Real-time Ultrasound Fusion Imaging–Guided Interventions: a Review
PERSPECTIVE
Real-time Ultrasound Fusion Imaging–Guided Interventions: a Review
CPY Chien, KH Lee, V Lau
Department of Radiology, Queen Mary Hospital, Hong Kong
Correspondence: Dr CPY Chien, Department of Radiology, Queen Mary Hospital, Hong Kong. Email: chienpyc@gmail.com
Submitted: 2 Dec 2020; Accepted: 22 Feb 2021.
Contributors: All authors designed the study. CPYC acquired the data. All authors analysed the data, drafted the manuscript, and critically
revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the final
version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have no conflicts of interest to declare.
Funding/Support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics Approval: This study was approved by Hong Kong West Cluster Research Ethics Committee and the requirement to obtain informed
consent was waived (IRB Ref UW19-267).
Disclosure: This topic was presented at the European Congress of Radiology 2020, 15-19 Jul 2020, Vienna, Austria. (Poster C-03874).
Acknowledgement: We thank Dr Tina PW Lam for general supervision and administrative support throughout the project.
Abstract
Ultrasound fusion imaging is a novel technique that allows the fused synchronous display of computed tomography or
magnetic resonance images during real-time ultrasound scanning. It has been widely applied in various ultrasound-guided
interventions to enhance lesion detectability, thereby improving procedural accuracy and safety. In this article,
we describe the current status and our institutional experience of the application of ultrasound fusion imaging in
hepatobiliary, renal, and musculoskeletal interventions. We also discuss techniques, challenges, and recommendations
for ultrasound fusion–guided interventions.
Key Word: Biopsy
中文摘要
實時超聲融合成像引導介入綜述
錢珮恩、李錦浩、劉泳恆
超聲融合成像是一種新技術,允許在實時超聲掃描期間同步融合顯示電腦斷層掃描或磁共振圖像。這種新技街已被廣泛應用於各種超聲引導介入,以提高病變檢出,從而提高手術的準確性和安全性。本文描述超聲融合成像在肝膽、腎臟和肌肉骨骼介入中應用的現狀和我們機構的經驗。我們討論超聲融合引導介入的技術、挑戰和建議。
INTRODUCTION
Ultrasound is a widely used imaging modality to guide
percutaneous interventional procedures due to its easy
accessibility, real-time capability, and lack of radiation.
During ultrasound-guided interventions, interventional
radiologists often localise the target lesion and carry
out procedures by cognitive fusion with reference to
the computed tomography (CT) or magnetic resonance
(MR) images. Despite advancements in ultrasound
technology, visualisation of the target lesion can be
difficult due to isoechogenicity with background
parenchyma, obscuration by gas/calcification-related
posterior acoustic shadowing, or attenuation of the
ultrasound beam in obese patients.[1]
Medical image fusion is defined as the registration and
overlaying of images from the same or different imaging
modalities. Ultrasound fusion offers the opportunity of
better localisation by displaying CT/MR images with
real-time ultrasound side-by-side in the same plane and
position.[2] [3] It improves the accuracy and confidence of
the interventional radiologists during procedures.[3]
Real-time ultrasound fusion–guided intervention has
gained popularity in recent years. Previous studies
have evaluated the clinical applications of real-time
ultrasound image fusion in different anatomical regions
including liver, kidney, pancreas, breast, prostate and
musculoskeletal system.[2] [3] [4] [5] [6] [7] [8] Contrast-enhanced ultrasound
(CEUS) can be used as an adjunct to further increase the
sensitivity of lesion detection.[9]
In this review, we describe the real-time ultrasound
fusion imaging technique used in our institution, and
our experience of applications of ultrasound fusion imaging in hepatobiliary, renal, and musculoskeletal
interventions. We also discuss the challenges associated
with ultrasound fusion–guided interventions and provide
recommendations.
REAL-TIME ULTRASOUND FUSION
IMAGING TECHNIQUE
There are several tracking methods for ultrasound probes,
including electromagnetic, optical, and image-based.[2]
In our institution, an electromagnetic tracking system
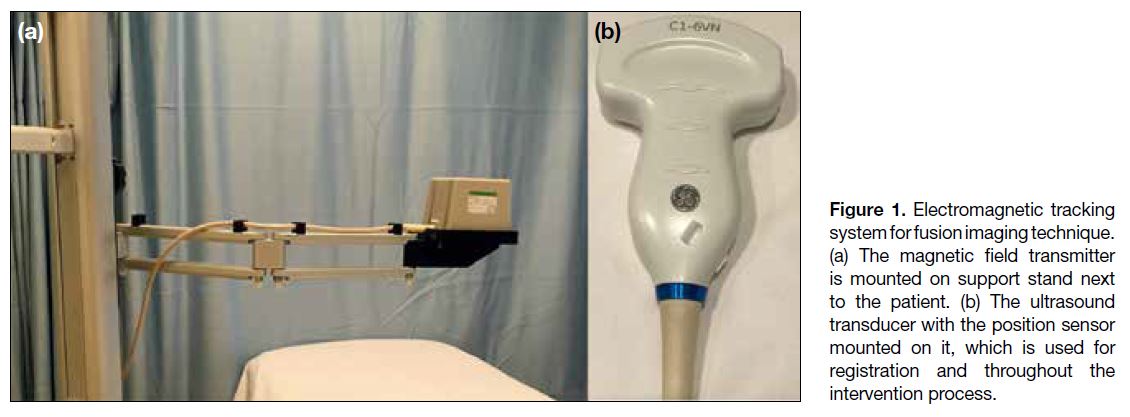
is used as the tracking method for ultrasound fusion–guided percutaneous interventions (Figure 1). The
tracking system consists of a magnetic field transmitter, a
position sensor, and a position sensor unit. The magnetic
field transmitter, which is positioned next to the patient,
creates a position-varying magnetic field. This induces
electric currents in the position sensor mounted on
the ultrasound transducer. During ultrasound probe
movement, information regarding the magnitude of the
induced current in the position sensor, which changes
with the magnetic field strength, is transmitted back to the
position sensor unit of the ultrasound machine, enabling
tracking of probe position relative to the magnetic field
transmitter.
