Ultrasound-guided Vacuum-assisted Excision of Papillary Breast Lesions as an Alternative to Surgical Excision: 7-year Experience
ORIGINAL ARTICLE CME
Ultrasound-guided Vacuum-assisted Excision of Papillary Breast Lesions as an Alternative to Surgical Excision: 7-year Experience
CM Chau1, EPY Fung2, CW Wong2, KM Kwok2, AYH Leung2, LKM Wong2, KCK Wong2, WS Mak2, HS Lam2, DHY Cho2
1 Department of Radiology, Princess Margaret Hospital, Hong Kong
2 Department of Diagnostic and Interventional Radiology, Kwong Wah Hospital, Hong Kong
Correspondence: Dr CM Chau, Department of Radiology, Princess Margaret Hospital, Hong Kong. Email: ccm152@ha.org.hk
Submitted: 1 Jan 2019; Accepted: 12 Feb 2019.
Contributors: All authors designed the study, acquired the data, analysed the data, drafted the article, and critically revised the article for
important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for publication, and take
responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics Approval: This retrospective study was approved by the Research Ethics Committee of Kowloon Central/Kowloon East Cluster (Ref KC/
KE-18-0128/ER-3). Owing to the retrospective nature of the study, the requirement for informed consent was waived.
Acknowledgement: We thank Ms Ellen LM Yu, Research Officer, Clinical Research Centre, Princess Margaret Hospital, Hong Kong, who
advised on the statistical methods for data analysis and assisted in implementing these methods.
Abstract
Objective
Removal of papillary breast lesions following percutaneous biopsy is advocated due to their diagnostic
challenges. Image-guided vacuum-assisted excision is a treatment option for managing papillary breast lesions.
The aim of our study was to review our experience in ultrasound-guided vacuum-assisted excision (US-VAE) of
papillary breast lesions.
Methods
This was a retrospective review of patients with biopsy-proven papillary breast lesions who underwent
US-VAE from January 2011 to April 2018. The clinical, radiological, and initial biopsy findings and the US-VAE
procedure data were collected. The final histology was reviewed for any evidence of malignancy. Patients were
followed up by clinical assessment and ultrasound. Lesion excision was considered successful removed if no residual
or recurrence was found at follow-up.
Results
A total of 71 patients with 76 papillary lesions underwent US-VAE over a 7-year period. No major
complications were observed. The overall cancer upgrade rate on pathology was 5.3%, with cancer upgrade rates
for papillary lesions with atypia and without atypia of 16.7% and 3.1%, respectively. Mean follow-up time was 12.8
months. Two residual lesions were found during follow-up, for a successful lesion removal rate of 97.2%.
Conclusion
The highly successful lesion removal rate with low residual or recurrence for benign papillary lesions
confirms that US-VAE avoids surgical excision in patients with biopsy-proven papillary lesions. It remains a safe
and effective alternative to surgical excision in managing biopsy-proven papillary lesions.
Key Words: Benign neoplasms/therapy; Breast/surgery; General surgery/instrumentation; Papilloma/therapy;Ultrasonography
中文摘要
超聲引導真空輔助抽吸乳頭狀乳房病灶切除術作為手術切除的替代方法:七年經驗回顧
周智敏、馮寶恩、黃鎮威、郭勁明、梁燕霞、黃嘉敏、黃卓琦、麥詠詩、林漢城、曹慶恩
目的
由於診斷上的困難和挑戰,一般建議經皮活檢診斷的乳頭狀乳房病灶以切除術治療。影像引
導真空輔助抽吸切除術是乳頭狀乳房病灶的治療方案之一。本研究回顧我院進行超聲引導真空輔助
抽吸乳頭狀乳房病灶切除術(US-VAE)的經驗。
方法
回顧分析2011年1月至2018年4月期間進行US-VAE的活檢證實乳頭狀乳房病灶患者。收集臨
床、影像學和初步活檢結果以及US-VAE手術記錄,核對組織學是否有惡性依據。患者進行臨床評估
和超聲檢查隨訪。如在隨訪中未發現殘留或復發會被視為已成功切除病灶。
結果
7年內71名患者共76個乳頭狀乳房病灶接受了US-VAE。圴無嚴重併發症。總體按照病理結果
的癌變升級率為5.3%,而具有不典型增生和無不典型增生的乳頭狀病灶的癌變升級率分別為16.7%和
3.1%。平均隨訪時間為12.8個月。在隨訪期間發現兩個殘留病灶。病灶清除成功率為97.2%。
結論
良性乳頭狀乳房病灶的高成功清除率且低殘留病灶或復發率,表明對於活檢證實乳頭狀乳房病灶患者使用US-VAE可避免手術治療。US-VAE治療活檢證實的乳頭狀乳房病灶是安全有效替代手術切除的方案。
INTRODUCTION
Papillary breast lesions comprise a broad spectrum
ranging from benign papilloma through atypical
papilloma or carcinoma in situ to papillary carcinoma,
and the distinctions among these lesions represent
a continuum that can be difficult to distinguish both
radiologically and pathologically.[1] Together with the
underestimation from fine needle aspiration (FNA) and
core needle biopsy (CNB),[2] [3] [4] which are the commonest
initial diagnostic procedures for ultrasound-detected
breast lesions, this imposes a diagnostic difficulty for
papillary breast lesions.
