Efficacy of Intravenous Iron in Cancer Patients with Moderate to Severe Iron Deficiency Anaemia
ORIGINAL ARTICLE
Efficacy of Intravenous Iron in Cancer Patients with Moderate to Severe Iron Deficiency Anaemia
W Chan, FAS Lee, WWY Tin, SF Yip, FCS Wong
Department of Clinical Oncology, Tuen Mun Hospital, Hong Kong
Correspondence: Dr W Chan, Department of Clinical Oncology, Tuen Mun Hospital, Hong Kong. Email: ac_wai@hotmail.com
Submitted: 16 Jul 2020; Accepted: 10 Nov 2020.
Contributors: All authors contributed to the concept and design of the study, acquisition and analysis of the data, drafting of the manuscript, and
critical revision of the manuscript for important intellectual content.
Conflicts of Interest: As an editor of the journal, FCS Wong was not involved in the peer-review process. Other authors have disclosed no
conflicts of interest.
Funding/Support: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethics Approval: This study was approved by the Research Ethics Committee of North Territory West Cluster, Hospital Authority (Ref NTWC/
REC/19055). The need for patient consent was waived owing to the retrospective nature of the study.
Abstract
Introduction
Iron deficiency anaemia is common in patients with cancer. Intravenous iron is approved for treatment
of iron deficiency anaemia when oral iron preparations are ineffective. Few data are available on the rapidity of
haemoglobin correction in patients with cancer and moderate to severe iron deficiency anaemia who are given
intravenous iron.
Methods
We retrospectively reviewed the efficacy and safety of ferric carboxymaltose (FCM) in cancer patients
with iron deficiency anaemia who were treated in our centre from January to June 2019. The primary endpoint was
the rise in haemoglobin levels at day 7, day 14, and day 28 after the first dose of FCM. The secondary endpoints
included the change in iron profile, the sustainability of haemoglobin response at day 60, and the changes in patients’
transfusion requirements following FCM.
Results
The mean baseline haemoglobin level of the 34 patients given FCM during this period was 7.8 g/dL. The
mean haemoglobin rise at day 7, day 14, and day 28 was 0.5 g/dL, 1.1 g/dL, and 2.1 g/dL, respectively. The rise in
haemoglobin level was sustainable at day 60 and accompanied by rises in ferritin and iron saturation (p < 0.001).
There was a statistically significant reduction in patients’ transfusion requirements (p = 0.016). No hypersensitivity
reaction or abnormality of vital signs was reported.
Conclusions
In patients with cancer and moderate to severe iron deficiency anaemia, FCM induced a prompt rise
in haemoglobin levels. This treatment may be a viable option for patients with iron deficiency anaemia who may
otherwise require transfusion.
Key Words: Blood transfusion; Ferric Compounds; Neoplasms
中文摘要
靜脈注射鐵劑對中重度缺鐵性貧血癌症患者的療效
陳偉、李安誠、佃穎恩、葉仕輝、黃志成
引言
缺鐵性貧血在癌症患者中很常見。當口服鐵劑無效時,靜脈注射鐵劑可用於治療缺鐵性貧血。有關中重度缺鐵性貧血癌症患者在靜脈注射鐵劑後的血紅蛋白校正的快速性尚無數據。
方法
我們回顧分析靜脈注射鐵劑羧甲基麥芽糖(FCM)在缺鐵性貧血癌症患者中的有效性和安全
性。患者於2019年1月至2019年6月接受治療,研究的主要終點是注射第一劑FCM後第7天、第14天和
第28天的血紅蛋白升幅。次要終點包括鐵譜的變化、第60天時血紅蛋白反應的可持續性,以及FCM
後輸血需求的變化。
結果
34名患者在研究期間接受FCM。平均基線血紅蛋白為7.8 g/dL。第7天、第14天和第28天的血
紅蛋白平均升幅分別為0.5 g/dL、1.1 g/dL和2.1 g/dL。血紅蛋白的增幅在第60天是可持續的,伴有鐵
蛋白和鐵飽和度的增加(p < 0.001)。輸血量顯著減少(p = 0.016)。患者沒有出現過敏反應或生命
體徵異常。
結論
對於中重度缺鐵性貧血癌症患者,FCM可迅速增加血紅蛋白。對於可能需要輸血的缺鐵性貧血患者,FCM是一種可行的治療選擇。
INTRODUCTION
Iron deficiency has been reported to be present in up to
42.6% of patients with cancer.[1] Randomised controlled
trials have shown that intravenous iron was superior
to oral iron at correcting iron deficiency anaemia in
