Single-Pass Split-Bolus Whole-Body Contrast-Enhanced Computed Tomography Protocol for Trauma Patients
ORIGINAL ARTICLE
Single-Pass Split-Bolus Whole-Body Contrast-Enhanced Computed Tomography Protocol for Trauma Patients
HKH Lui, DTF Lee, JHM Cheng, KYK Tang, CY Chu, WKW Leung, WK Kan
Department of Radiology, Pamela Youde Nethersole Eastern Hospital, Hong Kong
Correspondence: Dr HKH Lui, Department of Radiology, Pamela Youde Nethersole Eastern Hospital, Hong Kong. Email: horace.lkh@gmail.com
Submitted: 26 Feb 2020; Accepted: 2 Jun 2020.
Contributors: HKHL and DTFL designed the study and acquired the data. All authors analysed the data. HKHL and DTFL drafted the
manuscript. JHMC, KYKT, CYC, WKWL and WKK critically revised the manuscript for important intellectual content. All authors had full
access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics Approval: This study was approved and supervised by the Hong Kong East Cluster Research Ethics Committee (Ref
HKECREC-2019-083). The requirement for informed consent was waived because this retrospective study used anonymous clinical data.
Abstract
Objective
We sought to improve the aortic enhancement to allow proper assessment and detection of vascular injury
in whole-body contrast-enhanced computed tomography (WBCT) of trauma patients without increasing the radiation
dose by using a single-pass combined arterial and venous phase split-bolus protocol, instead of a conventional
venous phase single-bolus protocol.
Methods
A retrospective study assessed consecutive trauma patients who underwent WBCT in two 6-month periods,
one in which patients underwent the single-bolus protocol and one in which they underwent the split-bolus protocol.
In the split-bolus group, 80 mL iohexol (300 mgI/mL) was injected after the plain CT scan and additional 40 mL
iohexol was injected 60 s later. Post-contrast CT images were acquired at 90 s after starting the first contrast injection.
Enhancement of the aorta, liver, spleen and kidneys and dose-length product (DLP) of WBCT were measured and
compared between patients in the two scan protocols.
Results
A total of 95 patients were included. There was statistically significant improvement of the enhancement
of the ascending aorta, descending thoracic aorta (177±36 vs. 249±63 HU, p < 0.001), infrarenal aorta, spleen
(123±16 vs. 146±21 HU, p < 0.001), right renal cortex (194±34 vs. 227±42 HU, p < 0.001) and left renal cortex
(193±37 vs. 228±40 HU, p < 0.001) in the split-bolus group. In the split-bolus group, the aortic enhancement was
>200 HU, which was considered to be sufficient for proper assessment and detection of vascular injury in literature.
There was no statistically significant difference in the DLP (3406±1076 vs. 3194±1261 mGycm, p = 0.152), which
is an effective dose of 64.7 mSv in the single-bolus group and 60.7 mSv in the split-bolus group.
Conclusion
The split-bolus protocol increased the aortic enhancement to allow proper assessment and detection of
vascular injury of trauma patients without increasing the radiation dose. Our study used the lowest possible contrast
dose to achieve diagnostic images among different studies.
