Lutetium-177 DOTATATE Therapy for Neuroendocrine Tumours: Report of Two Cases
CASE REPORT
Lutetium-177 DOTATATE Therapy for Neuroendocrine Tumours:
Report of Two Cases
YH Hui, BT Kung, TK Au Yong
Pong Ding Yuen Clinical PET-CT Centre and Nuclear Medicine Unit, Queen Elizabeth Hospital, Jordan, Hong Kong
Correspondence: Dr YH Hui, Nuclear Medicine Unit, Queen Elizabeth Hospital, Jordan, Hong Kong. Email: yancol82@yahoo.com.hk
Submitted: 18 Mar 2019; Accepted: 23 May 2019.
Contributors: All authors designed the study. YHH acquired the data, analysed the data, and drafted the manuscript. All authors critically revised
the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for
publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: As editors of the journal, YH Hui and TK Au Yong were not involved in the peer-review process. Other authors have
disclosed no conflicts of interest.
Funding/Support: This case report received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Ethics Approval: The patients were treated in accordance with the tenets of the Declaration of Helsinki. The patients provided written informed
consent for all treatments and procedures.
Declaration: The results of this report were presented in part at the 26th Annual Scientific Meeting of the Hong Kong College of Radiologists,
Hong Kong, 17-18 November 2018 and the 5th Theranostics World Congress, Jeju, South Korea, 1-3 March 2019.
INTRODUCTION
Neuroendocrine tumours (NETs) are rare but the
incidence has been reported to be increasing in recent
years.[1] NETs are characterised by their ability to produce
and secrete a variety of peptide hormones. The primary
treatment modality for NETs is surgical[2] but most
patients present with disseminated inoperable disease.[3]
Owing to the high-level expression of somatostatin
receptors in NETs, especially subtypes 2 and 5, peptide
receptor radionuclide therapy (PRRT) with radioactive
somatostatin analogues have recently been developed
and show promising results.[4] Yttrium SST2 analogues
have been used in our hospital for PRRT in the past.
Lutetium-177 (Lu-177) has been available in Hong
Kong only since 2016. Two patients were referred to our