patients with renal failure, heavy uterine bleeding,
and inflammatory bowel disease.[2] [3] [4] A meta-analysis
primarily involving patients with non-malignant causes
of iron deficiency anaemia suggested that intravenous
iron may reduce patients’ transfusion needs.[5]
Patients with cancer have distinct patterns of iron
metabolism and complex pathogenesis of iron deficiency
anaemia.[6] In patients with cancer, iron deficiency can be
caused by tumour bleeding, poor oral intake or impaired
iron absorption. Two large prospective observational
studies showed that intravenous iron could correct iron
deficiency anaemia effectively in patients with cancer.[7] [8]
However, previous studies in patients with cancer
focused on anaemia with baseline haemoglobin levels of
9 to 11 g/dL.[7] [8] [9] [10] [11] [12] In addition, the majority of the
aforementioned studies assessed the trend of haemoglobin
levels at a monthly interval and therefore did not provide
much information about haemoglobin levels during the
first 4 weeks after treatment.
The evidence for intravenous iron in patients with cancer
and more severe anaemia (e.g., haemoglobin 7-9 g/dL) is more limited. These patients may have higher rates of
bleeding and more prominent anaemic symptoms that
require rapid correction of haemoglobin levels. Data on
the rate of haemoglobin response following intravenous
iron administration in these patients are lacking. Blood
transfusion can correct anaemia quickly, but it is limited
by supply, carries risk, and may be associated with
poorer oncological outcomes.[13] It is not known whether
intravenous iron can correct anaemia rapidly to avoid
transfusion in patients with lower baseline haemoglobin
levels.
Ferric carboxymaltose (FCM) is a form of intravenous
iron. It was approved by the United Kingdom Medicines
and Healthcare products Regulatory Agency in 2007 for
iron deficiency anaemia when oral iron preparations are
ineffective or cannot be used. Since January 2019, our
department has been using FCM to correct iron deficiency
anaemia. The aim of this study was to determine the
efficacy of FCM in patients with cancer and moderate to
severe anaemia, particularly during the first 4 weeks of
treatment. Use of intravenous iron is part of the patient
blood management approach to reduce transfusion and
improve clinical outcomes.[14]
METHODS
Study Design and Population
This single-centre retrospective study included all consecutive patients with cancer who received at least
one dose of FCM between 1 January 2019 and 30 June
2019 in our centre. The study was approved by the
Research Ethics Committee of North Territory West
Cluster, Hospital Authority (Ref NTWC/REC/19055).
The STROBE guidelines were used to ensure the
reporting of this study.[15]
In our centre, clinicians prescribed FCM and arranged
follow-up blood tests according to our department
protocol. The eligibility criteria in our department
protocol were Karnofsky Performance Status ≥60, iron
saturation <20% with ferritin <220 pmol/L, and either
haemoglobin <8 g/dL or haemoglobin 8 to 9 g/dL with
at least one of the following: active bleeding, on regular
proton pump inhibitors/histamine 2 blockers, or failure
of oral iron to induce adequate haemoglobin rise. These
criteria were devised to select patients in whom oral
iron would likely be ineffective. Patients with low
haemoglobin (<8 g/dL) or active bleeding with stable
haemodynamics require rapid replacement of the
iron store, which could not be achieved by oral iron.
Absorption of oral iron is enhanced by gastric pH and is
reduced by medications that inhibit gastric acid release.
