Iterative Model Reconstruction in Lumbar Spine Image Retrieval from Computed Tomography of the Abdomen and Pelvis
ORIGINAL ARTICLE CME
Iterative Model Reconstruction in Lumbar Spine Image Retrieval from Computed Tomography of the Abdomen and Pelvis
SY Tan1, A Kuganesan1, K Buchan1, KK Lau1,2
1 Diagnostic Imaging Department, Monash Health, Victoria, Australia
2 Faculty of Medicine, Nursing and Health Sciences, Monash University, Victoria, Australia
Correspondence: Dr SY Tan, Diagnostic Imaging Department, Monash Health, Victoria, Australia. Email: tanshuyi@gmail.com
Submitted: 1 Sep 2019; Accepted: 18 Nov 2019.
Contributors: SYT and KKL designed the study. SYT acquired the data. SYT and KKL analysed the data. SYT drafted the manuscript. All
authors critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study,
approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics Approval: This study was approved by the Monash Health Human Research Ethics Committee (Ref 16-0000-478Q), and the requirement
for patient consent was waived.
Abstract
Objective
We sought to compare the image quality of iterative model reconstruction (IMR) with statistical iterative
reconstruction (SIR) in lumbar spine (L-spine) images reconstructed from computed tomography (CT) image data
from examinations of the abdomen and pelvis compared with routine L-spine CT images reconstructed with SIR.
Methods
We conducted a retrospective study to compare image noise in L-spine reconstructions using SIR and IMR
techniques in consecutive CT abdomen and pelvis (CTAP) examinations, with L-spine CT images reconstructed using
SIR. Hounsfield units and their standard deviations in areas of image noise were measured in bone, cerebrospinal fluid
(CSF), and lumbar discs. The results of SIR and IMR were compared using paired t tests. The results of CTAP and
CT L-spine were compared using the Mann-Whitney U test. A p value <0.05 was considered significant. Qualitative
assessment of the bone/CSF, disc/CSF, and disc/bone interface was performed by two readers.
Results
IMR generated less image noise than SIR with CTAP and L-spine CT with SIR, particularly on bone windows.
There was also significant improvement in the clarity of interfaces between vertebral bodies, discs, epidural fat, and
CSF with IMR. The mean radiation dose was much higher for L-spine CT compared with CTAP.
Conclusion
IMR is superior to SIR for the image quality of reconstructed lumbar spine images from CTAP. The
IMR lumbar spine image from CTAP image data acquired with a lower radiation dose was also shown to be clearer
when compared with routine SIR L-spine CT images.
Key Words: Artifacts; Image processing, computer-assisted; Radiometry; Spine/diagnostic imaging; Tomography, X-ray
computed
中文摘要
迭代模型重建腹部和盆腔CT的腰椎圖像
SY Tan、A Kuganesan、K Buchan、KK Lau
目的
比較迭代模型重建(IMR)和統計迭代重建(SIR)腹部和盆腔CT的腰椎圖像質量,並與常
規SIR 腰椎CT圖像質量作比較。
方法
這項回顧性研究比較以SIR和IMR方法從連續腹部和盆腔CT重建出腰椎圖像的噪聲,以及比
較SIR重建直接腰椎掃描圖像的噪聲,測量骨、腦脊液和腰椎間盤的圖像噪聲(Hounsfield單位及其
標準差)。使用配對t 檢驗比較SIR和IMR重建腹部和盆腔CT的結果,以及使用Mann-Whitney U檢驗
比較腹部/盆腔CT與腰椎直接CT掃描的結果,p < 0.05為差異有統計學意義。由兩名閱片者對骨與
腦脊液、椎間盤與腦脊液,以及椎間盤與骨界面清晰度進行定性評估。
結果
相比SIR重建腹部和盆腔CT圖像和SIR重建腰椎CT圖像,IMR重建腹部和盆腔CT圖像的噪聲
較少,尤以骨視窗測量為甚。透過IMR重建,椎體、椎間盤、硬膜外脂肪和腦脊液間的界面清晰度
也得到顯著改善。與腹部和盆腔CT相比,腰椎CT的平均放射劑量明顯較多。
結論
以IMR重建腹部和盆腔CT的腰椎圖像質量較SIR為佳。與常規SIR腰椎CT圖像相比,以較低輻射劑量採集腹部和盆腔CT圖像數據的IMR腰椎圖像更加清晰。
INTRODUCTION
Part of the process of optimising a radiographic
examination involves reducing the radiation dose to
as low as reasonably achievable whilst maintaining
adequate image quality for diagnostic purposes.[1] It is
well known that reducing radiation dose increases image
noise, and reduces image quality.[2] Current radiology
practice concentrates on techniques of dose reduction
whilst optimising image quality.[2]
Computed tomography of the abdomen and pelvis
(CTAP) has become the mainstay of a diagnostic
workup in trauma settings,[3] particularly if there is a high
probability of an abdominal or pelvic injury. Lumbar
