Transient Blindness after Endovascular Parent Artery Occlusion to Treat Giant Aneurysm of Internal Carotid Artery: a Case Report
CASE REPORT
Transient Blindness after Endovascular Parent Artery Occlusion to Treat Giant Aneurysm of Internal Carotid Artery: a Case Report
A Padmanabhan1, SN Keshava1, M Ahmed1, RK Moorthy2
1 Department of Interventional Radiology, Christian Medical College, Vellore, India
2 Department of Neurological Sciences, Christian Medical College, Vellore, India
Correspondence: Prof SN Keshava, Department of Interventional Radiology, Christian Medical College, Vellore, India. Email: aparna_shyam@yahoo.com
Submitted: 30 Jan 2020; Accepted: 3 Jun 2020.
Contributors: SNK designed the study. AP and SNK acquired the data. AP, SNK and MA analysed the data. AP and SNK drafted the manuscript.
SNK, MA and RKM critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the
study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: authors have disclosed no conflicts of interest.
Funding/Support: The case report received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics Approval: Ethical committee approval was not required for this case report as standard care was provided for the clinical scenario. The patient was treated in accordance with the tenets of the Declaration of Helsinki. The patient provided written informed consent for all treatments
and procedures.
INTRODUCTION
Giant aneurysm of the cerebral circulation is an arterial
outpouching ≥25 mm. Cavernous internal carotid artery
(ICA) aneurysms account for approximately 15% of all
aneurysms arising from the ICA.[1] Owing to their large
size, they may present with symptoms due to compression
of adjoining nerves but are less likely to rupture.[2]
We present a patient who underwent endovascular
proximal parent artery occlusion for management
of a giant ICA aneurysm. Aneurysmal thrombosis,
which is an expected outcome, caused an unexpected
complication of delayed-onset blindness. We describe
the mechanism of complication, and its management and
follow-up. Use of steroid as a preventive and therapeutic
option in similar cases is proposed.
CASE REPORT
A 45-year-old woman with no previous co-morbidities
presented with a 2-month history of headache and
vomiting. Her headaches were dull and aching, and
centred towards the left hemicranium. The intermittent
episodes of vomiting were non-projectile. She had no
history of loss of consciousness, seizures or other motor deficits. Non-contrast computed tomography (CT) scan
and magnetic resonance imaging performed prior to her
visit to our institution had revealed a giant aneurysm
involving the cavernous segment of the left ICA.
General examination was unremarkable. Power was
5/5 in bilateral upper and lower limbs. Cranial nerve
examination detected visual acuity of 6/12 bilaterally.
Perimetry evaluation revealed deficit in the temporal
field of the left eye.
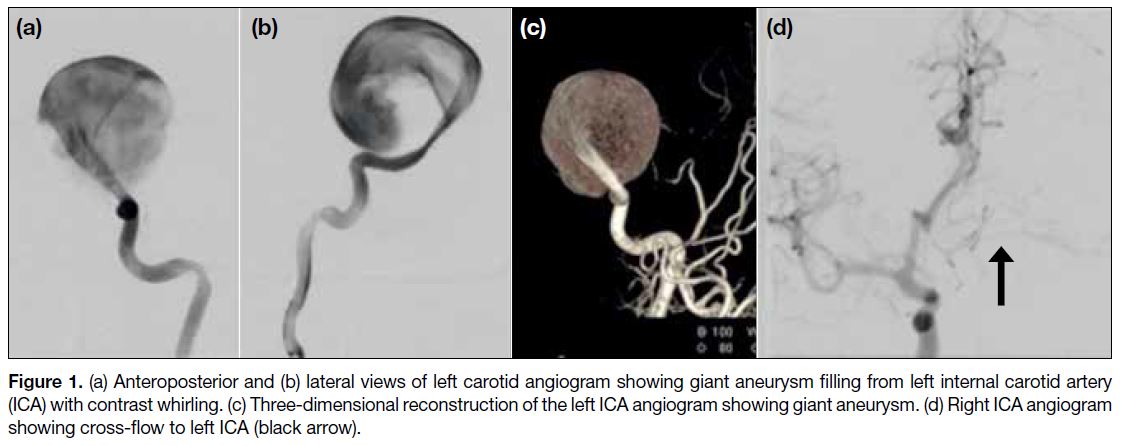
Digital subtraction cerebral angiography revealed a giant
saccular aneurysm involving the cavernous segment
of the left ICA measuring 30 mm × 35 mm × 30 mm
(Figure 1). The ICA distal to the aneurysm was faintly
filling. Left cerebral perfusion was maintained from the
right ICA across the anterior communicating artery. The
ophthalmic artery on the left side was not opacified.