The exclusion criteria included allergy to intravenous
iron, active infection, asthma, allergy to more two or
more drugs, inflammatory joint disease, cirrhosis, and
hypophosphataemia at baseline.
Patients receiving FCM had baseline and follow-up
blood tests according to our department protocol. At
baseline, blood tests including complete blood count
(CBC), reticulocyte count, liver and renal function tests,
and levels of calcium, phosphate, iron saturation, and
ferritin were performed. At 1 week and 2 weeks after
the first dose of FCM, CBC and reticulocyte count were
repeated. At 4 weeks after the first dose of FCM, blood
tests including CBC, reticulocyte count, and calcium,
phosphate, iron saturation, and ferritin levels were
repeated. Calcium and phosphate levels were measured
at baseline and at day 28 because intravenous iron is
known to cause hypophosphataemia by increasing
urinary phosphate excretion.[16] Thereafter, the frequency
of blood tests was decided by the treating clinician.
Transfusion of packed cells was arranged by the treating
clinician, as clinically indicated.
The dose of FCM was determined according to the
patient’s body weight. A single dose of 500 mg was
given to patients whose body weight was <40 kg.
Two doses of 750 mg one week apart were given to patients whose body weight was 40 to 70 kg. Two doses
of 1000 mg one week apart were given to patients whose
body weight was >70 kg. Oral iron was withheld for at
least 4 weeks after intravenous iron administration. The
presence of any anaemic symptoms, including dizziness,
fatigue, shortness of breath or palpitation was assessed at
baseline and at follow-up visits. The interval of follow-up
visits was decided by the treating clinicians.
Parameters
Baseline characteristics including age, gender,
performance status, cancer type, treatment intent
(curative or palliative), and concurrent cancer treatment
(e.g., chemotherapy, target therapy, radiotherapy) were
accessed from the clinical notes. The dose and date
of FCM administration were retrieved from the drug
dispensing history within the electronic patient record.
The blood results at baseline, day 7, day 14, and
day 28 were evaluated to investigate the changes in
haemoglobin, mean corpuscular volume, reticulocyte
count, iron saturation, ferritin, and phosphate levels.
Blood results were recorded as corresponding to day 7,
day 14, and day 28 if they were obtained at 7±3 days,
14±4 days, and 28±4 days after the first dose of
intravenous FCM, respectively. When several blood
results from a particular week were available, the results
closest to the pre-determined dates (i.e., day 7, day
14, and day 28) were used. These blood results were
retrieved from the electronic patient record.
To assess the sustainability of haemoglobin response,
the patients’ haemoglobin levels at day 60 (defined as
60±10 days after the first dose of FCM) were recorded.
The consultation notes were reviewed to assess any
reported side effects and improvement of anaemic
symptoms including dizziness, palpitation, fatigue, and
shortness of breath. Information regarding transfusions
was assessed using the Clinical Data Analysis and
Reporting System, which was able to retrieve all
of the patients’ blood transfusion history given in
hospitals under the Hospital Authority, Hong Kong.
The transfusion history was verified by reviewing the
consultation notes.
Objectives
The primary objective was to evaluate the haemoglobin
increase from baseline to day 7, day 14, and day 28.
The secondary objectives were to investigate the
sustainability of haemoglobin at day 60, the change in iron profile, the safety profile of FCM, and its effects on
the patients’ transfusion requirements. Each patient’s
transfusion requirement was assessed by comparing the
number of packed cells transfused within 60 days before
and 60 days after FCM.
Confounding Factors
Transfusion could be an important confounding factor
that modulates changes to haemoglobin levels. Patients
who received transfusions before day 28 were excluded
from analysis of the haemoglobin trend, change of
iron profile, and improvement of anaemic symptoms.
Transfusion can raise haemoglobin levels, alter the iron
profile, and improve anaemic symptoms. If patients who
received transfusions were not excluded, this would
overestimate the effects of FCM on the above parameters.