Removal of percutaneously diagnosed papillary lesions
is advocated by many studies due to the substantial
cancer upgrade rate.[5] [6] [7] However, the drawbacks of
surgical excision for all percutaneously diagnosed
papillary lesions are the surgical risks, high costs, and
long recovery time from surgical operations.
Vacuum-assisted excision has consistently been shown
to be effective.[8] [9] [10] [11] [12] The National Health Service (NHS)
of the UK has recommended the use of image-guided
vacuum-assisted excision to remove benign breast
lesions such as fibroadenomas since 2006 and also for
management of some histological indeterminate B3
lesions.[13] [14]
The aim of our study was to review the outcome and
effectiveness of ultrasound-guided vacuum-assisted
excision (US-VAE) of papillary breast lesions at our
institution.
METHODS
We reviewed the data of patients that underwent
US-VAE in Kwong Wah Hospital from January 2011
to April 2018. Patients with prior biopsy-proven benign
papillary lesions were included. We excluded patients
with known untreated malignancy in the same breast.
Data on each patient’s age at diagnosis, sex, and
presenting symptoms were collected from the electronic
patient record. The ultrasound characteristics of the
lesions, including size and distance from the nipple,
were recorded from the ultrasound images or reports.
The assessment categories of the lesions were retrieved
from the reports. The reporting radiologists were using
either the Breast Imaging Reporting and Data System
(BI-RADS) by the American College of Radiology or the
UK five-point breast imaging classification by the Royal