Figure 1. Electromagnetic tracking system for fusion imaging technique. (a) The magnetic field transmitter is mounted on support stand next to the patient. (b) The ultrasound transducer with the position sensor mounted on it, which is used for registration and throughout the intervention process.
To start the fusion procedure, the CT or MR images
best depicting the target lesion and its anatomic
relationship are uploaded to the ultrasound system.
Next, coregistration—the process of overlaying real-time
ultrasound and CT/MR images—is performed
by using either external fiducial markers or internal
anatomic landmarks. External fiducial markers contain
position sensors, which allow automatic ultrasound
image fusion when placed to the body surface close to
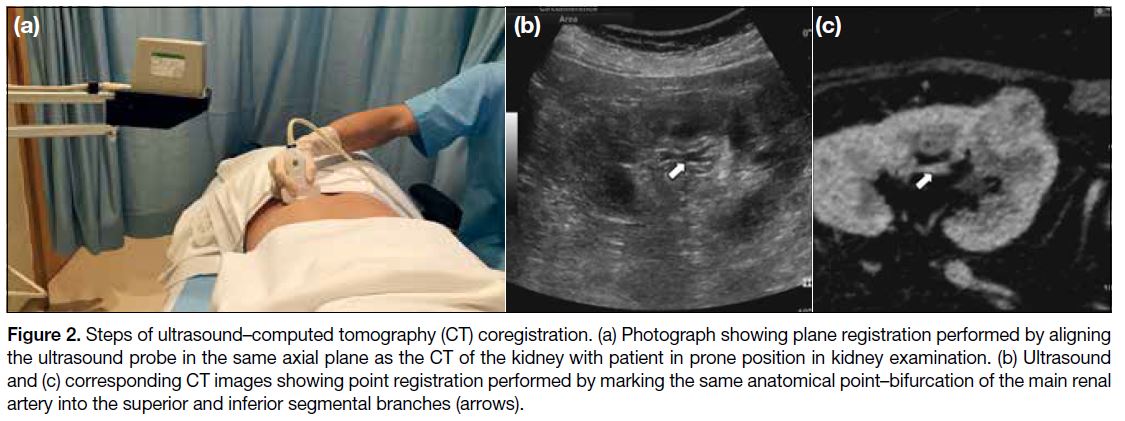
the target organ when performing CT/MR. For internal anatomic landmarks, plane and point registrations are
performed manually (Figure 2). Plane registration is
performed by aligning the ultrasound probe in the same
plane (usually the axial plane) as the uploaded CT/MR
images. Point registration is performed by marking
standardised anatomical landmarks within the target
organ/area (e.g., vessels, calcifications, cysts) manually
on both the ultrasound and CT/MR images. Finally, the
operator should check if accurate registration has been
achieved by scrutinising the region of interest, including
the target lesion and its surrounding anatomic structures.
The process of point registration can be repeated until
optimal registration is obtained. After coregistration, the
CT/MR images are displayed on the monitor side-by-side
with the real-time ultrasound images in a synchronous
manner and updated simultaneously according to the
change in position and imaging plane of ultrasound
probe.
Figure 2. Steps of ultrasound–computed tomography (CT) coregistration. (a) Photograph showing plane registration performed by aligning
the ultrasound probe in the same axial plane as the CT of the kidney with patient in prone position in kidney examination. (b) Ultrasound
and (c) corresponding CT images showing point registration performed by marking the same anatomical point–bifurcation of the main renal
artery into the superior and inferior segmental branches (arrows).
To avoid registration errors, movement of the transmitter
and patient should be avoided after coregistration.
Therefore, a stable and comfortable body position
should be ensured to minimise patient movement. A
short time interval between the CT/MR examination
and the interventional procedure is also preferred to
minimise interval changes in anatomy. In our institution,
we usually perform the ultrasound fusion–guided
interventions within 1 to 2 months after acquisition of
the CT/MR images.
APPLICATIONS OF FUSION
IMAGING
Hepatobiliary Interventions
Common ultrasound-guided hepatobiliary interventions
include lesion biopsy, tumour ablation, and abscess drainage. Deep, small and isoechoic liver lesions are
difficult to be visualised in ultrasound. Synchronous
visualisation of both real-time ultrasound and
corresponding CT/MR images improves diagnostic
and therapeutic accuracy. Park et al[10] reported that
ultrasound fusion allowed accurate localisation of target
lesion in the cirrhotic liver and decreased false sampling
of pseudolesion in the background of coarsened
parenchymal echogenicity.