Figure 1. (a) Anteroposterior and (b) lateral views of left carotid angiogram showing giant aneurysm filling from left internal carotid artery
(ICA) with contrast whirling. (c) Three-dimensional reconstruction of the left ICA angiogram showing giant aneurysm. (d) Right ICA angiogram
showing cross-flow to left ICA (black arrow).
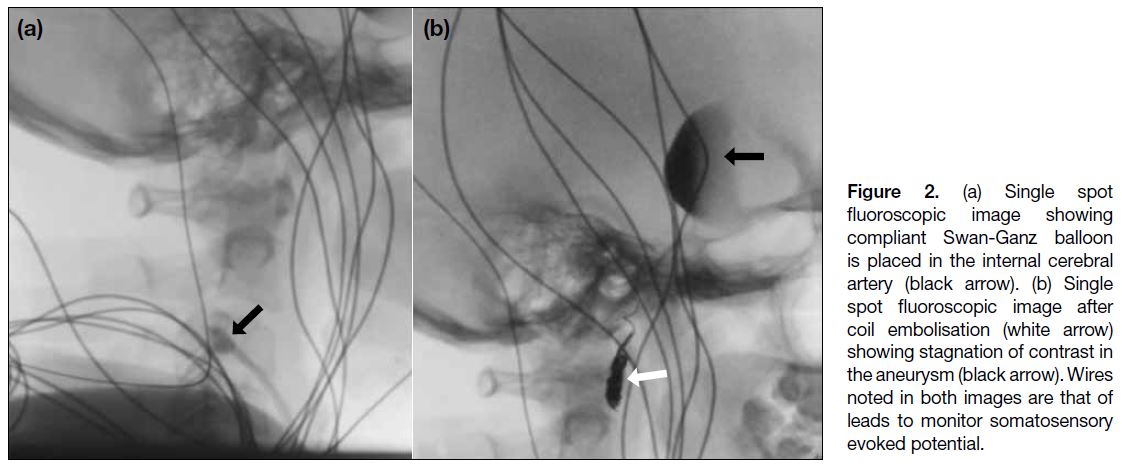
The patient gave consent. Balloon occlusion test was
performed using a 7-Fr Swan-Ganz balloon. Occlusion
was applied for 10 minutes at baseline blood pressure and
10 minutes of hypotensive challenge was given, reducing
the mean blood pressure by 20 mm Hg. Adequacy of collaterals was evaluated by periodic clinical testing
approximately every 2 minutes. Somatosensory evoked
potential monitoring was done. The venous delay in
the contralateral ICA angiogram was <2 seconds. After
successful completion of the test, a 2.7-Fr Progreat
microcatheter (Terumo) was passed along the side of the
balloon and then pushable coils (0.018-inch) were used to
tightly pack a short segment of petrous ICA maintaining
the balloon inflated in the cervical ICA (Figure 2). Her
immediate postoperative recovery was uneventful and
she was discharged home on the fourth day.