Patients who received haemostatic interventions (e.g.,
radiotherapy, embolisation) within 60 days of the
first dose of FCM were excluded from analysis of the
transfusion requirement. Haemostatic radiotherapy to the
tumour or embolisation of bleeding vessels could alter
the bleeding rate and be a significant confounding factor
that modulates the change in transfusion requirements
after FCM.
Statistical Analysis
Categorical data are summarised as number and
percentage. Continuous variables are presented as
mean±standard deviation unless otherwise stated.
Paired-samples t tests were used to assess the changes in
haemoglobin levels, iron profile, and number of packed
cells transfused before vs after FCM.
Patients with missing data for any of the primary or
secondary objectives for any reason, including death,
were excluded from analysis for that study objective.
RESULTS
Patient Characteristics
All of the 34 patients given FCM from January to
June 2019 were included for analysis. Their baseline
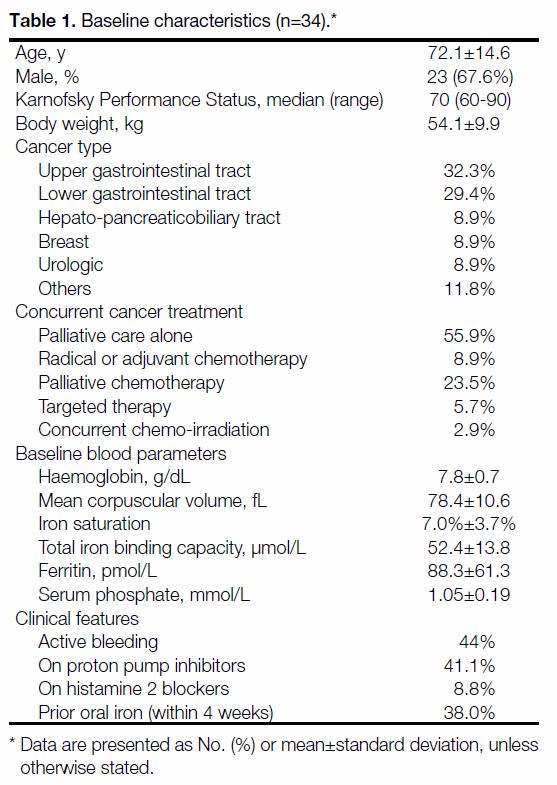
characteristics are shown in Table 1. The mean baseline haemoglobin level was 7.8 g/dL.
Table 1. Baseline characteristics (n=34).
Among the patients, 82.4% received 1500 mg divided
into two infusions. The other doses given included a
single infusion of 500 mg (8.8%), a single infusion of
750 mg (6%), and 2000 mg divided into two infusions
(3%).
Efficacy
After patients who received transfusions (n = 6) or died
(n = 3) within 28 days of the first dose of FCM were
excluded, data from 25 patients could be analysed for
changes of haemoglobin and iron profile. They all had
CBC available at day 0, day 7, day 14, and day 28. The
median intervals between the dates of the ‘day 0’, ‘day
7’, and ‘day 14’ CBC time points and that of the baseline
CBC were 7 days (range, 7-10 days), 14 days (range,
13-18 days), and 28 days (range, 26-32 days),
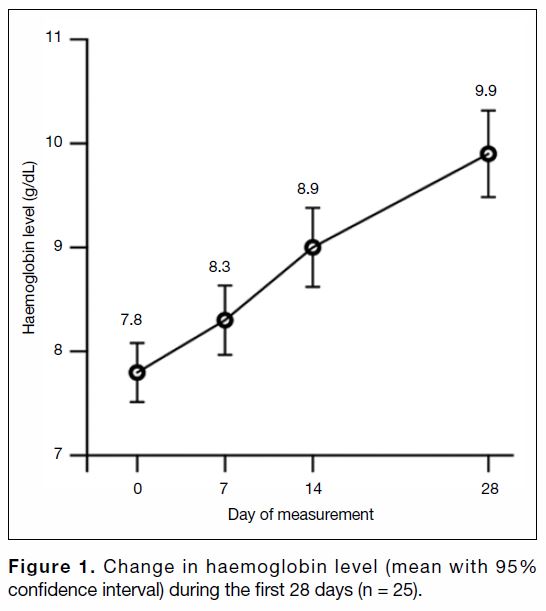
respectively. The trend of mean haemoglobin levels
with 95% confidence intervals is shown in Figure 1.