Tumours located near the hepatic dome are technically
challenging to ablate due to the deep location and
suboptimal ultrasound visualisation, resulting in
increased risk of thermal injury to lung and diaphragm
and incomplete ablation.[11] [12] Lee et al[13] demonstrated
that ultrasound fusion with CT/MR images could reduce
false-positive detection for lesions <2 cm, and enhanced
lesion detectability of small hepatocellular carcinoma
for percutaneous radiofrequency ablation. Song et al[14]
showed that fusion imaging improved the conspicuity of
hepatocellular carcinoma and feasibility of ablation of
tumours that were not identifiable on ultrasound alone.
The authors found that 26 out of 82 tumours poorly seen
on fusion imaging could still be ablated by placing the
electrode based on peritumoral anatomical landmarks.[14]
In hepatobiliary fusion imaging, the images/sequences
best showing the target lesion is used for coregistration,
such as arterial phase for hypervascular mass and
portovenous phase for hypovascular mass on CT and
hepatobiliary phase on MR imaging. The common
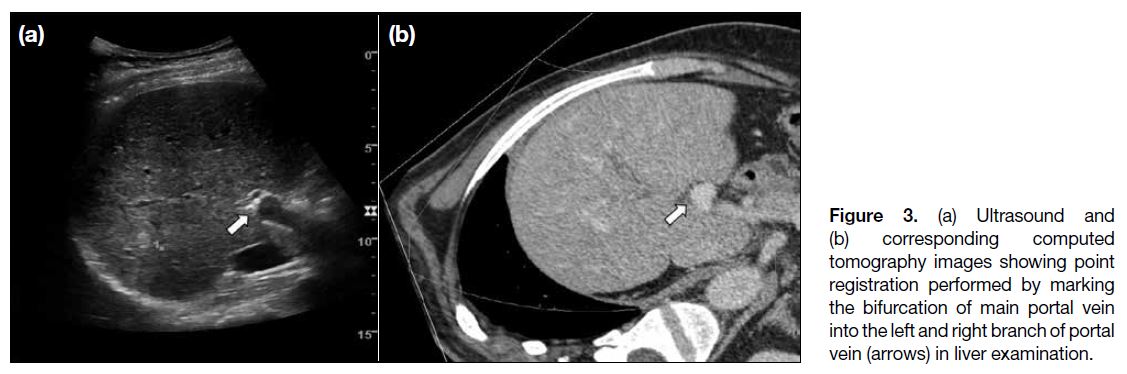
anatomical landmarks used for registration include
vascular bifurcations, such as right hepatic vein–inferior
vena cava junction and portal vein bifurcation (Figure 3),
or non-index lesions, such as liver cysts.
Figure 3. (a) Ultrasound and
(b) corresponding computed tomography images showing point registration performed by marking the bifurcation of main portal vein into the left and right branch of portal vein (arrows) in liver examination.
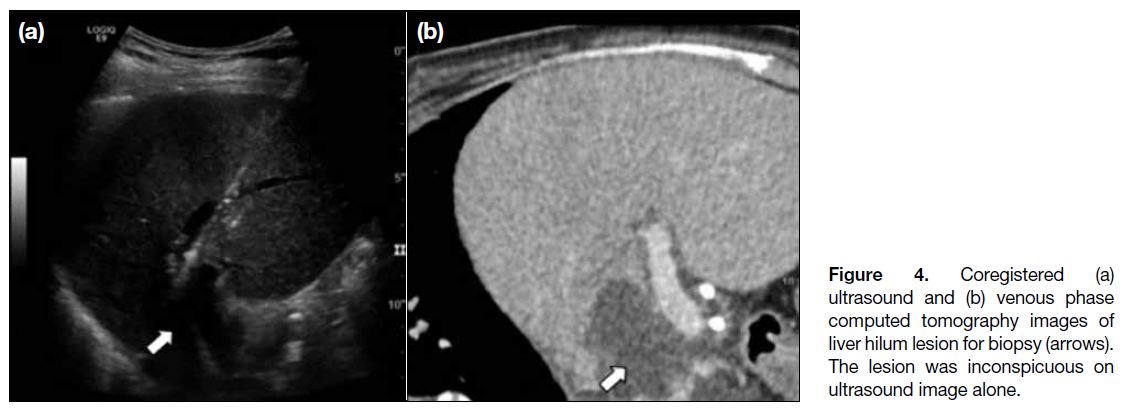
In our experience, fusion imaging improves the detection
of deep and small isoechoic lesions in the liver (Figure 4),
hence reducing sampling errors during biopsy. In
patients with hepatocellular carcinoma who have
previously received transarterial chemoembolisation,
ultrasound fusion allows more accurate localisation of
active residual or recurrent disease in the background
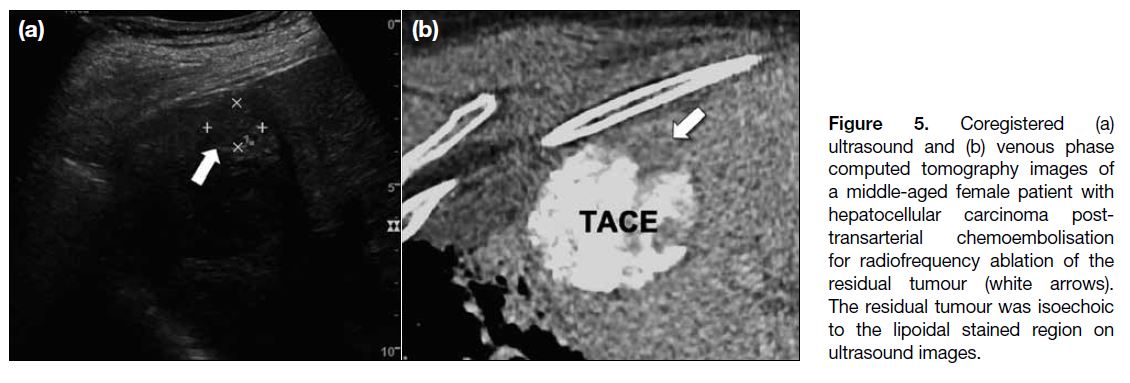
of echogenic parenchymal/tumoral lipiodol uptake (Figure 5). This technique also allows ablation of small
and difficult lesions to be done under real-time ultrasound
instead of CT guidance, potentially reducing radiation
exposure to patients and radiologists.