Key Words: Contrast media; Radiation dosage; Tomography, X-ray computed; Vascular system injuries; Whole body
imaging
中文摘要
用於創傷患者的全身顯影電腦斷層掃描單程分次推注方案
呂錦浩、李騰飛、鄭希敏、鄧業勤、朱志揚、梁錦榮、簡偉權
目的
在不增加輻射劑量的情況下,使用單程動靜脈期分次推注分案(而非傳統靜脈期單次推注分案)對創傷患者進行全身顯影電腦斷層掃描(WBCT),尋求改善主動脈造影強化,從而正確評估和檢測血管損傷。
方法
這項回顧性研究評估創傷患者於兩段6個月期間分別接受WBCT單次推注方案和分次推注方案。分次推注方案組患者在平掃CT掃描後注射80毫升非離子造影劑iohexol(300 mgI/mL),60秒後再注射40毫升iohexol。注射第一次造影劑後 90 秒採集造影後CT圖像。測量並比較兩種掃描方案中患者的主動脈、肝、脾和腎的造影結果以及WBCT的劑量長度乘積(DLP)。
結果
共納入95例患者。相比單次推注方案,分次推注方案使升主動脈、胸降主動脈(177±36
比249±63 HU,p < 0.001)、腎下主動脈、脾(123±16比146±21 HU,p < 0.001)、右腎皮質(194±34比227±42 HU,p < 0.001)和左腎皮質(193±37比228±40 HU,p < 0.001)的造影強化均有顯著改善。在分次推注組中,主動脈造影強化超過200 HU,這在文獻中被認為足以正確評估和檢出血管損傷。DLP沒有明顯差異(3406±1076比3194±1261 mGycm,p = 0.152)。單次推注組的有效劑量為 64.7 mSv,分次推注組的有效劑量則為60.7 mSv。
結論
分次推注方案改善主動脈造影強化,允許在不增加輻射劑量的情況下正確評估和檢出創傷患者的血管損傷。本研究在不同檢查中使用盡可能少的造影劑量採集到可以適合診斷的圖像。
INTRODUCTION
Whole-body contrast-enhanced computed tomography
(WBCT) is a well-established tool for the rapid diagnosis
of trauma patients.[1] [2] [3] [4] Different WBCT protocols have
been published for the detection of truncal vascular and
organ injury. However, there is no single universally
accepted protocol, as different patients’ demographic
data, contrast dose, scan timing, and computed
tomography (CT) scanner models affect the image
quality and radiation dose significantly. In our centre,
the standard WBCT protocol for traumatic truncal injury
was a conventional venous phase single-bolus protocol
that included unenhanced and venous phase scans of the
chest, abdomen, and pelvis.
It has been suggested that an additional arterial phase
scan increases the accuracy of truncal vascular and
splenic injury when compared with the venous phase
scan alone.[5] [6] [7] [8] [9] [10] However, an extra set of arterial phase
images will increase the radiation dose, workload of
the radiologists, imaging software requirements, and
imaging storage capacity requirements. This study
was aimed at improving the diagnostic accuracy in truncal vascular and splenic injury in trauma patients
without increasing the radiation dose by using a single-pass
combined arterial and venous phase split-bolus
protocol[11] [12] [13] [14] [15] [16] [17] [18] [19] [20] instead of a conventional venous phase
single-bolus protocol.
METHODS
Patient clinical records were retrieved to review data
of mechanism of injury, clinical outcome, and any
adverse reactions to intravenous contrast. All data were
measured or retrieved by fellows of The Royal College
of Radiologists. Enhancement of the vasculature and
solid organs was measured by a radiologist with 5 years’
experience in CT imaging.
This was a retrospective study in which consecutive
trauma patients who underwent WBCT with a single-bolus
protocol between October 2018 and March 2019
(single-bolus group) were compared with consecutive
trauma patients who underwent WBCT with a split-bolus
protocol between May 2019 and October 2019 (split-bolus
group). All patients were scanned with the same
scanner (Toshiba Aquilion 64, 1-mm section thickness, 120 kVp, 0.7 pitch, and automatic exposure control) in
our centre. Patients aged <18 years or who had an extra
set of arterial phase images were excluded. Iohexol
300 mgI/mL (Omnipaque 300, GE Healthcare, Waukesha
[WI], United States) was used as an intravenous contrast
agent.
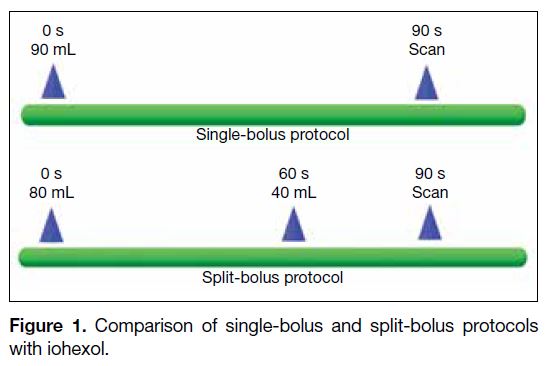
In the single-bolus protocol, 90 mL iohexol was injected
after the plain CT chest, abdomen and pelvis scan. Post-contrast
venous phase images were acquired 90 s after
starting the contrast injection.