College of Radiologists Breast Group. The initial biopsy
methods and their pathology results were collected.
All the US-VAEs were performed by one of five
breast radiologists with 2 to 11 years’ specialised experience in breast imaging. The vacuum-assisted
systems used were either EnCor (7-gauge or 10-gauge
needle) or Mammotome (8-gauge or 11-gauge needle).
The ultrasound images from the biopsy procedure
were reviewed, and the lesion identified using a highfrequency
linear array ultrasound transducer. A solution
of 2% lidocaine with 1:200 000 epinephrine diluted
with normal saline in 1:1 ratio (mean 14 mL) was
injected under the skin and around the lesion as local
anaesthesia. The needle was placed underneath the
lesion and US-VAE of the lesion was performed with a
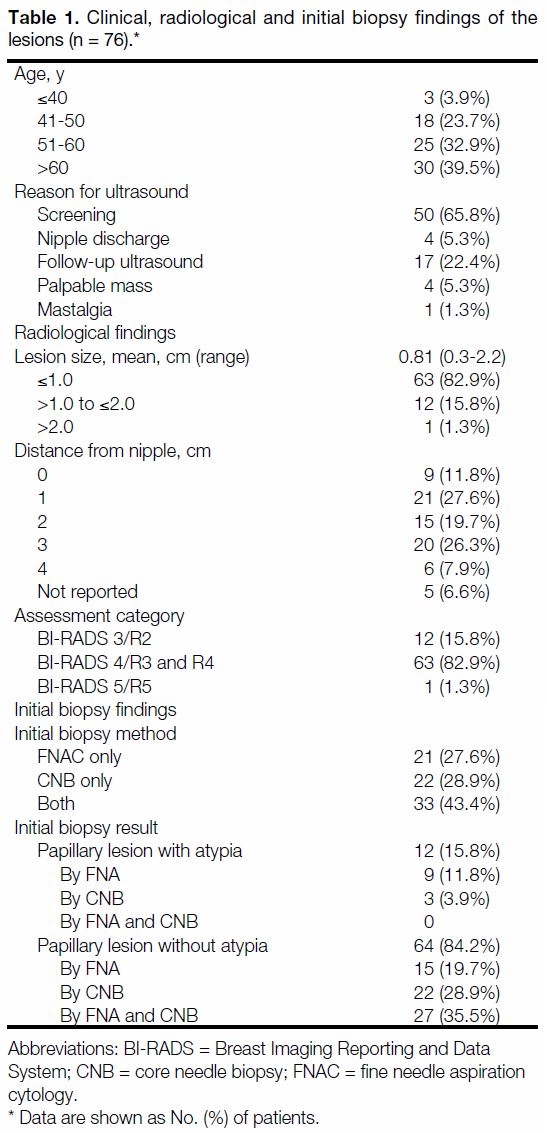
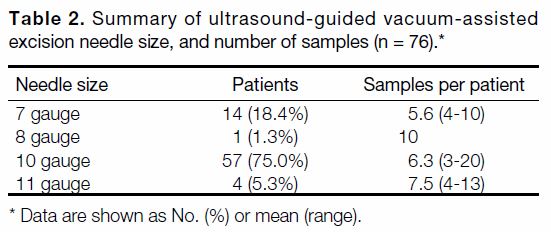
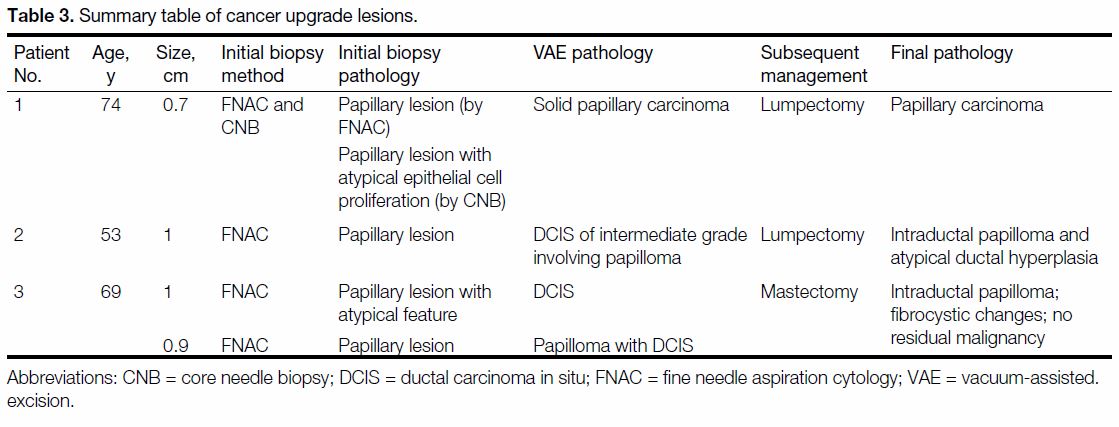
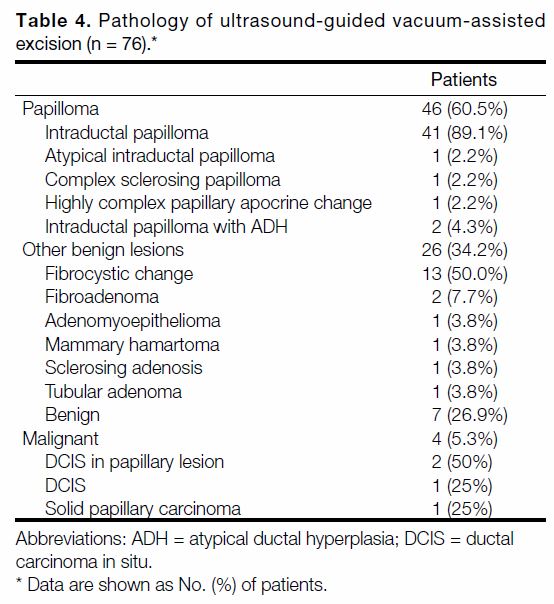
180° sweep (Figure). Complete removal was attempted
and evidenced by real-time ultrasound. Any incomplete
removal was recorded. A localisation marker was placed
at the excision site after lesion removal. Haemostasis
was first achieved by manual compression; then the
wound was closed with sterile strips followed by a
compression dressing around the chest. Any immediate complications during the procedure were recorded. The
procedure was performed in an outpatient breast clinic
setting and the patient was discharged on the same day
after the procedure. Patients were advised to return to
our breast clinic if there were any complications, such
as breast swelling, bleeding or fever, after the procedure.