unit between July 2017 and September 2018, and have
completed treatment cycles with PRRT. The aims of
this report are to summarise and present our treatment
results and to assess the clinical safety and potential
complications in those patients who received Lu-177
DOTATATE PRRT at our unit.
CASE 1
A 57-year-old man presented in August 2015 with a
3-month history of obstructive jaundice. Investigations
revealed a pancreatic head mass with cytology suggestive
of NET. Total pancreatectomy was performed in
February 2016 with histopathology showing grade 1
NET and one-quarter of the peripancreatic lymph nodes
were positive. He was closely monitored. Unfortunately,
Ga-68 DOTATATE positron emission tomography/computed tomography in December 2016 showed
bilobar hepatic metastases. He was initially started on
lanreotide but follow-up imaging confirmed disease
progression with abdominal discomfort. He was then
referred in May 2017 for PRRT at our unit. Initial
chromogranin A (CgA) was 276 ng/mL (normal reference
range, 27-94 ng/mL). He received 5 cycles of 150 mCi
Lu-177 DOTATATE at 8- to 10-week intervals, with the
first cycle given in July 2017. Renal protective amino
acids were infused 30 minutes before administration of
the radiopharmaceutical. He experienced no immediate
adverse effects or hormone-related crisis. Scintigraphy with single-photon emission computed tomography/computed tomography was performed 4 days after each
cycle. Initially there was intense Lu-177 DOTATATE
uptake over the hepatic metastases. Subsequently, scintigraphy revealed interval decrease in size and tracer
uptake of the lesions. His last treatment cycle was in
March 2018 and only minimal uptake was seen in the
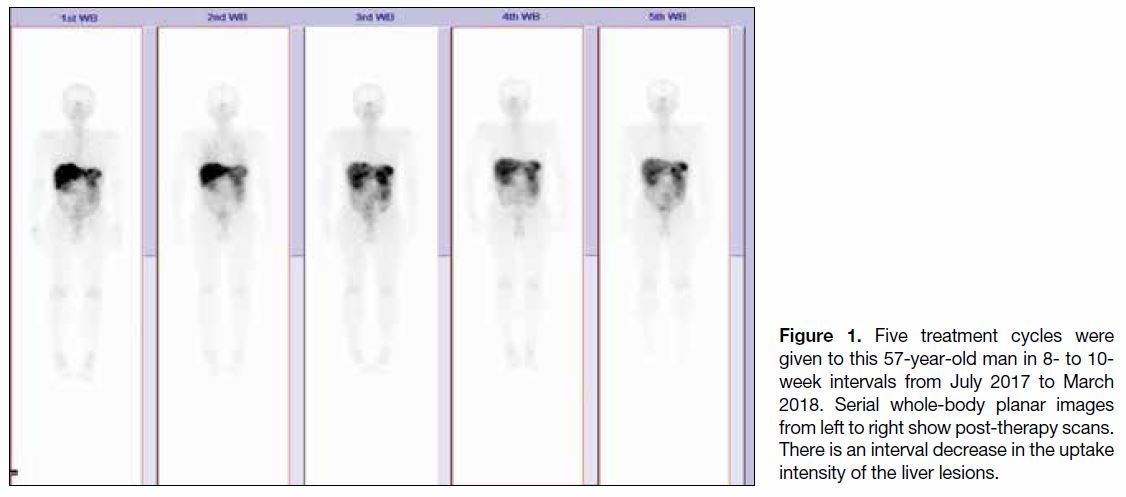
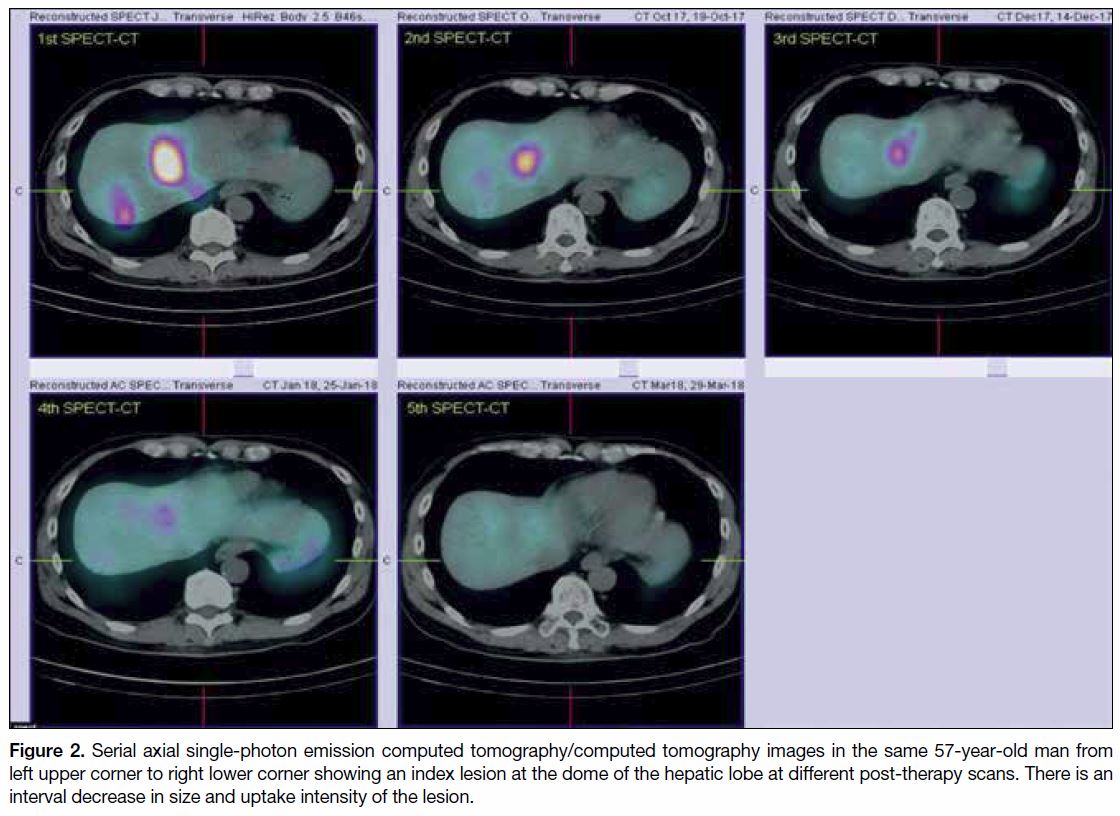
liver lesions on the final (fifth cycle) post-therapy scan (Figures 1 and 2). Blood test results at 2-week intervals
after each cycle for complete blood count, liver and
renal function were unremarkable. His symptoms had
subsided after the second cycle of treatment. His CgA
had reduced to 127 ng/mL at his last follow-up in July
2018 and he was symptom-free.