In the split-bolus protocol, 80 mL iohexol was injected
after the plain CT chest, abdomen and pelvis scan. An
additional 40 mL contrast agent was injected 60 s later.
Post-contrast combined arterial and venous phase images
were acquired at 90 s after starting the first contrast
injection (Figure 1).
Figure 1. Comparison of single-bolus and split-bolus protocols with iohexol.
Post-contrast enhancement (in Hounsfield units; HU) of
vasculature and abdominal solid organs was measured
to provide an objective assessment of image quality.11,15
Enhancement of the ascending and descending aorta
(at the level of the pulmonary trunk), infrarenal aorta
(between the right renal artery and inferior mesenteric
artery), common iliac arteries (1 cm from the aortic
bifurcation), liver (segment 7), spleen (superior to the
splenic hilum), and kidneys (posterior cortex of the
upper poles) was measured using commercial software
(Carestream PACS Client Version 11.1) on the PACS
monitor.
The dose-length products (DLPs) of plain and postcontrast
CT chest, abdomen and pelvis were recorded.
The two protocols were compared in terms of the post-contrast
enhancement and DLP, using the Mann-Whitney U test. A p value <0.05 was considered statistically
significant.
RESULTS
In the single-bolus group, there were 44 patients
(9 women, 35 men) with median age 48.5 years (range,
22-91 years). In the split-bolus group, there were 41 patients
(12 women, 29 men) with median age 48 years (range,
20-96 years).
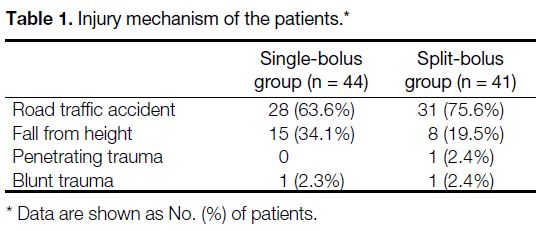
In the single-bolus group, there were 28 road traffic
accident cases, 15 cases of falling from a height, and one
blunt trauma case. In the split-bolus group, there were
31 road traffic accident cases, eight cases of falling from
a height, one penetrating trauma case, and one blunt
trauma case (Table 1). In the WBCT findings, the majority
of trauma patients in both groups had no vascular injury,
solid organ injury, or pelvic fracture. In the single-bolus
group, there were two cases of vascular injury, one case
of solid organ injury (liver and right kidney) and three
cases of pelvic fracture with no contrast extravasation.
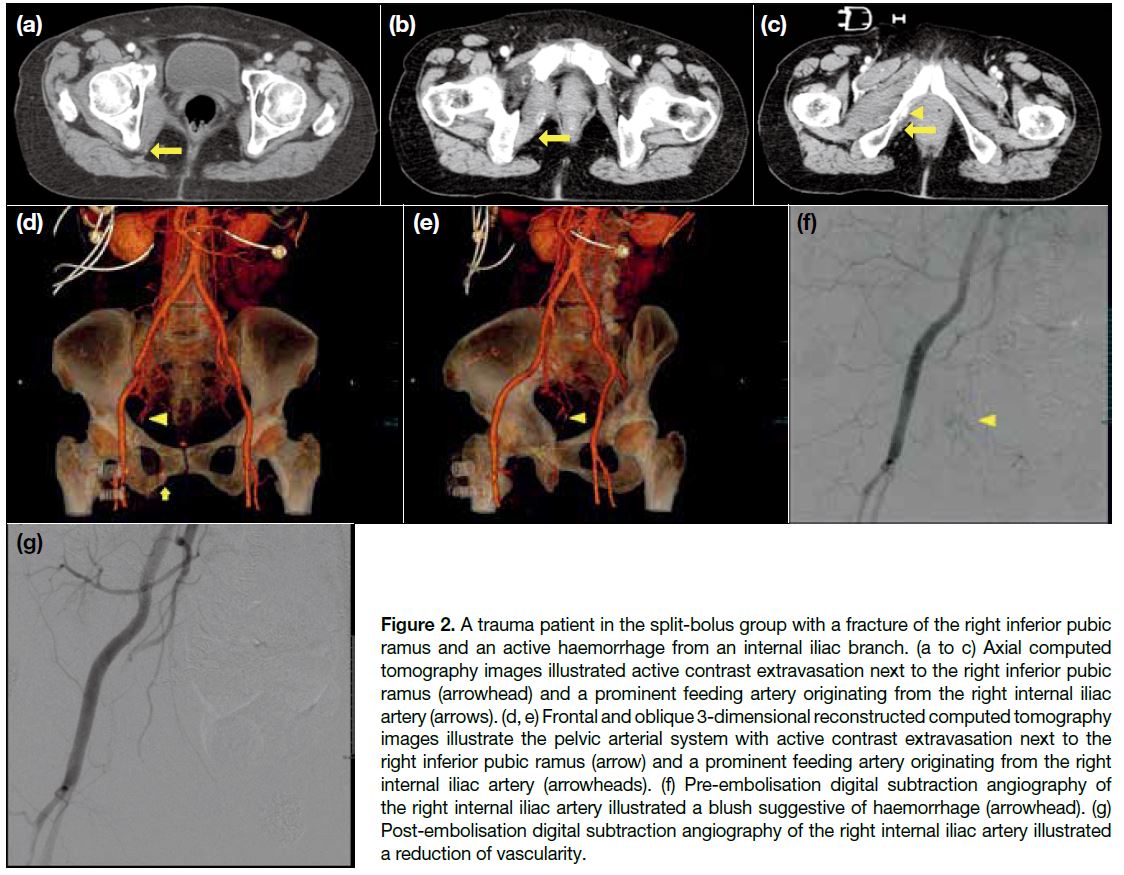
In the split-bolus group, there were four cases of
vascular injury, two cases of solid organ injury (right
kidney; liver and right adrenal gland) and two cases of
pelvic fracture. One of the pelvic fracture cases had an