Compared with day 0, the mean haemoglobin level had
increased by 0.5 g/dL at day 7 (p < 0.01), 1.1 g/dL at day
14 (p < 0.001), and 2.1 g/dL at day 28 (p < 0.001).
Figure 1. Change in haemoglobin level (mean with 95% confidence interval) during the first 28 days (n = 25).
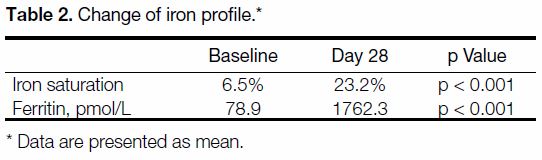
All 25 patients had baseline iron profiles including
iron saturation and ferritin available. Of the patients,
92% had a repeated iron profile and ferritin available at
28 days±4 days after the first dose of FCM. There was
a statistically significant (p < 0.001) increase in iron
saturation and ferritin at that time point. The mean iron
saturation and ferritin levels at baseline and day 28 are
shown in Table 2.
Table 2. Change of iron profile
Among the six patients who received transfusion
within 28 days of the first dose of FCM, five had
upper gastrointestinal tract tumours with bleeding.
They were admitted for haematemesis or melaena with
haemoglobin level drops that required transfusion.
One of them underwent embolisation of the bleeding
vessel by an interventional radiologist after transfusion
to stop the massive bleeding. Another patient had
haemoperitoneum that required an emergency
angiogram and transfusion.
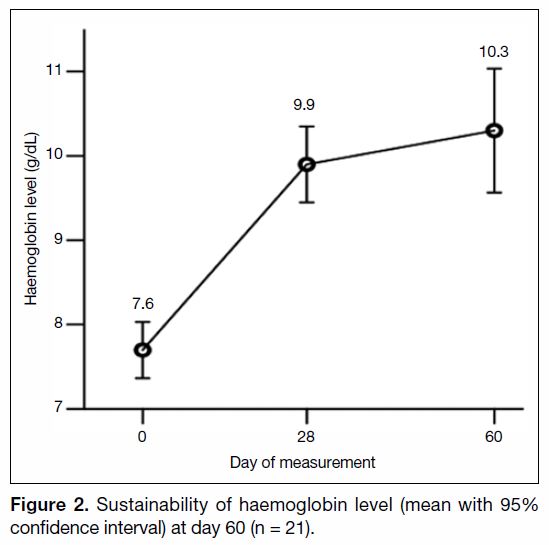
There was a sustainable rise of haemoglobin level at
60±10 days after the first dose of FCM. Among the
25 patients included above, two received transfusions
and one died between day 28 and day 60. One patient did
not have a haemoglobin level check at day 60. The two
patients who had transfusions between day 28 and day
60 had tumour progression in the upper gastrointestinal
tract that caused increases in tumour bleeding. They both
had metastatic cancer without effective cancer treatment.
The haemoglobin levels of the remaining 21 patients were analysed to assess the sustainability of FCM-induced
haemoglobin rise. Their mean haemoglobin
levels with 95% confidence intervals at baseline, day 28,
and day 60 are shown in Figure 2.
Figure 2. Sustainability of haemoglobin level (mean with 95% confidence interval) at day 60 (n = 21).
There was also a statistically significant (p = 0.016)
reduction in the number of packed cells transfused
following FCM administration. After excluding patients
who received haemostatic intervention, the numbers of
packed cells transfused in each patient within 60 days
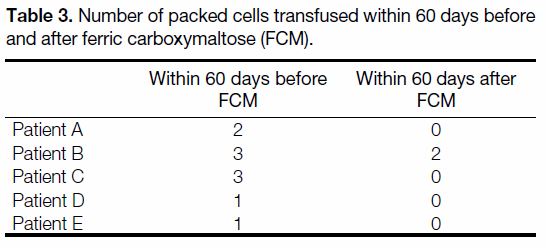
before and after FCM are shown in Table 3.