Figure 1. Five treatment cycles were
given to this 57-year-old man in 8- to 10-week intervals from July 2017 to March
2018. Serial whole-body planar images
from left to right show post-therapy scans.
There is an interval decrease in the uptake
intensity of the liver lesions.
Figure 2. Serial axial single-photon emission computed tomography/computed tomography images in the same 57-year-old man from
left upper corner to right lower corner showing an index lesion at the dome of the hepatic lobe at different post-therapy scans. There is an
interval decrease in size and uptake intensity of the lesion.
CASE 2
In May 2015, a 69-year-old woman underwent surgery
in China for removal of a rectal NET (medical records
are incomplete). Histopathology showed grade 2 NET.
An octreotide scan in July 2015 was negative and she
was kept under observation. Multiple hepatic metastases
were found on computed tomography in February
2016. She was given lanreotide followed by everolimus
but subsequent imaging revealed persistent disease
progression. She was then referred to our unit for PRRT.
The patient’s chief complaint was of mild abdominal
pain. Octreotide scan in August 2017 showed multiple
liver and left common iliac nodal metastases. Her initial
CgA was 195 ng/mL. She was treated with 5 cycles of
150 mCi Lu-177 DOTATATE at 8- to 10-week intervals
with the first cycle beginning in October 2017. Renal
protective amino acid infusion, post-therapy scintigraphy,
and follow-up blood tests were similar to those of
Case 1. In the first 3 cycles, the patient complained of
mild nausea after infusion of the radiopharmaceutical,
resolved with antiemetics, but no hormone-related crisis.
The first scan after therapy demonstrated intense uptake
over the liver, left iliac nodal and right pubic bony
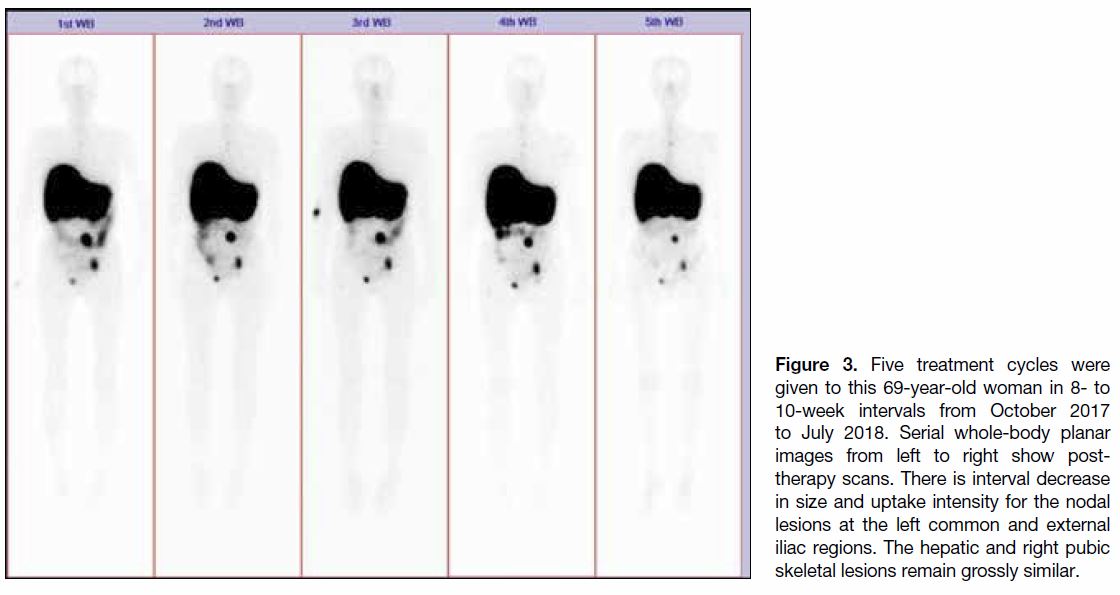
metastases. Subsequent scintigraphy showed interval
reduction in the nodal lesion size and uptake intensity
while the skeletal and hepatic lesions remained relatively
unchanged (Figure 3). Her last treatment cycle was in
July 2018. Only transient grade 1 marrow toxicity was
encountered in the last blood test, otherwise there was
no persistent hepatic or renal impairment. The patient’s
symptoms improved after each treatment cycle. Her
CgA had reduced to 22 ng/mL at her last follow-up visit
in September 2018.