Figure 4. Coregistered (a)
ultrasound and (b) venous phase computed tomography images of liver hilum lesion for biopsy (arrows). The lesion was inconspicuous on ultrasound image alone.
Figure 5. Coregistered (a)
ultrasound and (b) venous phase computed tomography images of a middle-aged female patient with hepatocellular carcinoma post-transarterial chemoembolisation for radiofrequency ablation of the residual tumour (white arrows). The residual tumour was isoechoic to the lipoidal stained region on ultrasound images.
Pancreatic Interventions
The pancreas, especially the pancreatic tail, is often
obscured by overlying bowel gas on ultrasound owing to its retroperitoneal location. CT or MR are better
modalities for assessment of the pancreas and its
relationship with adjacent structures such as the stomach,
duodenum, portal vein, aorta, and celiac axis. Therefore,
combining ultrasound and CT/MR allows real-time
visualisation of the needle path and increases the
accuracy during interventions. Zhang et al[15] reported that
applying ultrasound-CT fusion in percutaneous drainage
of walled-off necrosis was associated with fewer
complications and a higher success rate when compared
with ultrasound alone, resulting in shorter hospital stay
and lower costs. To the best of our knowledge, there is
no evidence on ultrasound fusion–guided interventions
of pancreatic tumours in the literature, likely because
the deep retroperitoneal location of the pancreas renders
percutaneous access difficult.
Renal Interventions
Ultrasound is good for discriminating solid renal
lesions from cystic lesions. However, it has limitations
in the detection and characterisation of solid lesions,
especially when the lesion is isoechoic to the renal
parenchyma.[16] Up to 35% of small (<3 cm) renal cell
carcinomas are isoechoic to renal parenchyma on
ultrasound.[17] Helck et al[18] reported that ultrasound image
fusion improved the identifiability and assessment of
renal lesions compared with other modalities. Another
study found improved image-guided tumour ablation
in the ultrasound fusion-guided treatment of patients
with renal tumours, particularly for lesions <4 cm and
in patients with a solitary kidney, in terms of avoiding
surgical nephrectomy and preserving renal function.[19]
Andersson et al[20] reported improved outcomes with
ultrasound-CT fusion guidance compared with ultrasound
alone in radiofrequency ablation of small renal masses.
In renal ultrasound fusion, the patient is usually positioned
in the lateral decubitus or prone position. The images/sequences best showing the target lesion are used for
registration, such as arterial and nephrographic phases on
CT imaging, or T1-weighted post-contrast sequences on
MR imaging. Standardised anatomical landmarks used
for registration include the bifurcation of the main renal
artery into the superior and inferior segmental branches,
or the confluence of the superior and inferior segmental
renal veins into the main renal vein. Stable non-target
lesions such as renal cysts or calcifications could also be
used for registration.
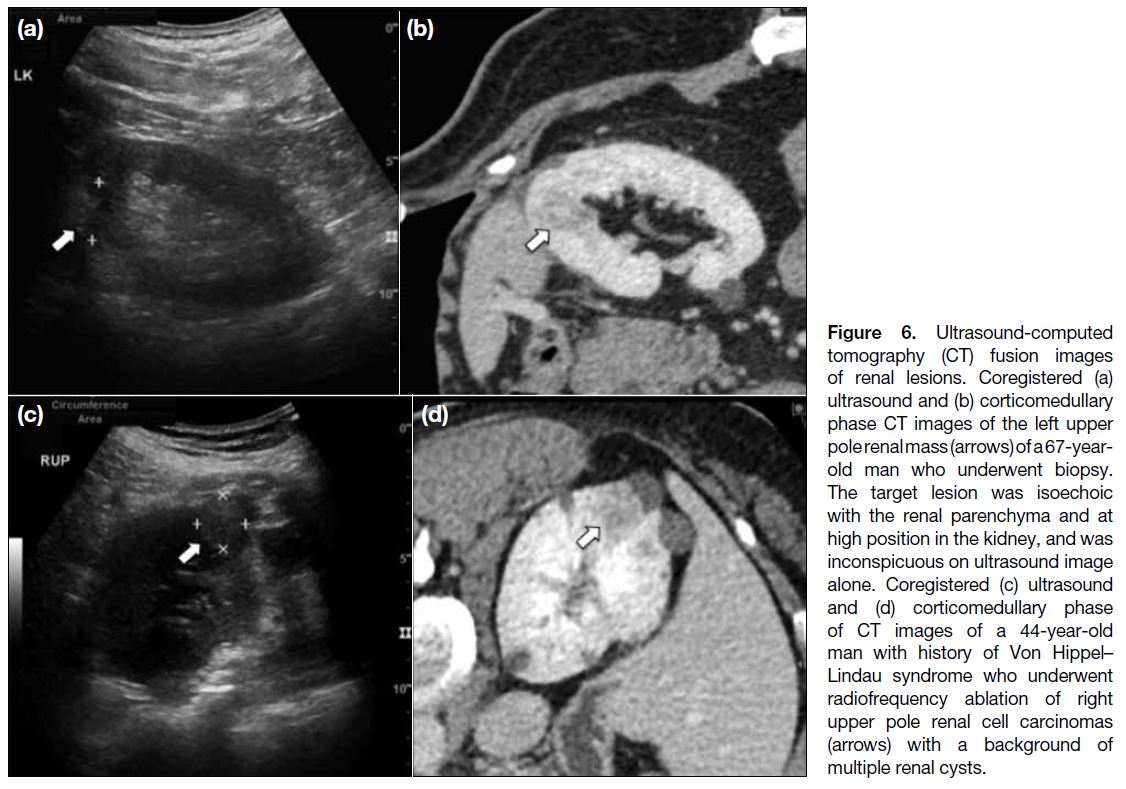
In our experience, ultrasound fusion imaging guidance is