Table 3. Number of packed cells transfused within 60 days before and after ferric carboxymaltose (FCM).
In total, 88% of patients reported improvement of at least
one of their anaemic symptoms.
Safety
No hypersensitivity reactions or abnormalities of vital
signs were reported following FCM administration.
All patients had baseline phosphate levels available.
Phosphate levels measured at 28±4 days after the first dose of FCM were available for 20 patients. There was a
statistically significant (p = 0.002) reduction of phosphate
levels from baseline to day 28 (from 1.01 mmol/L
to 0.72 mmol/L). No clinical symptoms attributed to
hypophosphataemia were reported.
DISCUSSION
Our study showed a prompt response in the haemoglobin
levels of patients with cancer and moderate to severe
anaemia who were given FCM. Compared with baseline,
their haemoglobin levels rose by 0.5 g/dL and 1.1 g/dL
at day 7 and day 14, respectively. By day 28, the
haemoglobin rise reached 2.1 g/dL and was sustained
at 60 days after FCM. This haemoglobin response was
accompanied by a statistically significant improvement
of the iron profile and a corresponding reduction in
transfusion requirements. The patients’ mean baseline
haemoglobin level was 7.8 g/dL, which was close to the
transfusion threshold in clinical practice.
The results of the present study suggest that intravenous
iron could be a viable treatment option for patients
with cancer and symptomatic iron deficiency anaemia
who may otherwise require transfusions. This result is
particularly meaningful in the context of a worldwide
shortage of blood products. The demand for donated
blood has been rising worldwide as a result of the ageing
population.[17] The use of intravenous iron may help to
avoid the cost and risks of transfusion of donated blood,
which is limited in supply.
The rapid rise of haemoglobin levels induced by
intravenous iron could be particularly useful in patients
who will receive radical radiotherapy. Anaemia has
been shown to correlate with poor tumour oxygenation
and may confer radioresistance.[18] Consequently, before
radical radiotherapy, some institutions (including ours)
arrange transfusions of packed cells for patients whose
haemoglobin levels are <10 g/dL.[19] As intravenous
iron can raise haemoglobin levels promptly, patients
with cancer and iron deficiency anaemia can be treated
with intravenous iron while radiotherapy planning is
in progress. This may obviate or reduce the need for
transfusions to top up haemoglobin to the target level,
particularly if the period between intravenous iron
infusion and the start of radiotherapy is more than
2 weeks. If an immediate rise of haemoglobin is desired,
transfusion followed by correction of the iron deficiency
by either the oral or intravenous route can be considered.
Of our patients, 24% required transfusions within the 60 days after FCM. Most of them had tumours in the
upper gastrointestinal tract that were causing significant
bleeding. In these patients, the rate of blood loss likely
exceeded the rate of restored erythropoiesis following
intravenous iron replacement. This highlighted the
importance of control of the bleeding source, for
instance by haemostatic radiotherapy or embolisation of
the bleeding vessel.
Our study has several strengths. First, the vast majority
of our patients had critical haematological parameters
(including CBC and iron profile) at baseline and at
scheduled intervals. For instance, all of our patients
had baseline haemoglobin, iron saturation, and ferritin
levels available. In comparison, among the three largest
reported series of patients with cancer who received
FCM, baseline iron saturation and ferritin levels were
only available in 54% to 74% and 54% to 57% of
patients, respectively.[7] [8] [11] Excluding patients who died
or received transfusions, all of our patients underwent
assessment of haemoglobin levels at the pre-specified
intervals in the first 4 weeks. The relative completeness
of the data enhanced the study’s statistical precision.