Major complications were defined as hospitalisation or
surgery related to the procedure.
Figure. Ultrasonography images during the ultrasound-guided vacuum-assisted excision (US-VAE) procedure. (a) The needle is placed deep
into the lesion. (b) The needle aperture (arrow) is opened to ensure lesion was touching the needle aperture. (c) Real-time ultrasonograph
during the VAE procedure showing that the lesion was being sucked into the needle aperture. (d) Ultrasonograph taken to confirm complete
removal of the lesion at the end of excision.
Pathology results from US-VAE were reviewed. Ductal
carcinoma in situ (DCIS) and invasive carcinoma were
considered as cancer upgrades and were referred for
surgical excision. Patients with nonmalignant lesions
were followed up by clinical assessment and ultrasound.
A lesion was considered as successfully removed if no
residual or recurrence was found at follow-up.
Fisher’s exact test was used for statistical analysis.
RESULTS
Between January 2011 and April 2018, of 236 breast
lesions in 228 patients removed with US-VAE, there
were 77 biopsy-proven papillary breast lesions in
72 patients. One lesion was excluded as the patient had
known DCIS in the same breast. In total, there were
76 papillary breast lesions in 71 patients in our study
(Table 1).
Table 1. Clinical, radiological and initial biopsy findings of the lesions (n = 76).
Clinical, Radiological, and Initial Biopsy
Findings of Lesions
The mean age at presentation was 56.3 years (range,
36-75 years). All were female patients. The majority of
the lesions were found during routine screening (n = 50,
65.8%) or follow-up ultrasound for other breast lesions
(n = 17, 22.4%).
The mean lesion size was 0.81 cm (range, 0.3 cm-2.2 cm).
The majority of the lesions were ≤1.0 cm (n = 63, 82.9%)
and only one lesion was >2.0 cm. The recorded locations
of the lesions in terms of distance from the nipple were
0 to 4 cm. Subareolar or periareolar lesions were marked
as 0 cm from nipple. There was no indication of the
location in five lesions.
The assessment category of each lesion was assigned
by the radiologist that performed the initial diagnostic
ultrasound before the biopsy. A total of 71 lesions
were categorised according to BI-RADS. Five lesions
were categorised as R3 (indeterminate/probably benign
findings; n = 2) or R4 (findings suspicious of malignancy;
n = 3), according to the UK five-point breast imaging
classification. We had mapped the UK five-point breast
imaging classification to BI-RADS according to the
study by Taylor et al.[15] The overall lesion assessment
categories are listed in Table 1. The majority of the
lesions were categorised as BI-RADS 4 (n = 63, 82.9%).
All the papillary lesions had undergone prior ultrasound-guided
biopsy, either by FNA, CNB, or both, depending
on the operator’s preference. The needle sizes and the
number of samples are summarised in Table 2. The
number of samples obtained from one lesion biopsied
with a 10-gauge needle was not specified. All the
pathology results from initial biopsy showed papillary
lesions. The lesions were further categorised as with
or without atypia. If there was a discrepancy between
the FNA and CNB pathology results, the lesions were
categorised based on the more suspicious pathology. A
total of 12 (15.8%) showed atypia and 64 (84.2%) did
not have atypia. Seven lesions biopsied with both FNA and CNB had had discrepant pathology results, in which
FNA showed atypia but CNB did not in five lesions and
CNB showed atypia but FNA did not in two lesions.
Thus, overall there were nine papillary lesions with
atypia diagnosed by FNA and three by CNB.
Table 2. Summary of ultrasound-guided vacuum-assisted
excision needle size, and number of samples (n = 76)
Ultrasound-guided Vacuum-assisted Excision
Results
All the lesions were confirmed as completely removed
by ultrasonogram. The sample weight of each lesion was
calculated by multiplying the weight of each core16 by
the number of cores. The mean sample weight for lesions
<1 cm was 1.345 g (±0.530; range, 0.336-2.904 g) and
for lesions ≥1 cm was 1.920 g (±1.033; range, 0.588-4.420 g).
No major complication was noted during the
procedures and no patient returned to the breast clinic
for complications after being discharged. One patient
required skin sutures due to a laceration during the
procedure with uneventful wound healing.