Figure 3. Five treatment cycles were
given to this 69-year-old woman in 8- to
10-week intervals from October 2017
to July 2018. Serial whole-body planar
images from left to right show post-therapy
scans. There is interval decrease
in size and uptake intensity for the nodal
lesions at the left common and external
iliac regions. The hepatic and right pubic
skeletal lesions remain grossly similar.
DISCUSSION
Because of the high-level expression of somatostatin
receptors, NETs are ideal neoplasms for treatment with
PRRT. Since the publication of the landmark randomised
NETTER-1 study in 2017 that showed a markedly
longer progression-free survival and significantly
higher response rate compared with high-dose long-acting
release octreotide among patients with advanced
midgut NET, there has been growing interest in the
therapeutic application of radio-labelled somatostatin
analogue therapy for these patients.[5] Lu-177 has been
available in Hong Kong only since 2016. To the best
of our knowledge, we are the first public hospital in
Hong Kong to provide this treatment. The inclusion
and exclusion criteria for PRRT in our institution are mainly in accordance with recommendations of the
joint International Atomic Energy Agency, European
Association of Nuclear Medicine and Society of Nuclear
Medicine and Molecular Imaging.[6] Patients referred
with inoperable NET and proven somatostatin receptor
avidity on scintigraphy without severely compromised
bone marrow and renal function, as suggested in the
guideline,[6] are considered candidates for such therapy.
A total of 10 cycles of therapy were given to our patients.
The most frequent acute adverse effect observed in the
first 24 to 48 hours after administration of PRRT was
nausea (in 3 out of 10 cycles, 30%) but it was transitory
and mild in severity and alleviated by prescription of an
antiemetic. Neither patient reported increased abdominal
pain or any hormone-related crisis. Our finding of few
and mild adverse effects are consistent with reported
findings.[7] [8] [9] During serial serological monitoring after
therapy, in 10 treatment cycles only 1 (10%) instance
of transient grade 1 marrow toxicity was encountered.
Öberg[8] and Seregni et al[10] reported transient low-grade
marrow toxicity in 20% and 23% of patients,
respectively. No myelodysplastic syndrome was
observed in our patients. This may partly be related to
the relative short follow-up period as this serious adverse
effect may take up to months or years to develop.11 There
was no hepatic or renal impairment in our patients. In
one study by Kwekkeboom,[7] more than half of the
patients complained of alopecia. Nonetheless this was
not evident in our patients. Overall, the treatment was
well tolerated.
Despite being a preliminary study, our results based on
scintigraphic findings are quite promising. One of our
patients achieved a partial treatment response while
the other remains stable according to RECIST criteria.
A response rate of 18% was achieved in the Lu-177
DOTATATE treatment groups in the NETTER-1
study.[5] Other studies by Matović,[12] Kwekkeboom et al,[7]
and Basu et al[13] reported patient response rates of 26%,
46%, and 60%, respectively. Our observed scintigraphic
improvements were slightly superior to those reported
in the literature, although our study is preliminary with
only two patients. Improved scintigraphic findings in
our patients was coupled with improved symptoms
and biochemical findings. Both patients demonstrated
improvement or resolution of symptoms after treatment.
At a biochemical level, our patients presented with
elevated tumour marker levels before therapy, and both
had an interval decrease in tumour marker level after
treatment. Hervás et al[9] evaluated treatment response in seven neuroendocrine patients treated with PRRT, five
of whom presented with raised tumour markers before
therapy and all demonstrated interval improvement after
treatment. Biochemical improvement after treatment was
also observed by Basu et al[13] with reduced CgA level in
three of five reported cases.