Second, all patients in our study were selected to receive
FCM based on our department protocol’s pre-specified
eligibility criteria. Our eligibility criteria for FCM,
described above, targeted patients in whom oral iron
would likely be ineffective. The eligibility criteria for
intravenous iron applied by the two large multi-centred
series of FCM in patients with cancer were not well
defined.[7] [8] The lack of consistent eligibility criteria in
those multi-centred studies likely reflected variation
in the study centres’ practices of selecting patients
for intravenous iron. Our results, based on the clear,
pre-specified eligibility criteria outlined in our
department protocol, can be informative to other
oncology departments that are planning to incorporate
the use of intravenous iron into their practice.
Our study also has some weaknesses. First, there
could be confounding factors that affect the changes in
haemoglobin levels and transfusion requirements. These
confounding factors could include tumour progression or
shrinkage, which could lead to variability of the bleeding
rate, and concurrent myelosuppressive cancer treatment.
The lack of a comparator or a randomised design could
make interpretation of the haemoglobin rise difficult.
Consequently, the changes in haemoglobin levels
observed in our study might not be fully attributable to
the effects of FCM.
Second, oral iron is a cheap and convenient treatment for
iron deficiency anaemia. Our study does not answer the
question of how to select patients for intravenous iron.
Randomised trials comparing intravenous iron with oral
iron, using pre-specified eligibility criteria, are needed to
guide decisions about patient selection for intravenous
iron. A published randomised controlled trial failed to
show the superiority of intravenous iron over oral iron
in patients with cancer.[20] This result could be related
to the eligibility criteria of that trial, which included all
patients with cancer and iron deficiency anaemia who
had haemoglobin levels <12 g/dL. The superiority of
intravenous iron over oral iron would likely be most
obvious in patients who showed unsatisfactory response
to oral iron, such as those fulfilling our study’s eligibility
criteria.
Third, the assessment of anaemic symptoms in our
patients was performed by the treating clinicians only.
Assessment of anaemic symptoms and quality of life is
best performed by a validated patient-reported outcome
instrument. Examples of such instruments to assess iron
deficiency anaemia include the Functional Assessment
of Cancer Therapy−Anaemia and the 36-item Short
Form Health Survey.[21] Without such instruments, our
assessment of anaemic symptoms is less reliable.
Our study’s external validity is limited by its small sample
size and the lack of a comparator group. Nevertheless, the
encouraging result of rapid haemoglobin rise following
intravenous iron administration can still be informative
to oncologists, who might want to consider alternatives
to blood transfusion.
CONCLUSION
In patients with cancer and symptomatic iron deficiency
anaemia, FCM can correct iron deficiency, raise
haemoglobin levels promptly, and reduce transfusion
requirements. This may reduce the demand for blood
products and avoid risks related to blood transfusion.
REFERENCES
1. Ludwig H, Müldür E, Endler G, Hübl W. Prevalence of iron
deficiency across different tumors and its association with poor
performance status, disease status and anemia. Ann Oncol.
2013;24:1886-92. Crossref
2. Shepshelovich D, Rozen-Zvi B, Avni T, Gafter U, Gafter-Gvili A.
Intravenous versus oral iron supplementation for the treatment of
anemia in CKD: an updated systematic review and meta-analysis.
Am J Kidney Dis. 2016;68:677-90. Crossref
3. Van Wyck DB, Mangione A, Morrison J, Hadley PE, Jehle JA,
Goodnough LT. Large-dose intravenous ferric carboxymaltose
injection for iron deficiency anemia in heavy uterine bleeding: a randomized, controlled trial. Transfusion. 2009;49:2719-28. Crossref
4. Lindgren S, Wikman O, Befrits R, et al. Intravenous iron sucrose is
superior to oral iron sulphate for correcting anaemia and restoring
iron stores in IBD patients: a randomized, controlled, evaluator-blind,
multicentre study. Scand J Gastroenterol. 2009;44:838-45. Crossref
5. Litton E, Xiao J, Ho KM. Safety and efficacy of intravenous iron
therapy in reducing requirement for allogeneic blood transfusion:
systematic review and meta-analysis of randomised clinical trials.