active haemorrhage and underwent pelvic embolisation
(Figure 2). In each group, there were two deaths due
to severe neurological injury or prolonged intubation-related
infection.
Table 1. Injury mechanism of the patients.
Figure 2. A trauma patient in the split-bolus group with a fracture of the right inferior pubic ramus and an active haemorrhage from an internal iliac branch. (a to c) Axial computed tomography images illustrated active contrast extravasation next to the right inferior pubic ramus (arrowhead) and a prominent feeding artery originating from the right internal iliac artery (arrows). (d, e) Frontal and oblique 3-dimensional reconstructed computed tomography images illustrate the pelvic arterial system with active contrast extravasation next to the right inferior pubic ramus (arrow) and a prominent feeding artery originating from the right internal iliac artery (arrowheads). (f) Pre-embolisation digital subtraction angiography of the right internal iliac artery illustrated a blush suggestive of haemorrhage (arrowhead). (g) Post-embolisation digital subtraction angiography of the right internal iliac artery illustrated a reduction of vascularity.
There were two patients in the single-bolus group with
elevated serum creatinine levels on admission with a
transient increase in creatinine level within 48 hours after
WBCT and subsequent normalisation of renal function
after rehydration. There was one patient in the split-bolus
group with normal renal function on admission with a
transient increase in creatinine level within 48 hours
after WBCT and subsequent normalisation of renal
function after rehydration. Otherwise, there were no
documented adverse reactions related to the intravenous
contrast injection.
There was no statistically significant difference in mean
(± standard deviation) DLP between the single-bolus and
split-bolus groups (3406±1076 vs. 3194±1261 mGycm,
p = 0.152), which is an effective dose of 64.7 mSv in
the single-bolus group and 60.7 mSv in the split-bolus
group.[21]
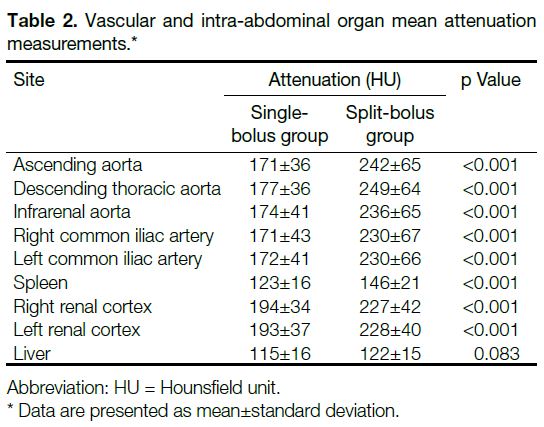
There were statistically significant increases between the
single-bolus and split-bolus groups in the enhancement of
ascending aorta (171 vs. 242 HU, p < 0.001); descending
thoracic aorta (177 vs. 249 HU, p < 0.001); infrarenal
aorta (174 vs. 236 HU, p < 0.001); right common iliac
artery (171 vs. 230 HU, p < 0.001); left common iliac
artery (172 vs. 230 HU, p < 0.001); spleen (123 vs.