spine reconstructions from CTAP datasets are frequently
requested by emergency physicians for assessment of
bony injury or pathology.[4] [5] The image quality of the
reconstructions is generally inferior to that of dedicated
CT of the lumbar spine (L-spine CT). However, the
better image quality of L-spine CT is at the expense of
higher radiation dose and potentially missing other intra-abdominal
or pelvic pathology outside the field of view.[6]
Data from the Australian Radiation Protection and
Nuclear Safety Agency have documented higher dose
levels for CT lumbar spine (26 mGy) compared with
CTAP (13 mGy).[7]
The rise of iterative reconstruction (IR) algorithms for
CT in recent years has decreased the need for radiation
dose increases in order to improve image quality.[8] [9]
Filtered back projection was previously the most
commonly used reconstruction algorithm for
conventional CT imaging. It was associated with
increased image noise in larger patients, but its
advantage was a faster image reconstruction rate.[10]
In comparison, IR techniques generated better image
quality with reduced image noise at lower radiation
doses (though longer reconstruction times) compared
with the filtered back projection technique.[9] [11] Dose
reductions from IR techniques range widely in the
available literature,[11] [12] [13] [14] some quoting as high as a 76%
reduction in dose.[15] This wide range of dose reduction
is partially due to patient body habitus. Different
models of IR techniques have been developed over the
years, including statistical IR (SIR) and iterative model
reconstruction (IMR). SIR utilises a set of algorithms to
reduce the dose whereas IMR requires a more complex
set of algorithms, taking into account the data statistics,
image statistics, and system models.[16] IMR generally
takes a longer time to compute,[17] which is usually
offset by the faster processing capacities of current
computers.
IMR has been shown to reduce objective noise and
improve subjective image quality.[8] It also improves
detection of small nodules on CT scans of the chest.[18]
Although there is a vast amount of literature available
documenting the benefits of IR techniques, assessment
of image quality improvement of the lumbar spine
reconstruction from routine CTAP using IMR compared
with SIR and its comparison with routine L-spine CT
has not been documented in the current literature. This
application of spinal reconstruction is particularly
important in trauma and oncology settings.
The aim of this study was to determine the efficacy of
IMR compared with SIR in image noise reduction on
spinal reconstructions of CTAP and dedicated L-spine
CT.
METHODS
Study Design and Setting
A retrospective study was performed on consecutive
adult cases from the accident and emergency department
who underwent CTAP, and another group of consecutive
adult cases that underwent dedicated L-spine CT at the
same tertiary hospital from July to September 2016.
Scan Properties
All patients had been scanned using a 256 multi-slice CT
scanner (ipatient CT [iCT]; Philips Healthcare, Cleveland
[OH], US) using the standard CTAP or L-spine CT
protocol in our institution. All CTAPs were performed
with intravenous contrast as per our institution’s standard
protocol, whereas L-spine CTs were performed without
contrast. Lumbar spine reconstructions from CTAP
image data were reconstructed in the axial, sagittal, and
coronal planes with 3 mm section thickness using SIR
(iDose4, Philips Healthcare, Cleveland [OH], US) and
level 1 (lowest level) IMR (Philips Healthcare, Cleveland
[OH], US) techniques. An average pitch of 1 was used
at our centre.
Dedicated L-spine CT images were reconstructed with
SIR in the axial, sagittal, and coronal planes with 3-mm
section thickness.
Scanning parameters such as kVp, mAs, volume CT
dose index (CTDIvol in mGy) and dose length product
(DLP in mGy·cm) were documented for each patient.