Four malignant lesions were found in three patients
from the final pathology of US-VAE. These included
one DCIS, two DCIS with papilloma, and one solid
papillary carcinoma. The overall cancer upgrade rate
was 5.3%. Among the four malignant lesions, three
lesions had FNA only and one had FNA and CNB as
initial biopsies. Two of them had atypical features on
initial biopsy, identified by FNA and CNB (Table 3).
Thus, the cancer upgrade rate for papillary lesions with
atypia was 16.7% and that for papillary lesions without
atypia was 3.1% (p = 0.115). The pathologies excised
with US-VAE are listed in Table 4.
Table 3. Summary table of cancer upgrade lesions.
Table 4. Pathology of ultrasound-guided vacuum-assisted excision (n = 76).
Follow-up
Four patients had subsequent surgery. The four malignant
lesions in three patients were removed with lumpectomy
or mastectomy (Table 3). Two patients with DCIS
showed no residual malignancy in the final pathology
from surgical excision. One benign lesion underwent
lumpectomy as the pathology from VAE showed
atypical intraductal papilloma. The final pathology from
the lumpectomy was benign intraductal papilloma.
The rest of the 67 patients were followed up clinically
and 51 (76.1%) patients had follow-up ultrasound. The mean follow-up time was 12.8 months (range, 1.3-67.4 months). The overall rate of successful lesion
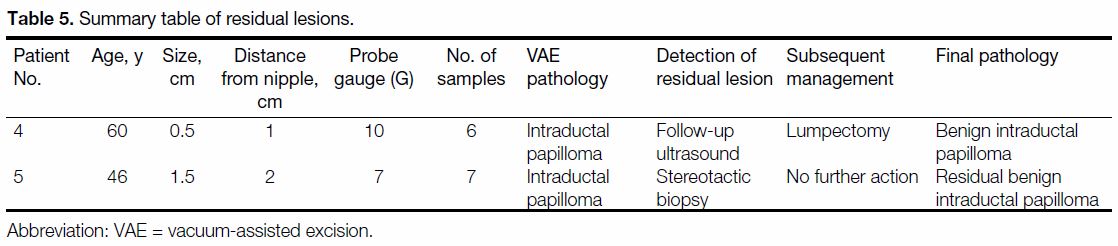
removal was 97.2%. Table 5 shows the handling of
residual pathology in two patients.
Table 5. Summary table of residual lesions.
Lesion size, location, and probe gauge were not
statistically significant predictors of residual lesions.
DISCUSSION
Management of percutaneously diagnosed papillary
lesions has been a challenge for years due to its diagnostic
difficulty and substantial rate of cancer upgrade. The
reported cancer upgrade rate has ranged from 3.1% to
15.8%[5] [6] [7] and lesion removal was advocated in many
different studies.[5] [6] [7] [17] [18] [19] [20] Before the advent of percutaneous
VAE, surgical excision was the treatment of choice for
lesion removal. As more and more papillary lesions were
diagnosed in breast screening, the high costs of labour
and operation theatre time would be a burden to the
healthcare system.[21] Moreover, since the papillary lesions
were indeterminate in biopsy histology with chances
of being a benign papilloma, patients may be reluctant
to undergo surgical excision due to the morbidity and
mortality from surgical and anaesthetic risks.
Image-guided VAE was initially used to improve
diagnostic accuracy for percutaneous biopsy due to
larger cores obtained when compared to conventional
FNA and CNB.[9] [10] Its use has been extended to remove
benign lesions because a larger amount of tissue can be
obtained in one pass, and with better cosmetic results.
US-VAE can be performed as an outpatient procedure in
the clinic and the patient can be discharged on the same
day, resulting in much lower costs than those incurred
with surgical excision.[22]
In our series, a success rate of 97.2% for benign papillary
lesion removal by US-VAE without residual or recurrent
disease was achieved, in line with the reported overall
success rate of 97% to 100% in the literature.[23] [24]
Two residual lesions were found, which raises the
question of how complete removal can be achieved
confidently during the procedure. For US-VAE,
the only evidence of lesion removal is by real-time
ultrasound imaging. However, during the procedure,
there are inevitable small haematomas and oedema at
the procedure site,[25] which may obscure the field and
make it difficult for the operators to determine whether
the lesion is completely removed or not. Some operators
remove breast tissue surrounding the lesions at four more
sampling sites to ensure complete lesion removal but the
results varied.[26] [27] The NHS Breast Screening working
group had suggested a specimen of approximately 4 g
to be equivalent to surgical excision.[28] Also, the working
group did not state the size range of the lesion for their
recommendation. Smaller lesions can be completely
excised with a lower specimen weight.