Since NETs are rare and our PRRT service with Lu-177
was started only in 2017, our study is limited to two
cases. The preliminary nature of this report also limits
evaluation of long-term toxicity and treatment response.
Future studies with a larger number of patients and longer
follow-up period are needed to enable more in-depth
assessment of adverse effects and outcome parameters
such as time to progression or overall survival.
CONCLUSION
Our initial experience suggests that PRRT is a safe and
effective treatment for metastatic inoperable NETs.
REFERENCES
1. Lawrence B, Gustafsson BI, Chan A, Svejda B, Kidd M, Modlin IM.
The epidemiology of gastroenteropancreatic neuroendocrine
tumors. Endocrinol Metab Clin North Am. 2011;40:1-18, vii. Crossref
2. Norton JA. Endocrine tumours of the gastrointestinal tract. Surgical
treatment of neuroendocrine metastases. Best Pract Res Clin
Gastroenterol. 2005;19:577-83. Crossref
3. Sahani DV, Bonaffini PA, Fernández-Del Castillo C, Blake MA.
Gastroenteropancreatic neuroendocrine tumors: role of imaging in
diagnosis and management. Radiology. 2013;266:38-61 Crossref
4. Sabet A, Haslerud T, Pape UF, Sabet A, Ahmadzadehfat H,
Grünwald F, et al. Outcome and toxicity of salvage therapy with
177Lu-octreotate in patients with metastatic gastroenteropancreatic
neuroendocrine tumours. Eur J Nucl Med Mol Imaging.
2014;41:205-10. Crossref
5. Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B,
et al. Phase 3 trial of 177Lu-Dotatate for midgut neuroendocrine
tumors. N Engl J Med. 2017;376:125-35. Crossref
6. Bodei L, Mueller-Brand J, Baum RP, Pavel ME, Hörsch D,
OʼDorisio MS, et al. The joint IAEA, EANM, and SNMMI practical
guidance on peptide receptor radionuclide therapy (PRRNT)
in neuroendocrine tumours. Eur J Nucl Med Mol Imaging.
2013;40:800-16. Crossref
7. Kwekkeboom DJ, de Herder WW, Kam BL, van Eijck CH,
van Essen M, Kooij PP, et al. Treatment with the radiolabeled
somatostatin analog [177Lu-DOTA0,Tyr3] Octreotate: toxicity,
efficacy, and survival. J Clin Oncol. 2008;26:2124-30. Crossref
8. Öberg K. Molecular imaging radiotherapy: theranostics for
personalized patient management of neuroendocrine tumors
(NETs). Theranostics. 2012;2:448-58. Crossref
9. Hervás I, Bello P, Falgas M, Del Olmo MI, Torres I, Olivas C, et al.
177Lu-DOTATATE treatment in neuroendocrine tumors. A
preliminary study. Rev Esp Med Nucl Imagen Mol. 2017;36:91-8. Crossref
10. Seregni E, Maccauro M, Chiesa C, Mariani L, Pascali C, Mazaferro V,
et al. Treatment with tandem [90Y] DOTA-TATE and [177Lu]
DOTA-TATE of neuroendocrine tumours refractory to conventional
therapy. Eur J Nucl Med Mol Imaging. 2014;41:223-30. Crossref
11. Valkema R, De Jong M, Bakker WH, Breeman WA, Kooij PP,
Lugtenburg PJ, et al. Phase I study of peptide receptor radionuclide
therapy with [In-DTPA] octreotide: the Rotterdam experience.
Semin Nucl Med. 2002;32:110-22. Crossref
12. Matović M. Peptide receptor radionuclide therapy of neuroendocrine tumors: Case series. Arch Oncol. 2012;20:143-8. Crossref
13. Basu S, Ranade R, Thapa P. Metastatic neuroendocrine tumor with
extensive bone marrow involvement at diagnosis: evaluation of
response and hematological toxicity profile of PRRT with (177)
Lu-DOTATATE. World J Nucl Med. 2016;15:38-43. Crossref
| Attachment | Size |
|---|---|
| v23n4_Lutetium.pdf | 207.88 KB |