Quantitative Assessment
Quantitative assessment of image quality was performed by quantifying the image noise (standard deviation
[SD] of the CT number [Hounsfield units]) centrally
at the vertebral bodies, disc spaces, and cerebrospinal
fluid (CSF) using 20 mm2 regions of interest. These
measurements were performed in the L2, L3 and L4
vertebral bodies; the L2/3, L3/4 and L4/5 disc spaces, and
the corresponding CSF spaces, unless visible pathology
was identified at any of these levels. In those instances,
measurements of regions of interest were taken at a lower
level. Mean noise was calculated. These measurements
were performed with both bone and soft tissue windows,
yielding results for SIR and IMR for CTAP and SIR
for L-spine CT. The contrast-to-noise ratio (CNR) was
measured using the formula:
CNR=(CT number [disc or bone] – CT number [CSF])/Noise (CSF)
CNR=(CT number [disc or bone] – CT number [CSF])/Noise (CSF)
Although CNR measurements do not include the effect
of image resolution and texture of noise, there was no
other satisfactory quantitative measurement of image
quality.[19]
Qualitative Assessment
Qualitative assessment was performed by two
radiologists (a CT radiologist with 28 years of CT
experience and a radiologist with 5 years of CT
experience). The more experienced radiologist has
an in-depth knowledge of IR techniques and has
been working with these techniques for over 3 years.
The other radiologist recruited in the study has lesser
knowledge of IR techniques. Both independently read
all CT lumbar spine images in a random order. The
readers were required to allocate a score between 1
and 5 to the bone/CSF, intervertebral disc/CSF, and
intervertebral disc/bone interfaces of the lumbar spine
CT images, and from the different IR methods of CTAP.
A score of 5 represented excellent demarcation of the
interface and a score of 1 represented poor delineation
of the interface. Median scores were obtained. Reading
was performed on both bone and soft tissue windows.
The readers were blinded to the patient details, clinical
history, type of study, and original report.
Inter-reader Variability
Inter-reader variability was calculated using Cohen’s
kappa statistics: a κ value of ≤0.20 indicated poor
agreement and a κ value of >0.80 indicated good
agreement.
Data Analysis
Data analysis was performed using Microsoft Excel®
(Microsoft Corporation, Redmond, Washington [WA], US) and STATA 14 (StataCorp, College Station [TX],
US). The IMR lumbar spine CT images were compared
with SIR images of lumbar spine from CTAP and
L-spine CT. Both quantitative and qualitative assessment
results of the different lumbar spine reconstructions from
CTAP and the L-spine CT were compared using the
Mann-Whitney U test. The results from the quantitative
and qualitative assessment between the different IR
techniques for CTAP were compared using paired t tests.
A p value of <0.05 was considered significant.
RESULTS
A total of 91 cases (46 women and 45 men; mean age
61±19 years, range 19-95) were selected for the CTAP
group. Two cases with significant beam hardening
artefact from spinal fusion screws were excluded from
the L-spine CT group. After these two cases had been
excluded, a total of 100 cases (53 women and 47 men;
mean age 63±15 years, range 31-83) were included in
the L-spine CT group.
Inter-reader agreements were high, κ = 0.93 and 0.85,
respectively for soft tissue and bony windows (κ value of
≤0.20 indicated poor agreement and a κ value of >0.80
indicated good agreement).
The kVp for dedicated L-spine CT was generally higher
than that for CTAP. All L-spine CTs were routinely
performed at 120 kVp in our institution. In total, 77
CTAPs were performed at 100 kVp whereas 14 CTAPs
were performed at 120 kVp based on patients’ body
weights (100 kVp for those weighing <80 kg and
120 kVp for those weighing ≥80 kg as our departmental
CTAP scanning protocols). Mean mAs was significantly
higher for L-spine CT (386±180 mAs) than for CTAP (178±71 mAs) [p < 0.001]. The scan range of L-spine
CT was routinely from T12 to S2, which was shorter
compared with CTAP, which was typically from the
diaphragm (around T9 to T10 level) to the inguinal
region (below the coccyx). Even with a shorter scanning
range, DLP and CTDIvol were significantly higher for
L-spine CT compared with CTAP: The mean DLP was
significantly higher for L-spine CT (774.1±363 mGy·cm)
than for CTAP (473.3±308 mGy·cm) [p < 0.001], and
mean CTDIvol was significantly higher for L-spine CT
(26.1±12 mGy) than for CTAP (8.6±5 mGy) [p < 0.001].