146 HU, p < 0.001); right renal cortex (194 vs. 227 HU,
p < 0.001); units of liver (115 vs. 122 HU, p = 0.083)
demonstrated no statistically significant difference
(Table 2). All of the non-injured solid abdominal organs demonstrated homogenous parenchymal enhancement.
Table 2. Vascular and intra-abdominal organ mean attenuation measurements.
DISCUSSION
Aortic enhancement >200 HU is considered to be
sufficient for proper assessment and detection of
vascular injury.[22] [23] In our study, there was a statistically
significant improvement of the aortic enhancement in the
included segments, from <200 HU in the single-bolus
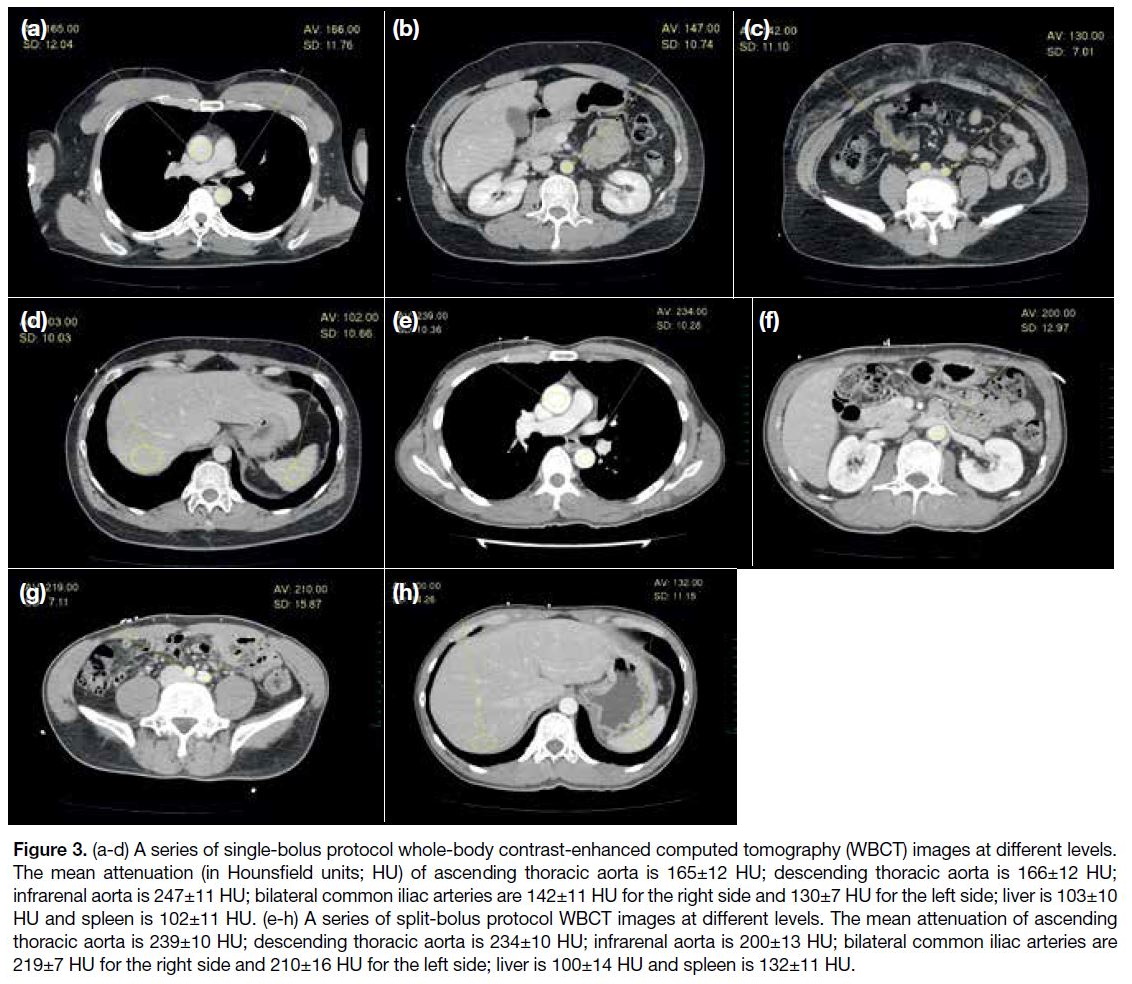
group to >200 HU in the split-bolus group (Figure 3).