particularly useful in the biopsy of isoechoic and upper pole renal lesions, which are difficult to localise on grey-scale
ultrasound (Figure 6a and b). Fusion imaging also
helps to target the most appropriate part of the cystic
lesion to be biopsied and to identify the best location
for electrode placement during ablation. In patients with
Von Hippel–Lindau syndrome, ultrasound fusion helps
to identify renal cell carcinomas for radiofrequency
ablation in the background of multiple renal cysts (Figure 6c and d). Furthermore, fusion imaging helps determine
the correct path for the needle, to avoid damaging the
renal vessels and pelvis.
Figure 6. Ultrasound-computed
tomography (CT) fusion images of renal lesions. Coregistered (a) ultrasound and (b) corticomedullary phase CT images of the left upper pole renal mass (arrows) of a 67-yearold man who underwent biopsy. The target lesion was isoechoic with the renal parenchyma and at high position in the kidney, and was inconspicuous on ultrasound image alone. Coregistered (c) ultrasound and (d) corticomedullary phase of CT images of a 44-year-old man with history of Von Hippel–Lindau syndrome who underwent radiofrequency ablation of right upper pole renal cell carcinomas (arrows) with a background of multiple renal cysts.
Musculoskeletal Interventions
In addition to abdominal applications, real-time
ultrasound fusion imaging guidance is helpful in
various musculoskeletal interventions, including
therapeutic injections and biopsy of mass and non-mass
lesions.[5] [6] Klauser et al[8] demonstrated the feasibility of
using ultrasound-CT fusion in therapeutic sacroiliac
joints injections in patients with chronic sacroiliitis,
obviating the need for repeated radiation exposure in
young patients with spondyloarthropathies who needed
repeated injections. Furthermore, Burke et al[6] reported
the use of ultrasound fusion in therapeutic injections of
the pudendal nerve, piriformis muscle, and sacroiliac
joints, as well as barbotage for calcific tendinopathy.
Fusion imaging complements ultrasound with augmented
anatomical details demonstrated on axial imaging,
thereby helping the operators plan the trajectory and
avoid critical structures such as neurovascular bundles
during soft tissue mass biopsy. Furthermore, lesions
poorly visualised on ultrasound, such as non-fatty
components of lipomatous tumours and areas of active
muscle oedema in patients with suspected myopathy, can
be targeted with confidence by side-by-side correlation
between real-time ultrasound and MR images. Van De
Vlekkert et al[21] demonstrated that using MR to select
target muscle with active oedema for biopsy decreased
the false-negative sampling rate from 23% to 19%
in patients with suspected idiopathic inflammatory
myopathy. Lee et al[5] demonstrated the feasibility and
benefits of performing muscle biopsy under ultrasound-MR fusion imaging guidance in the patients with
suspected myopathy, by sampling site of active muscle
oedema without significant fatty infiltration. Our
centre currently employs ultrasound-MR fusion when
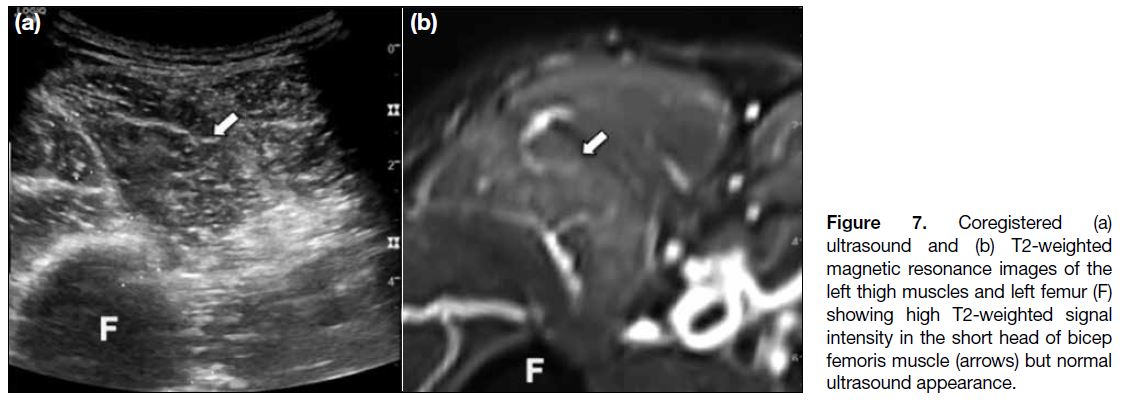
performing muscle biopsy for suspected myopathy
(Figure 7), obviating the need for open surgical biopsy,
which is more invasive and creates a larger wound.