The two residual lesions in our study occurred at the early
stage (2013 and 2014) since we started US-VAE in 2011.
Salazar et al[29] reported that 11 excisions were required
to acquire skills to perform complete excision in more
than 80% at the end of the US-VAE and 18 excisions
at 6 months. Thus, operator experience is one of the
important factors for successful lesion removal. Centres
performing US-VAE should formulate standardised
training and credentialing for better performance of
US-VAE.
Three papillomas with atypical features were found
(Table 4), namely, one atypical intraductal papilloma
and two intraductal papillomas with atypical ductal
hyperplasia. The atypical intraductal papilloma
underwent surgical excision and final pathology
showed benign papilloma with no atypical or invasive
features. The two intraductal papillomas with ADH
had no recurrence or cancer development during the
follow-up period of 11.0 months and 27.6 months. The
literature shows a low underestimation rate of 0% with
US-VAE.[23] [30] Thus, these lesions can be safely managed by close monitoring after multidisciplinary discussion
and this approach is supported by the consensus from the
UK and Europe.[28] [31]
No major complication was found after US-VAE in our
series. US-VAE has been reported as a safe procedure
with a low rate of complications, ranging from 0% to
9%.[10] [12] [23] [24] [27] [32] [33] The majority of complications included
hematoma and pain, which were usually self-limiting and
did not require further intervention. One of our patients
required suturing due to skin laceration during the
procedure because the lesion was superficially located.
This was considered as a minor complication and we
should inform patients about this potential complication,
especially when the lesion is close to the skin.
In our study, the cancer upgrade rate for papillary lesions
diagnosed by FNA or CNB prior to US-guided VAE was
5.3%, which is compatible with the literature.[6] [7] [18] For
diagnosing papillary lesions with atypia, pathologists
need to assess the size of the area of atypical epithelial
proliferation and sometimes it would be difficult to
distinguish atypical epithelial proliferation within a
papilloma from low-grade DCIS within a papilloma in
the tissue samples,[34] and FNA may miss the small foci of
carcinoma in situ or the foci that are invasive.[35] [36] Thus,
for papillary lesions with atypia diagnosed by FNA or
CNB, surgical excision would be more appropriate
as the next step of management, as included in the
recommendations from the NHS Breast Screening
working group.
There were a few limitations in this study. Our sample
size was small, as was the number of residual lesions.
Thus, we could not identify any statistically significant
factors associated with incomplete excision. Also,
the adequate sample weight for different lesion sizes
may need further study for validation. There would be
selection bias in a retrospective study as the lesion size,
location of the lesion and technical factors may affect
the clinicians’, radiologists’ and patients’ decision on
choosing US-VAE or surgical excision. Only 70.8% of
patients had follow-up ultrasonography examination,
which may have resulted in underestimation of the
residual rate.
In conclusion, our successful lesion removal rate with
low rates of residual or recurrence for benign papillary
lesions confirms that US-VAE spares most patients with
FNA- or CNB-proven papillary lesions from surgical
excision and provides adequate tissue samples for a confident diagnosis guiding subsequent management. It
is a safe and effective alternative to surgical excision in
managing biopsy-proven papillary lesions.
REFERENCES
1. Sohn V, Keylock J, Arthurs Z, Wilson A, Herbert G, Perry J, et al.
Breast papillomas in the era of percutaneous needle biopsy. Ann
Surg Oncol. 2007;14:2979-84. Crossref
2. Houssami N, Ciatto S, Ellis I, Ambrogetti D. Underestimation of
malignancy of breast core–needle biopsy: concepts and precise
overall and category-specific estimates. Cancer. 2007;109:487-95. Crossref
3. Ciatto S, Houssami N, Ambrogetti D, Bianchi S, Bonardi R,
Brancato B, et al. Accuracy and underestimation of malignancy of
breast core needle biopsy: the Florence experience of over 4000
consecutive biopsies. Breast Cancer Res Treat. 2007;101:291-7. Crossref
4. Youk JH, Kim EK, Kim MJ, Oh KK. Sonographically guided
14-gauge core needle biopsy of breast masses: a review of
2420 cases with long-term follow-up. AJR Am J Roentgenol.