The time for image reconstruction using IMR was 3 to 5
minutes and about 2 minutes for SIR.
As demonstrated in Table 1, the SDs representing image
noise varied widely between SIR and IMR. There were
significant reductions in image noise in the lumbar spine
vertebral bodies, intervertebral discs, and CSF on IMR
compared with SIR lumbar spine images from the same
CTAP (all p < 0.001). IMR lumbar spine images from
CTAP also produced significantly less image noise
than those of dedicated L-spine CT (p < 0.001). Further
comparison made between the IMR lumbar spine
images from the 14 CTAPs performed at 120 kVp with
L-spine CT (also performed at 120 kVp) again revealed
statistically significant less noise on IMR spine images
for this subgroup of CTAP at 120 kVp (p < 0.001).
Table 1. Image noise and contrast-to-noise ratio for computed tomography of the abdomen and pelvis (CTAP) and computed tomography
of the lumbar spine (L-spine CT).
Qualitatively, IMR spine images from CTAP
demonstrated significantly better interfaces between
bone/CSF (p < 0.001), disc/CSF (p < 0.001) and disc/bone (p < 0.001) than SIR images (Table 2). Compared
with L-spine CT, SIR images were significantly inferior
for all interfaces whereas IMR images were superior to L-spine CT for bone/CSF (p < 0.001) and disc/CSF
(p < 0.001) interfaces and of equal quality in delineating
disc/bone interface (Figures 1 2 3 and 4).
Table 2. Comparison of the quality of interfaces between
computed tomography of the abdomen and pelvis (CTAP) and
computed tomography of the lumbar spine (L-spine CT).
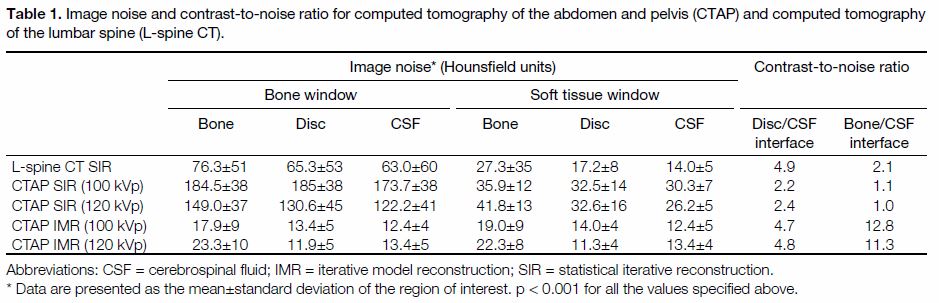
Figure 1. a) Computed tomography image of the lumbar spine (L-spine CT). (b) Statistical iterative reconstruction (SIR) of computed
tomography of the abdomen and pelvis (CTAP) images and (c) iterative model reconstruction (IMR) of CTAP images in soft tissue and bone
windows. The IMR image in (c) rendered less image noise and better low-contrast target visibility such that the interfaces between bone,
disc, and cerebrospinal fluid (CSF) were better delineated compared with those in CTAP in (b) and were also better in disc/CSF and bone/CSF interfaces compared with those of SIR images of dedicated L-spine CT in (a).
