al. Use of 18F-FDG PET/CT in the
preoperative evaluation of patients diagnosed with peritoneal
carcinomatosis of ovarian origin, candidates to cytoreduction and
hipec. A pending issue. Eur J Radiol. 2016;85:1824-8. Crossref
82. Kim HW, Won KS, Zeon SK, Ahn BC, Gayed IW. Peritoneal
carcinomatosis in patients with ovarian cancer: enhanced CT versus
18F-FDG PET/CT. Clin Nucl Med. 2013;38:93-7. Crossref
83. Rubini G, Altini C, Notaristefano A, Merenda N, Rubini D,
Ianora AA, et al. Role of 18F-FDG PET/CT in diagnosing
peritoneal carcinomatosis in the restaging of patient with ovarian
cancer as compared to contrast enhanced CT and tumor marker
Ca-125. Rev Esp Med Nucl Imagen Mol. 2014;33:22-7. Crossref
| Attachment | Size |
|---|---|
| v23n4_Current.pdf | 813.6 KB |