2008;190:202-7. Crossref
5. Rizzo M, Linebarger J, Lowe MC, Pan L, Gabram SG, Vasquez L,
et al. Management of papillary breast lesions diagnosed on core-needle
biopsy: clinical pathologic and radiologic analysis of 276
cases with surgical follow-up. J Am Coll Surg. 2012;214:280-7. Crossref
6. Jaffer S, Nagi C, Bleiweiss IJ. Excision is indicated for intraductal
papilloma of the breast diagnosed on core needle biopsy. Cancer.
2009;115:2837-43. Crossref
7. Chang JM, Moon WK, Cho N, Han W, Noh DY, Park IA, et al.
Management of ultrasonographically detected benign papillomas
of the breast at core needle biopsy. AJR Am J Roentgenol.
2011;196:723-9. Crossref
8. Rajan S, Shaaban AM, Dall BJ, Sharma N. New patient pathway
using vacuum-assisted biopsy reduces diagnostic surgery for B3
lesions. Clin Radiol. 2012;67:244-9. Crossref
9. Simon JR, Kalbhen CL, Cooper RA, Flisak ME. Accuracy and
complication rates of US-guided vacuum-assisted core breast
biopsy: initial results. Radiology. 2000;215:694-7. Crossref
10. Cassano E, Urban LA, Pizzamiglio M, Abbate F, Maisonneuve P,
Renne G, et al. Ultrasound-guided vacuum-assisted core breast
biopsy: experience with 406 cases. Breast Cancer Res Treat.
2007;102:103-10. Crossref
11. Chang JM, Han W, Moon WK, Cho N, Noh DY, Park IA, et al.
Papillary lesions initially diagnosed at ultrasound-guided vacuum-assisted
breast biopsy: rate of malignancy based on subsequent
surgical excision. Ann Surg Oncol. 2011;18:2506-14. Crossref
12. Fine RE, Boyd BA, Whitworth PW, Kim JA, Harness JK,
Burak WE. Percutaneous removal of benign breast masses using a
vacuum-assisted hand-held device with ultrasound guidance. Am
J Surg. 2002;184:332-6. Crossref
13. National Institute for Health and Care Excellence, UK Government.
Image-guided vacuum-assisted excision biopsy of benign breast
lesions. 2006. Available from: https://www.nice.org.uk/guidance/ipg156/resources/imageguided-vacuumassi.... Accessed 23 Sep 2018.
14. National Health Service. NHS Breast Screening Programme.
Clinical guidance for breast cancer screening assessment.
2016. Available from: https://associationofbreastsurgery.org.uk/media/1414/nhs-bsp-clinical-gu.... Accessed 23 Sep 2018.
15. Taylor K, Britton P, O’Keeffe S, Wallis MG. Quantification of the
UK 5-point breast imaging classification and mapping to BI-RADS to facilitate comparison with international literature. Br J Radiol.
2011;84:1005-10. Crossref
16. Preibsch H, Baur A, Wietek BM, Krämer B, Staebler A,
Claussen CD, et al. Vacuum-assisted breast biopsy with 7-gauge,
8-gauge, 9-gauge, 10-gauge, and 11-gauge needles: how many
specimens are necessary? Acta Radiologica. 2015;56:1078-84. Crossref
17. Mercado CL, Hamele-Bena D, Oken SM, Singer CI, Cangiarella J.
Papillary lesions of the breast at percutaneous core-needle biopsy.
Radiology. 2006;238:801-8. Crossref
18. Sakr R, Rouzier R, Salem C, Antoine M, Chopier J, Daraï E, et al.
Risk of breast cancer associated with papilloma. Eur J Surg Oncol.
2008;34:1304-8. Crossref
19. Tatarian T, Sokas C, Rufail M, Lazar M, Malhotra S, Palazzo JP,
et al. Intraductal papilloma with benign pathology on breast core
biopsy: to excise or not? Ann Surg Oncol. 2016;23:2501-7. Crossref
20. Leithner D, Kaltenbach B, Hödl P, Möbus V, Brandenbusch V,
Falk S, et al. Intraductal papilloma without atypia on image-guided
breast biopsy: upgrade rates to carcinoma at surgical excision.