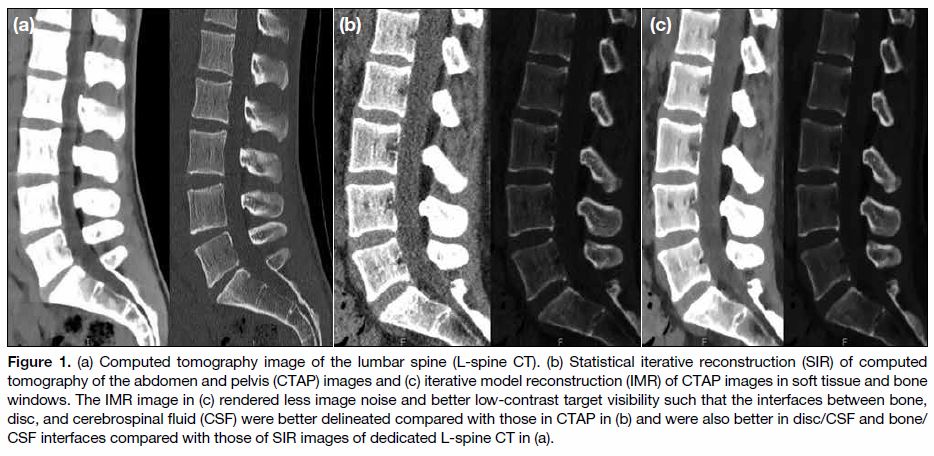
Figure 2. (a) Bone metastases (indicated by black arrows) on computed tomography image of the lumbar spine (L-spine CT) using
statistical iterative reconstruction (SIR) of an adult patient. Computed tomography images of the abdomen and pelvis (CTAP) of a different
patient using (b) SIR and (c) iterative model reconstruction (IMR). The IMR image in (c) shows better interfaces between metastasis, bone,
and cerebrospinal fluid compared with those of SIR images from (b) CTAP and dedicated (a) L-spine CT.
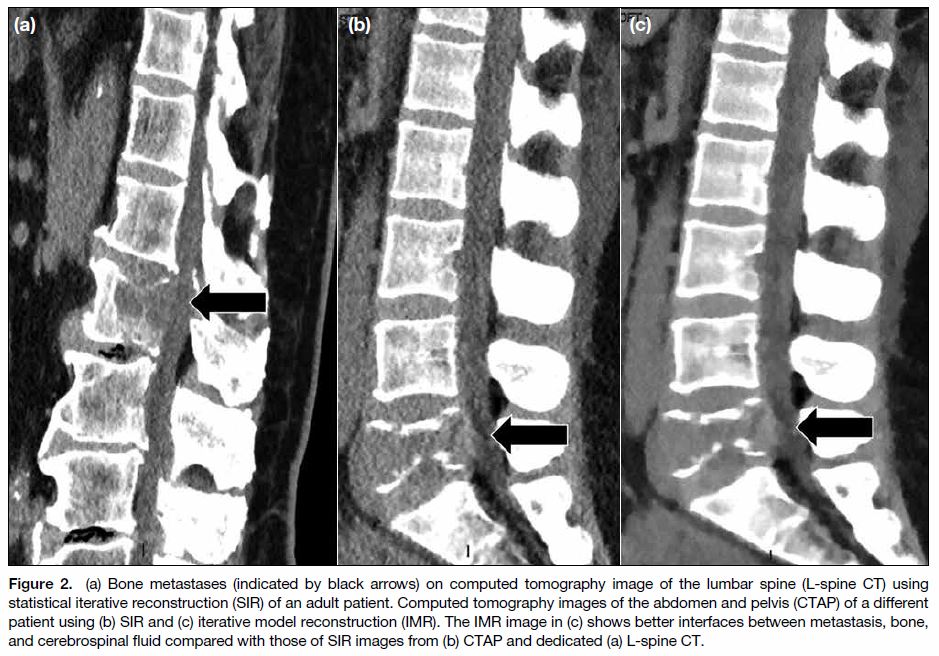
Figure 3. (a) Fractures (indicated by black arrows) on computed tomography image of the lumbar spine (L-spine CT) using statistical
iterative reconstruction (SIR) of an adult patient. Computed tomography images of the abdomen and pelvis (CTAP) of another adult patient
using (b) SIR and (c) iterative model reconstruction (IMR). The fracture lines and fragments are better defined on the IMR image in (c).