During the procedure, fat-suppressed T2-weighted images of the pelvis and thighs are used for image
fusion to target the area of active muscle inflammation.
Standardised anatomical landmarks for point registration
include the saphenofemoral junction, common femoral
artery bifurcation, and quadriceps tendon insertion at the
superior pole of the patella.
Figure 7. Coregistered (a)
ultrasound and (b) T2-weighted magnetic resonance images of the left thigh muscles and left femur (F) showing high T2-weighted signal intensity in the short head of bicep femoris muscle (arrows) but normal ultrasound appearance.
CONTRAST-ENHANCED
ULTRASOUND
There are additional benefits of CEUS in abdominal
imaging, including improved detectability and
conspicuity of liver and renal lesions.[22] [23] Because CEUS
allows real-time visualisation of contrast enhancement of the septum and nodules in cystic renal tumours, the
most representative part of the lesion can be identified
for biopsy or ablation.[22] Meloni et al[23] demonstrated the
use of CEUS in percutaneous treatment of hepatic and
renal tumours, including immediate visualisation and
real-time assessment of the ablation results.
In our experience, CEUS is particularly useful when the
target lesion is small, isoechoic to the parenchyma, and
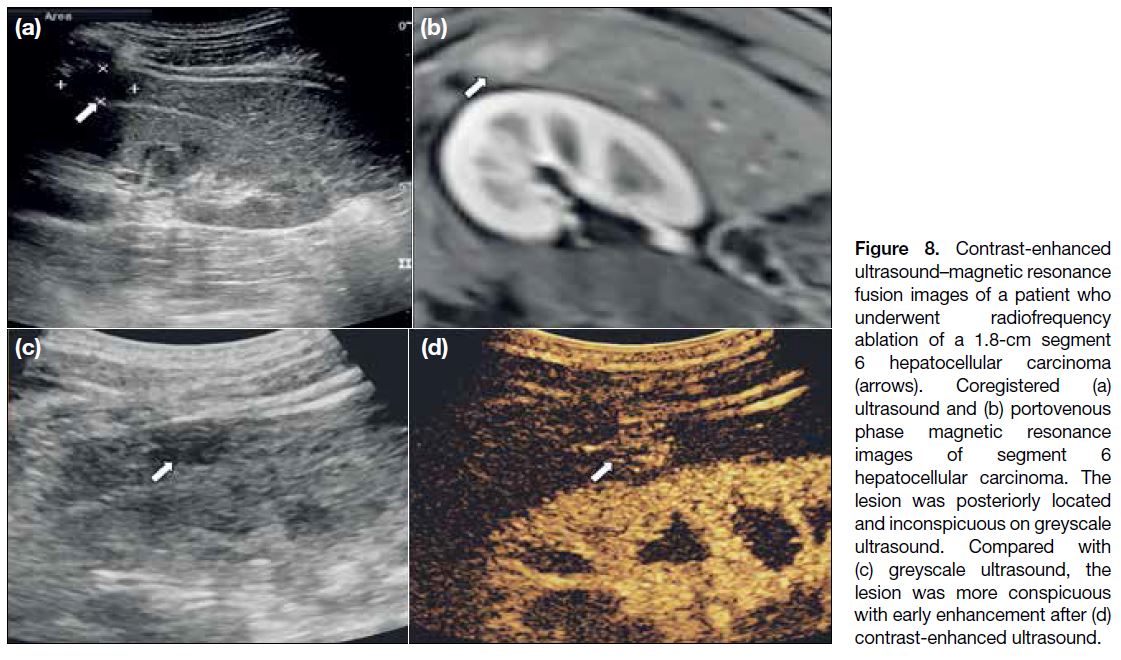
posteriorly located in the liver (Figure 8). Ultrasound
contrast can be injected during ablation screening for
tumour detection, as well as during the ablation process
(Figure 9). This further increases the interventional
radiologist’s confidence and accuracy when performing
ablation in inconspicuous lesions.
Figure 8. Contrast-enhanced
ultrasound–magnetic resonance fusion images of a patient who underwent radiofrequency ablation of a 1.8-cm segment 6 hepatocellular carcinoma (arrows). Coregistered (a) ultrasound and (b) portovenous phase magnetic resonance images of segment 6 hepatocellular carcinoma. The lesion was posteriorly located and inconspicuous on greyscale ultrasound. Compared with (c) greyscale ultrasound, the lesion was more conspicuous with early enhancement after (d) contrast-enhanced ultrasound.