Figure 2. (a) Single spot
fluoroscopic image showing compliant Swan-Ganz balloon is placed in the internal cerebral artery (black arrow). (b) Single spot fluoroscopic image after coil embolisation (white arrow) showing stagnation of contrast in the aneurysm (black arrow). Wires noted in both images are that of leads to monitor somatosensory evoked potential.
One-week post-procedure she presented to the
emergency department with rapidly worsening vision
in the left eye. Objective acuity testing revealed only perception of light in the left eye. On examination, the left
pupil was larger with sluggish reaction to light. Contrast
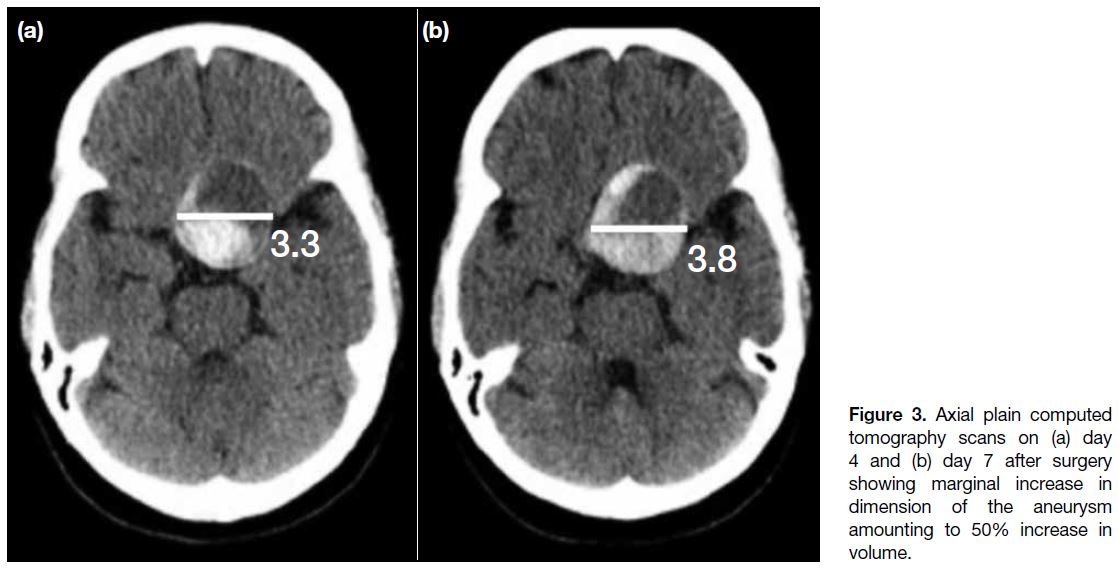
enhanced CT scan revealed increased thrombosis
within the aneurysm causing a marginal increase in
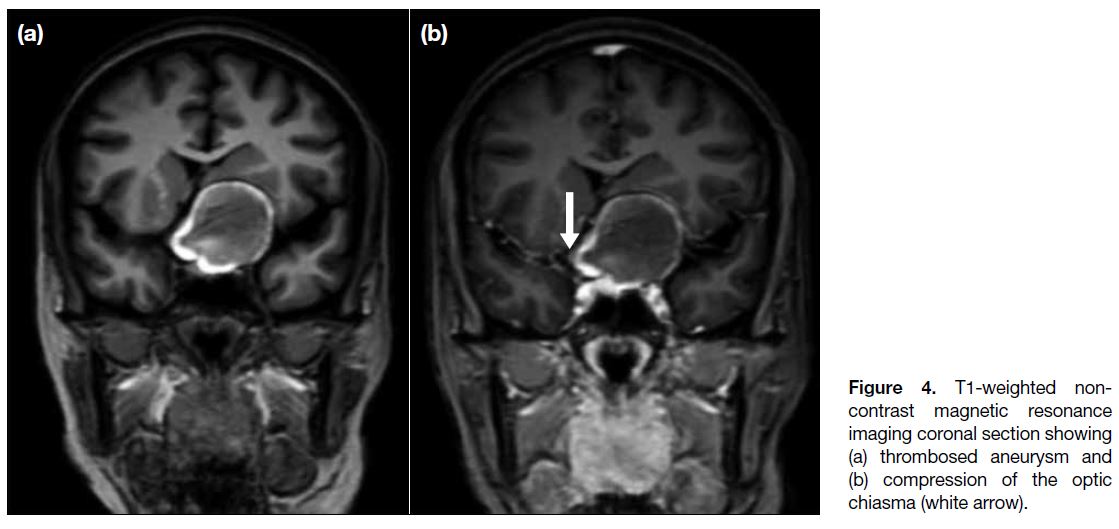
diameter of 5 mm (Figure 3). Magnetic resonance
imaging showed that because of its close proximity, the
aneurysm was compressing the optic chiasma (Figure 4).