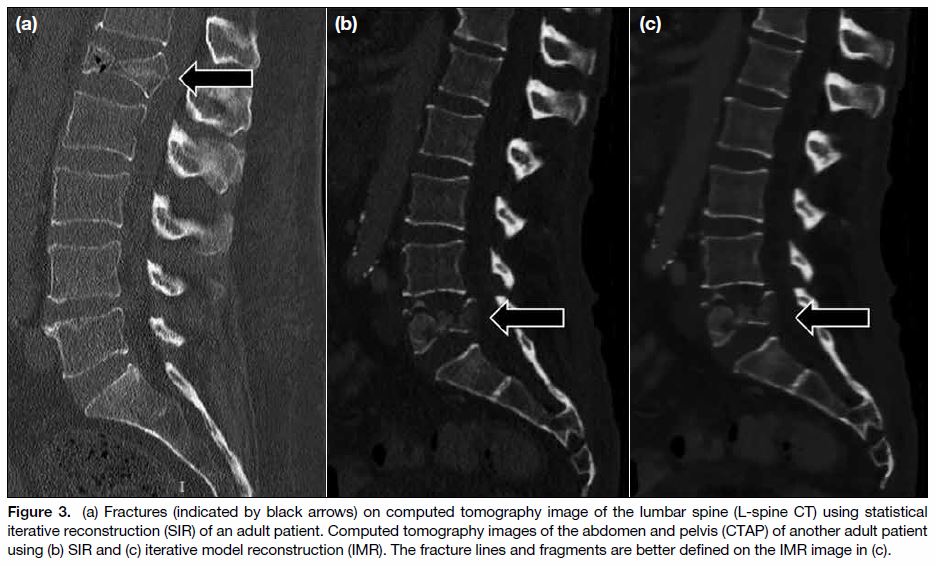
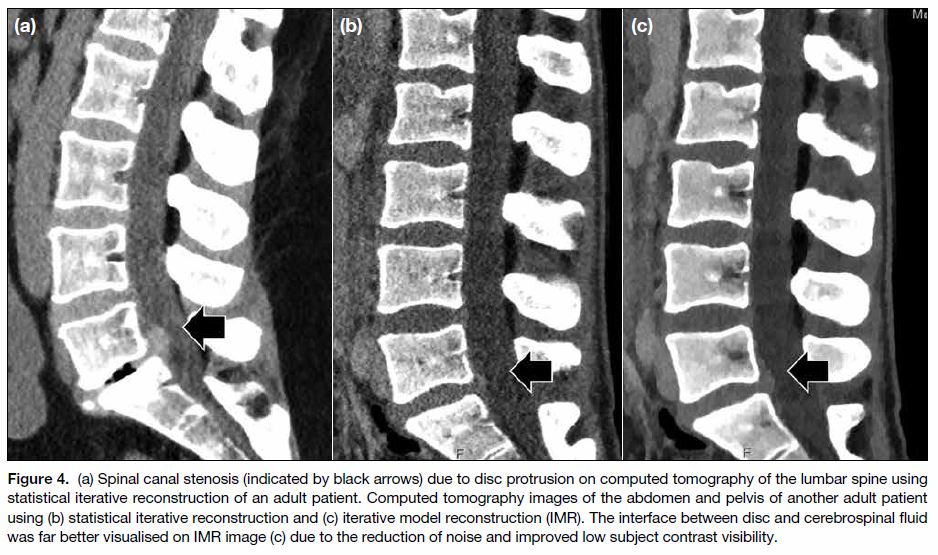
Figure 4. (a) Spinal canal stenosis (indicated by black arrows) due to disc protrusion on computed tomography of the lumbar spine using
statistical iterative reconstruction of an adult patient. Computed tomography images of the abdomen and pelvis of another adult patient
using (b) statistical iterative reconstruction and (c) iterative model reconstruction (IMR). The interface between disc and cerebrospinal fluid
was far better visualised on IMR image (c) due to the reduction of noise and improved low subject contrast visibility.
DISCUSSION
There is an increasing tendency of generating multiplanar
reconstructed spinal images from CTAP examinations,
particularly in the trauma setting.[4] This is primarily
because they are more sensitive in detecting fractures
compared with plain radiographs and avoid subjecting
patients to a second CT spine study.[4] Similarly, in a non-trauma
setting, especially oncology patients and patients
with pyrexia of unknown origin, spinal reconstructions
are routinely performed to assist in the detection of bone,
paravertebral, and disc pathologies.[20]
Image noise levels were significantly higher for
reconstructed lumbar spine images from CTAP compared with L-spine CT, when both were reconstructed using
SIR. As a result, the interfaces between bone, disc,
CSF, and soft tissues were less well defined in the
reconstructed spinal images from CTAP (Figure 2) due
to increased image noise, making detection of subtle
fractures and disc pathology more difficult. However,
the reduction in image noise on L-spine CT was at the
expense of patients’ radiation exposure.