BMJ. 2013;347:f4822. Crossref
6. Busti F, Marchi G, Ugolini S, Castagna A, Girelli D. Anemia and
iron deficiency in cancer patients: role of iron replacement therapy.
Pharmaceuticals (Basel). 2018;11:94. Crossref
7. Steinmetz T, Tschechne B, Harlin O, et al. Clinical experience
with ferric carboxymaltose in the treatment of cancer- and
chemotherapy-associated anaemia. Ann Oncol. 2013;24:475-82. Crossref
8. Toledano A, Luporsi E, Morere JF, et al. Clinical use of ferric
carboxymaltose in patients with solid tumours or haematological
malignancies in France. Support Care Cancer. 2016;24:67-75. Crossref
9. Abdel-Razeq H, Abbasi S, Saadi I, Jaber R, Abdelelah H.
Intravenous iron monotherapy for the treatment of non-iron-deficiency
anemia in cancer patients undergoing chemotherapy: a
pilot study. Drug Des Devel Ther. 2013;7:939-44. Crossref
10. Calleja JL, Delgado S, del Val A, et al. Ferric carboxymaltose
reduces transfusions and hospital stay in patients with colon cancer
and anemia. Int J Colorectal Dis. 2016;31:543-51 Crossref
11. Coussirou J, Debourdeau A, Stancu A, et al. Impact of ferric
carboxymaltose on the evolution of hemoglobin and ECOG
performance status in iron-deficient patients with solid tumors:
a 3-month follow-up retrospective study. Support Care Cancer.
2018;26:3827-34. Crossref
12. Lima J, Gago P, Rocha M, et al. Role of intravenous iron in the
treatment of anemia in patients with gastrointestinal tract tumors
undergoing chemotherapy: a single-center, observational study. Int
J Gen Med. 2018;11:331-6. Crossref
13. Goubran HA, Elemary M, Radosevich M, Seghatchian J, El-Ekiaby M,
Burnouf T. Impact of transfusion on cancer growth and outcome.
Cancer Growth Metastasis. 2016;9:1-8. Crossref
14. Franchini M, Marano G, Veropalumbo E, et al. Patient blood
management: a revolutionary approach to transfusion medicine.
Blood Transfus. 2019;17:191-5.
15. von Elm E, Altman DG, Egger M, et al. The Strengthening the
Reporting of Observational Studies in Epidemiology (STROBE)
statement: guidelines for reporting observational studies. J Clin
Epidemiol. 2008;61:344-9. Crossref
16. Hardy S, Vandemergel X. Intravenous iron administration
and hypophosphatemia in clinical practice. Int J Rheumatol.
2015;2015:468675. Crossref
17. Ali A, Auvinen MK, Rautonen J. The aging population poses a
global challenge for blood services. Transfusion. 2010;50:584-8. Crossref
18. Becker A, Stadler P, Lavey RS, et al. Severe anemia is associated
with poor tumor oxygenation in head and neck squamous cell
carcinomas. Int J Radiat Oncol Biol Phys. 2000;46:459-66. Crossref
19. Harrison LB, Chadha M, Hill RJ, Hu K, Shasha D. Impact of tumor
hypoxia and anemia on radiation therapy outcomes. Oncologist.
2002;7:492-508. Crossref
20. Birgegård G, Henry D, Glaspy J, Chopra R, Thomsen LL,
Auerbach M. A randomized noninferiority trial of intravenous
iron isomaltoside versus oral iron sulfate in patients with
nonmyeloid malignancies and anemia receiving chemotherapy:
the PROFOUND trial. Pharmacotherapy. 2016;36:402-14. Crossref
21. Strauss WE, Auerbach M. Health-related quality of life in patients
with iron deficiency anemia: impact of treatment with intravenous
iron. Patient Relat Outcome Meas. 2018;9:285-98. Crossref
| Attachment | Size |
|---|---|
| v23n4_Efficacy.pdf | 505.38 KB |