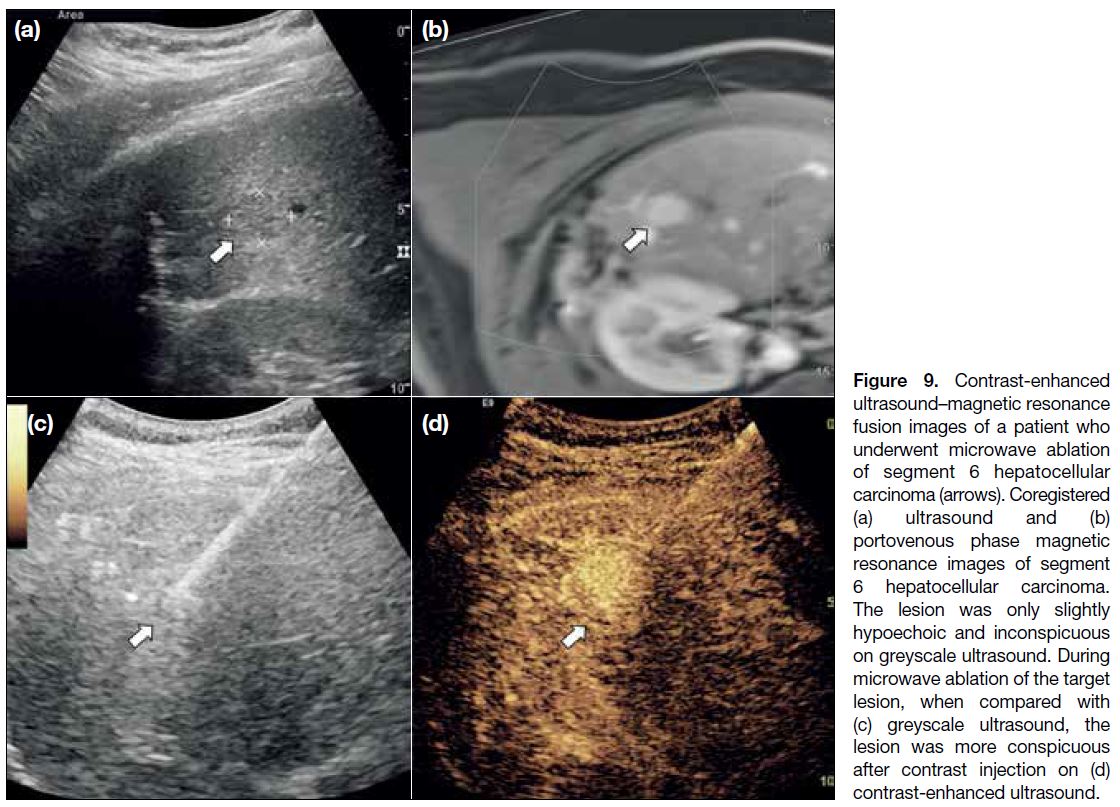
Figure 9. Contrast-enhanced
ultrasound–magnetic resonance fusion images of a patient who underwent microwave ablation of segment 6 hepatocellular carcinoma (arrows). Coregistered (a) ultrasound and (b) portovenous phase magnetic resonance images of segment 6 hepatocellular carcinoma. The lesion was only slightly hypoechoic and inconspicuous on greyscale ultrasound. During microwave ablation of the target lesion, when compared with (c) greyscale ultrasound, the lesion was more conspicuous after contrast injection on (d) contrast-enhanced ultrasound.
CHALLENGES AND RECOMMENDATIONS
There are some possible technical limitations to
real-time ultrasound fusion imaging techniques.
First, ultrasound fusion may potentially prolong the
procedure time owing to the extra time needed for image
coregistration. However, in our experience, the image
fusion step is simple and usually takes <10 minutes.
Moreover, ultrasound fusion guidance decreases
the time needed for trajectory planning, alleviating the need for re-positioning of the needle or ablation
electrode. Thus, operator confidence and accuracy are
increased and procedure time is shortened. Second,
misregistration of images can occur owing to patient
movement, uncooperative breathing movements, and
tissue deformation by probe compression.[2] One study
showed that the mean maximum registration error
between real-time ultrasound and fused CT images was
11.5 mm.[24] To minimise image misregistration, plane
registration should be performed in the same imaging
plane as the CT/MR examinations; patient movement
should be avoided after the image coregistration; and the
operator should maintain a steady and gentle force on
the ultrasound probe throughout the procedure to reduce
the tissue deformation, which is particular challenging
in superficial lesions. Third, the time interval between
the CT/MR examination and real-time ultrasound
fusion–guided intervention can affect the accuracy of
coregistration owing to disease progression or change in
patient’s body habitus. Therefore, it is crucial to use up-to-date CT/MR images for coregistration
CONCLUSION
In conclusion, real-time ultrasound fusion imaging
is a useful tool in various interventional procedures,
including the abdominal and musculoskeletal systems. It provides improved visualisation of the anatomy and
the target lesions by exploiting the strength of contrast
resolution of CT or MR and combining this with real-time
ultrasound imaging. This allows more precise
planning of needle paths, increases safety, and decreases
radiation exposure. In muscle biopsy, ultrasound fusion
imaging can be used to target the most inflamed tissue,
potentially increasing diagnostic yield and replacing
invasive surgical open biopsy. Future studies would be
helpful to explore new clinical applications of ultrasound
fusion imaging, such as its utility in diagnostic imaging
of other organs or during surgery.
REFERENCES
1. Baad M, Lu ZF, Reiser I, Paushter D. Clinical significance of US
artifacts. Radiographics. 2017;37:1408-23. Crossref
2. Lee MW. Fusion imaging of real-time ultrasonography with CT or
MRI for hepatic intervention. Ultrasonography. 2014;33:227-39. Crossref
3. Ewertsen C, Săftoiu A, Gruionu LG, Karstrup S, Nielsen MB.
Real time image fusion involving diagnostic ultrasound. AJR Am
J Roentgenol. 2013;200:W249-55. Crossref
4. Sumi H, Itoh A, Kawashima H, Ohno E, Itoh Y, Nakamura Y, et al. Preliminary study on evaluation of the pancreatic tail observable
limit of transabdominal ultrasonography using a position sensor
and CT fusion image. Eur J Radiol. 2014;83:1324-31. Crossref
5. Lee KH, Lau V, Gao Y, Li YL, Fang BX, Lee R, et al. Ultrasound-MRI fusion for targeted biopsy of myopathies. AJR Am J
Roentgenol. 2019 Feb 26. Epub ahead of print.