It was assumed that this increase in volume was due to
thrombus formation with consequent compression of the
adjoining left optic nerve. The patient was immediately
started on oral steroids (dexamethasone 4 mg every
6 hours). After 1 week she showed minimal improvement
in visual acuity and was discharged from the hospital on
tapering dose of steroids. At a follow-up examination
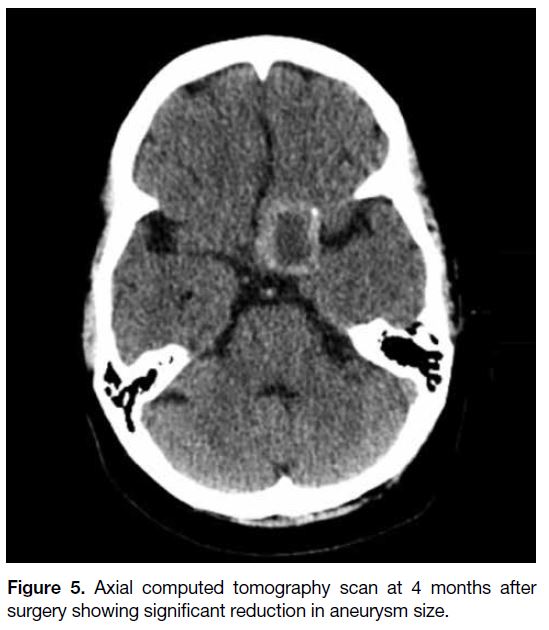
4 months after surgery, the patient’s visual acuity had recovered to 6/12. A CT scan of the brain revealed
reduction in the size of the aneurysm (Figure 5). She had
no new complaints.
Figure 3. Axial plain computed
tomography scans on (a) day 4 and (b) day 7 after surgery showing marginal increase in dimension of the aneurysm amounting to 50% increase in volume.
Figure 4. T1-weighted non-contrast
magnetic resonance imaging coronal section showing (a) thrombosed aneurysm and (b) compression of the optic chiasma (white arrow).
Figure 5. Axial computed tomography scan at 4 months after
surgery showing significant reduction in aneurysm size.
DISCUSSION
Owing to their interdural location, giant cavernous
ICA aneurysms are less likely to cause life-threatening
subarachnoid haemorrhage, unlike aneurysms in an
intradural location (distal to the cavernous segment).
Treatment of cavernous segment aneurysms is usually
restricted to symptomatic patients who present with
chronic headaches or compressive cranial nerve palsies
around the cavernous sinuses. Rarely, they may rupture
into the cavernous sinus leading to formation of type I
carotid-cavernous fistula.[3] Spontaneous intra-aneurysmal
eccentric thrombus sometimes can be seen and maybe a
source of embolism causing transient ischaemic attacks
or strokes.[4]
Two options were considered for the treatment of
aneurysm in this patient: parent artery occlusion
following balloon occlusion test or flow diversion. Flow
diversion involves placement of a braided stent across the
aneurysm neck in the parent artery to regulate the flow
into the aneurysm, thus causing gradual thrombosis.[5]
Flow diversion comes with a significant additional
cost and requires the patient to take anti-aggregatory
medications for a prolonged period.[6]
Parent artery occlusion of the vessel harbouring the giant
aneurysm is a less expensive but effective way noted in
early work by Drake et al.[7] Occlusion of the parent artery
alters the flow dynamics within the aneurysm causing it
to thrombose. Balloon occlusion test is a technique used
to temporarily occlude the parent artery using a compliant
balloon intended for permanent sacrifice. This testing
provides valuable information about the efficiency of
alternative circulatory pathways to maintain the brain
supply.