Image noise in reconstructed lumbar spine images from
CTAP can be improved by applying IMR. Our study
confirms that IMR is superior to SIR for both bone and
soft tissue assessment in lumbar spine reconstructions
from CTAP and those from routine L-spine CT due
to reduction of image noise. This was agreed upon by
both our radiologists of different years of experience
as evident from our high inter-reader agreement. Apart
from image noise and radiation dose reduction, IMR is
also capable of improving low-contrast target visibility,[8]
which is important when detecting disc pathology and
small fractures in osteoporotic bone. Therefore, using
IMR for lumbar spine reconstruction from CTAP may
negate the need for performing a separate L-spine CT in
trauma patients who have already undergone a CTAP. As
a consequence of image noise reduction and improved
low-contrast target visibility, traumatic (fractures) and
non-traumatic (disc bulges, metastases) pathologies may
become more conspicuous and better appreciated on
IMR images. This would be cost-effective in providing a
timely diagnosis without need for a second scan.
However, IMR CTAP images can produce a ‘plastic’
appearance or ‘blotchy’ texture as described by our
readers as well as radiologists in other studies.[18] The
learning curve for interpreting these images will vary
for different individuals and the degree of experience
and confidence plays an important role. The benefits are
more pronounced when radiologists are more familiar
with the IMR CT image appearance. Our radiologists,
with different lengths of experience, both adapted
quite quickly to the change in CT appearance. Other
radiologists from different settings and regions may have
different opinions and experiences on the reconstructed
images.
Radiation dose is generally higher in L-spine CT
compared with routine CTAP despite the shorter
scanning range due to two main reasons. First, a higher
mAs is required to generate better image quality for the
bone and soft tissue details, resulting in a linear increase
in radiation dose.[21] Second, utilisation of higher kVp in
order to improve image quality of the bones causes a
dose increase approximately proportional to the square
of the change in tube voltage.[21] Multiple other factors,
such as length of time of acquisition, may affect the
image quality, which could not be standardised in this
study due to its retrospective nature.
Limitations of this study included small number of
cases. Because this was a retrospective study, patients
did not undergo both CTAP and L-spine CT, making
direct comparisons in the same patient impossible.
Furthermore, the patients were scanned at different
scanning parameters such as kVp which might affect the
CNR. Despite this, the small subgroup CTAPs acquired
at 120 kVp confirmed improved image quality of the
lumbar spine with IMR. Future prospective studies with
a larger patient cohort, a larger group of radiologists,
as well as dedicated qualitative assessment of various
lumbar spine pathologies using different IR techniques,
would be helpful to confirm the benefits of IMR in the
reconstructed lumbar spine images from CTAP.
CONCLUSION
IMR lumbar spine images from CTAP were shown to
produce less image noise and better low-contrast target
detectability, and therefore, resulted in better image
quality even when compared with dedicated CT lumbar
spine images. These IMR lumbar spine images provided
better interface details compared with that of dedicated
SIR L-spine CT examinations. This may negate the
need for performing dedicated lumbar spine CT in some
patients and therefore, aids in the timely diagnosis,
which is important in trauma and oncology settings. It may also help streamline the workflow in a busy tertiary
imaging centre.
REFERENCES
1. The 2007 recommendations of the International Commission
on Radiological Protection. ICRP publication 103. Ann ICRP.
2007;37:1-332. Crossref
2. Tamm EP, Rong XJ, Cody DD, Ernst RD, Fitzgerald NE, Kundra V.
Quality initiatives: CT radiation dose reduction: how to implement
change without sacrificing diagnostic quality. Radiographics.
2011;31:1823-32. Crossref
3. Soto JA, Anderson SW. Multidetector CT of blunt abdominal
trauma. Radiology. 2012;265:678-93. Crossref
4. Carter B, Griffith B, Mossa-Basha F, Zintsmaster SA, Patel S,
Williams TR, et al. Reformatted images of the thoracic and lumbar
spine following CT of chest, abdomen, and pelvis in the setting of
blunt trauma: are they necessary? Emerg Radiol. 2015;22:373-8. Crossref
5. Gross EA. Computed tomographic screening for thoracic and
lumbar fractures: is spine reformatting necessary? Am J Emerg
Med. 2010;28:73-5. Crossref
6. Lee SY, Landis MS, Ross IG, Goela A, Leung AE. Extraspinal
findings at lumbar spine CT examinations: prevalence and clinical
importance. Radiology. 2012;263:502-9. Crossref
7. Lee K, Beveridge T, Sanagou M, Thomas P. Updated Australian
diagnostic reference levels for adult CT. J Med Radiat Sci.