6. Burke CJ, Bencardino J, Adler R. The potential use of ultrasound-magnetic
resonance imaging fusion applications in musculoskeletal
intervention. J Ultrasound Med. 2017;36:217-24. Crossref
7. Ukimura O, Mitterberger M, Okihara K, Miki T, Pinggera GM,
Neuruer R, et al. Real-time virtual ultrasonographic radiofrequency
ablation of renal cell carcinoma. BJU Int. 2008;101:707-11. Crossref
8. Klauser AS, De Zordo T, Feuchtner GM, Djedovic G, Weiler RB,
Faschingbauer R, et al. Fusion of real time US with CT images
to guide sacroiliac joint injection in vitro and in vivo. Radiology.
2010;256:547-53. Crossref
9. Chung YE, Kim KW. Contrast-enhanced ultrasonography: advance
and current status in abdominal imaging. Ultrasonography.
2015;34:3-18. Crossref
10. Park HJ, Lee MW, Lee MH, Hwang J, Kang TW, Lim S, et al.
Fusion imaging-guided percutaneous biopsy of focal hepatic lesions
with poor conspicuity on conventional sonography. J Ultrasound
Med. 2013;32:1557-64. Crossref
11. Sartori S, Tombesi P, Macario F, Nielsen I, Tassinari D,
Catellani M, et al. Subcapsular liver tumors treated with percutaneous radiofrequency ablation: a prospective comparison
with nonsubcapsular liver tumors for safety and effectiveness.
Radiology. 2008;248:670-9. Crossref
12. Patidar Y, Singhal P, Gupta S, Mukund A, Sarin SK. Radiofrequency
ablation of surface v/s intraparenchymal hepatocellular carcinoma
in cirrhotic patients. Indian J Radiol Imaging. 2017;27:496-502. Crossref
13. Lee MW, Rhim H, Cha DI, Kim YJ, Lim HK. Planning US for
percutaneous radiofrequency ablation of small hepatocellular
carcinomas (1-3 cm): value of fusion imaging with conventional
US and CT/MR images. J Vasc Interv Radiol. 2013;24:958-65. Crossref
14. Song KD, Lee MW, Rhim H, Cha DI, Chong Y, Lim HK. Fusion
imaging-guided radiofrequency ablation for hepatocellular
carcinomas not visible on conventional ultrasound. AJR Am J
Roentgenol. 2013;201:1141-7. Crossref
15. Zhang H, Chen GY, Xiao L, Ma X, Shi L, Wang T, et al. Ultrasonic/CT image fusion guidance facilitating percutaneous catheter
drainage in treatment of acute pancreatitis complicated with infected
walled-off necrosis. Pancreatology. 2018;18:635-41. Crossref
16. European Society of Radiology (ESR). Abdominal applications of
ultrasound fusion imaging technique: liver, kidney, and pancreas.
Insights Imaging. 2019;10:6. Crossref
17. Sidhar K, McGahan JP, Early HM, Corwin M, Fananapazir G,
Gerscovich EO. Renal cell carcinomas: sonographic appearance
depending on size and histologic type. J Ultrasound Med.
2016;35:311-20. Crossref
18. Helck A, D’Anastasi M, Notohamiprodjo M, Thieme S, Sommer W, Reiser M, et al. Multimodality imaging using ultrasound image fusion in renal lesions. Clin Hemorheol Microcirc. 2012;50:79-
89. Crossref
19. Breen DJ, Railton NJ. Minimally invasive treatment of small
renal tumors: trends in renal cancer diagnosis and management.
Cardiovasc Intervent Radiol. 2010;33:896-908. Crossref
20. Andersson M, Hashimi F, Lyrdal D, Lundstam S, Hellström M.
Improved outcome with combined US/CT guidance as compared
to US guidance in percutaneous radiofrequency ablation of small
renal masses. Acta Radiol. 2015;56:1519-26. Crossref
21. Van De Vlekkert J, Maas M, Hoogendijk JE, De Visser M,
Van Schnik IN. Combining MRI and muscle biopsy improves
diagnostic accuracy in subacute- onset idiopathic inflammatory
myopathy. Muscle Nerve. 2015;51:253-8. Crossref
22. Rübenthaler J, Paprottka KJ, Marcon J, Reiser M, Clevert DA. MRI
and contrast enhanced ultrasound (CEUS) image fusion of renal
lesions. Clin Hemorheol Microcirc. 2016;64:457-66. Crossref
23. Meloni MF, Smolock A, Cantisani V, Bezzi M, D’Ambrosio F,
Proiti M, et al. Contrast enhanced ultrasound in the evaluation and
percutaneous treatment of hepatic and renal tumors. Eur J Radiol.
2015;84:1666-74. Crossref
24. Hakime A, Deschamps F, De Carvalho EG, Teriitehau C,
Auperin A, De Baere T. Clinical evaluation of spatial accuracy
of a fusion imaging technique combining previously acquired
computed tomography and real-time ultrasound for imaging of
liver metastases. Cardiovasc Intervent Radiol. 2011;34:338-44. Crossref