Expansion of a blood vessel following acute thrombus
formation is a known phenomenon. Evidence of this may
be more prominent in veins owing to their non-muscular
walls. Such examples are obvious in cases of peripheral
deep venous thrombosis or renal vein thrombosis where increase in size of the vein is a common finding.
Similar changes may occur in arteries leading to a
marginal increase in size. This size increase is due to clot
formation that involves cytokines and clotting factors
and expands the volume. The size increase sometimes
results in compression on adjoining vital structures.
Steroids help to reduce this response of inflammation
during clot formation. They also play a role in reducing
inflammation of the compressed structure, which was the
left optic nerve in this case.[8]
A quick volumetric analysis with comparison on CT was
useful in our case. The marginal increase in diameter
caused an approximate 50% increase in overall volume.
This hypothesis correlates with the onset of visual
symptoms 1 week after the procedure. Improvement was
seen with steroid treatment with gradual recovery over a
few months.
CONCLUSION
Giant ICA aneurysm causing compressive symptoms
warrants treatment. Endovascular options include
parent artery occlusion or flow diversion. Parent artery
occlusion may cause thrombosis within the aneurysm
causing expansion and more compression. In our patient,
this presented as delayed loss of vision, subsequently
managed by steroids. Steroids may be useful in such
cases and may also be considered as prophylaxis in
similar cases.
REFERENCES
1. Rosi Junior J, Welling LC, Yeng LT, Caldas JG, Schafranski M,
Teixeira MJ, et al. Cavernous carotid artery aneurysms:
epidemiology, natural history, diagnostic and treatment. An
experience of a single institution. Clin Neurol Neurosurg.
2014;125:32-5. Crossref
2. Eddleman CS, Hurley MC, Bendok BR, Batjer HH. Cavernous carotid aneurysms: to treat or not to treat? Neurosurg Focus.
2009;26:E4 Crossref
3. Kam CK, Alvarez H, Lasjaunias P. Treatment of carotid
cavernous fistula secondary to rupture of a giant intracavernous
carotid aneurysm. Transarterial coiling of aneurysm and carotid
compression. A case report. Interv Neuroradiol. 2003;9:293-8. Crossref
4. McLaughlin N, Bojanowski MW. Unruptured cerebral aneurysms
presenting with ischemic events. Can J Neurol Sci. 2008;35:588-92. Crossref
5. Briganti F, Leone G, Marseglia M, Mariniello G, Caranci F,
Brunetti A, et al. Endovascular treatment of cerebral aneurysms
using flow-diverter devices: a systematic review. Neuroradiol J.
2015;28:365-75. Crossref
6. Tonetti DA, Jankowitz BT, Gross BA. Antiplatelet therapy in flow
diversion. Neurosurgery. 2020;86(Suppl 1):S47-S52. Crossref
7. Drake CG, Peerless SJ, Ferguson GG. Hunterian proximal
arterial occlusion for giant aneurysms of the carotid circulation. J
Neurosurg. 1994;81:656-65. Crossref
8. Barnes PJ. Anti-inflammatory actions of glucocorticoids: molecular
mechanisms. Clin Sci. 1998;94:557-72. Crossref
| Attachment | Size |
|---|---|
| v24n1_Transient.pdf | 250.12 KB |