2020;67:5-15. Crossref
8. Deák Z, Grimm JM, Treitl M, Geyer LL, Linsenmaier U,
Körner M, et al. Filtered back projection, adaptive statistical
iterative reconstruction, and a model-based iterative reconstruction
in abdominal CT: an experimental clinical study. Radiology.
2013;266:197-206. Crossref
9. Löve A, Siemund R, Höglund P, Van Westen D, Stenberg L,
Petersen C, et al. Hybrid iterative reconstruction algorithm in brain
CT: a radiation dose reduction and image quality assessment study.
Acta Radiol. 2014;55:208-17. Crossref
10. Willemink MJ, de Jong PA, Leiner T, de Heer LM, Nievelstein RA,
Budde RP, et al. Iterative reconstruction techniques for computed
tomography Part 1: technical principles. Eur Radiol. 2013;23:1623-
31. Crossref
11. Hara AK, Paden RG, Silva AC, Kujak JL, Lawder HJ, Pavlicek W. Iterative reconstruction technique for reducing body radiation dose
at CT: feasibility study. AJR Am J Roentgenol. 2009;193:764-71. Crossref
12. Gatewood MO, Grubish L, Busey JM, Shuman WP, Strote J. The
use of model-based iterative reconstruction to decrease ED radiation
exposure. Am J Emerg Med. 2015;33:559-62. Crossref
13. Klink T, Obmann V, Heverhagen J, Stork A, Adam G, Begemann P.
Reducing CT radiation dose with iterative reconstruction
algorithms: the influence of scan and reconstruction parameters
on image quality and CTDIvol. Eur J Radiol. 2014;83:1645-54. Crossref
14. Kordolaimi SD, Saradeas I, Ploussi A, Pantos I, Argentos S,
Efstathopoulos EP. Introduction of an effective method for
the optimization of CT protocols using iterative reconstruction
algorithms: comparison with patient data. Am J Roentgenol.
2014;203:W434-9. Crossref
15. Willemink MJ, Leiner T, de Jong PA, de Heer LM, Nievelstein RA,
Schilham AM, et al. Iterative reconstruction techniques for
computed tomography part 2: initial results in dose reduction and
image quality. Eur Radiol. 2013;23:1632-42. Crossref
16. Park SB, Kim YS, Lee JB, Park HJ. Knowledge-based iterative
model reconstruction (IMR) algorithm in ultralow-dose CT for
evaluation of urolithiasis: evaluation of radiation dose reduction,
image quality, and diagnostic performance. Abdom Imaging.
2015;40:3137-46. Crossref
17. Löve A, Olsson ML, Siemund R, Stålhammar F, Björkman-Burtscher IM, Söderberg M. Six iterative reconstruction algorithms
in brain CT: a phantom study on image quality at different radiation
dose levels. Br J Radiol. 2013;86:20130388. Crossref
18. Zhang M, Qi W, Sun Y, Jian Y, Liu X, Hong N. Screening for lung
cancer using sub-millisievert chest CT with iterative reconstruction
algorithm: image quality and nodule detectability. Br J Radiol.
2018;91:20170658. Crossref
19. Christianson O, Chen JJ, Yang Z, Saiprasad G, Dima A, Filliben JJ,
et al. An improved index of image quality for task-based
performance of CT iterative reconstruction across three commercial
implementations. Radiology. 2015;275:725-34. Crossref
20. Meyer CA, Vagal AS, Seaman D. Put your back into it: pathologic
conditions of the spine at chest CT. Radiographics. 2011;31:1425-41. Crossref
21. Kalra MK, Maher MM, Toth TL, Hamberg LM, Blake MA,
Shepard JA, et al. Strategies for CT radiation dose optimization.
Radiology. 2004;230:619-28. Crossref
| Attachment | Size |
|---|---|
| v24n1_Iterative.pdf | 652.46 KB |