Breast Care (Basel). 2018;13:364-8. Crossref
21. Fernández-García P, Marco-Doménech SF, Lizán-Tudela L,
Ibáñez-Gual MV, Navarro-Ballester A, Casanovas-Feliu E. The
cost effectiveness of vacuum-assisted versus core-needle versus
surgical biopsy of breast lesions. Radiologia. 2017;59:40-6. Crossref
22. Alonso-Bartolomé P, Vega-Bolívar A, Torres-Tabanera M,
Ortega E, Acebal-Blanco M, Garijo-Ayensa F, et al. Sonographically
guided 11-G directional vacuum-assisted breast biopsy as an
alternative to surgical excision: utility and cost study in probably
benign lesions. Acta Radiol. 2004;45:390-6. Crossref
23. Kim MJ, Kim EK, Kwak JY, Son EJ, Park BW, Kim SI, et al.
Nonmalignant papillary lesions of the breast at US-guided
directional vacuum-assisted removal: a preliminary report. Eur
Radiol. 2008;18:1774-83. Crossref
24. Ko KH, Jung HK, Youk JH, Lee KP. Potential application of
ultrasound-guided vacuum-assisted excision (US-VAE) for well-selected
intraductal papillomas of the breast: single-institutional
experiences. Ann Surg Oncol. 2012;19:908-13. Crossref
25. Fine RE, Whitworth PW, Kim JA, Harness JK, Boyd BA,
Burak Jr WE. Low-risk palpable breast masses removed using a
vacuum-assisted hand-held device. Am J Surg. 2003;186:362-7. Crossref
26. Kim SY, Kim EK, Lee HS, Kim MJ, Yoon JH, Koo JS, et al. Asymptomatic benign papilloma without atypia diagnosed at
ultrasonography-guided 14-gauge core needle biopsy: which
subgroup can be managed by observation? Ann Surg Oncol.
2016;23:1860-6. Crossref
27. Youk JH, Kim EK, Kwak JY, Son EJ, Park BW, Kim SI. Benign
papilloma without atypia diagnosed at US-guided 14-gauge core-needle
biopsy: clinical and US features predictive of upgrade to
malignancy. Radiology. 2011;258:81-8. Crossref
28. Pinder SE, Shaaban A, Deb R, Desai A, Gandhi A, Lee AH, et al.
NHS Breast Screening multidisciplinary working group guidelines
for the diagnosis and management of breast lesions of uncertain
malignant potential on core biopsy (B3 lesions). Clin Radiol.
2018;73:682-92. Crossref
29. Salazar JP, Miranda I, De Torres J, Rus MN, Espinosa-Bravo M,
Esgueva A, et al. Percutaneous ultrasound-guided vacuum-assisted
excision of benign breast lesions: A learning curve to assess
outcomes. Br J Radiol. 2019;92:20180626. Crossref
30. Grady I, Gorsuch H, Wilburn-Bailey S. Ultrasound-guided,
vacuum-assisted, percutaneous excision of breast lesions: an
accurate technique in the diagnosis of atypical ductal hyperplasia.
J Am Coll Surg. 2005;201:14-7. Crossref
31. Rageth CJ, O’Flynn EA, Comstock C, Kurtz C, Kubik R,
Madjar H, et al. First International Consensus Conference on lesions
of uncertain malignant potential in the breast (B3 lesions). Breast
Cancer Res Treat. 2016;159:203-13. Crossref
32. Maxwell AJ. Ultrasound-guided vacuum-assisted excision of
breast papillomas: review of 6-years experience. Clin Radiol.
2009;64:801-6. Crossref
33. Kim MJ, Park BW, Kim SI, Youk JH, Kwak JY, Moon HJ, et al.
Long-term follow-up results for ultrasound-guided vacuum-assisted
removal of benign palpable breast mass. Am J Surg. 2010;199:1-7. Crossref
34. Page DL, Salhany KE, Jensen RA, Dupont WD. Subsequent breast
carcinoma risk after biopsy with atypia in a breast papilloma.
Cancer. 1996;78:258-66. Crossref
35. Michael CW, Buschmann B. Can true papillary neoplasms of breast
and their mimickers be accurately classified by cytology? Cancer.
2002;96:92-100. Crossref
36. Gomez-Aracil V, Mayayo E, Azua J, Arraiza A. Papillary
neoplasms of the breast: clues in fine needle aspiration cytology.
Cytopathology. 2002;13:22-30. Crossref
| Attachment | Size |
|---|---|
| v23n4_Ultrasound.pdf | 550.51 KB |