Figure 3. (a-d) A series of single-bolus protocol whole-body contrast-enhanced computed tomography (WBCT) images at different levels.
The mean attenuation (in Hounsfield units; HU) of ascending thoracic aorta is 165±12 HU; descending thoracic aorta is 166±12 HU;
infrarenal aorta is 247±11 HU; bilateral common iliac arteries are 142±11 HU for the right side and 130±7 HU for the left side; liver is 103±10
HU and spleen is 102±11 HU. (e-h) A series of split-bolus protocol WBCT images at different levels. The mean attenuation of ascending
thoracic aorta is 239±10 HU; descending thoracic aorta is 234±10 HU; infrarenal aorta is 200±13 HU; bilateral common iliac arteries are
219±7 HU for the right side and 210±16 HU for the left side; liver is 100±14 HU and spleen is 132±11 HU.
Multiple factors can affect the enhancement of the aorta
and solid abdominal organs in CT, including the patient’s
body weight, cardiac function, contrast dose, injection
rate, tube voltage, and scan timing.[24] [25] In our study,
a standardised protocol instead of an individualised
protocol was used to reduce the complexity of workflow
and allow unstable trauma patients to complete the CT scan and to be managed within a short period. Therefore,
no individual adjustment of the contrast dose, tube
voltage, or scan time was done based on the patients’
body weight or cardiac function. This is a limitation
of the study. Using bolus tracking may minimise these
individual variances and achieve optimal enhancement.
This is a topic for future research.
Aortic time-attenuation curves from the literature
demonstrated a rapid increase in aortic enhancement
after contrast injection with peak enhancement at 30
to 50 s, followed by a rapid decrease.[25] The mean
aortic enhancement is significantly affected by the scan
timing after contrast injection. In our study, the split-bolus
protocol post-contrast images were acquired 30 s after the second bolus, which explains the statistically
significant improvement in aortic enhancement.
In the split-bolus group, renal and splenic enhancement
demonstrated a statistically significant increase as
venous parenchymal enhancement was superimposed on
the arterial parenchymal enhancement.
The hepatic time-attenuation curve was relatively steady
with a delayed peak from portal venous system supply.[25] [26]
As a result, there was no statistically significant change
in hepatic enhancement between the two groups.
The DLP between the two groups had no statistically
significant difference as no extra image data were
captured. For centres using a WBCT protocol with
separate arterial phase images acquisition, the split-bolus
significantly reduces the radiation dose.[11] [19]
According to the United States Food and Drug
Administration, the recommended intravascular dose
of iohexol for body CT is 15 to 60 g iodine (50-200 mL).
Contrast doses used in multiple studies ranged from
42 g iodine (120 mL, 350 mgI/mL) to 51.8 g iodine
(140 mL, 370 mgI/mL).[11] [12] [14] [15] [17] [18] [19] [27] The use of 36 g
iodine (120 mL iohexol) in our protocol was the lowest
among the different studies. In our study, only a few cases
in both protocol groups had transient renal dysfunction,
which did not require renal replacement therapy. These
findings may have been the result of confounding factors
that could alter renal function, such as blood loss from
limb fracture or inadequate hydration. Otherwise, there
was no documented adverse reaction related to the
intravenous contrast injection.
Some studies have suggested that it is important to
differentiate pelvic arterial and venous bleeding, as the
management is different.[28] In our centre, we adopted
a ‘3-in-1 Pelvic Damage Control Protocol’.[29] Patients
with a hemodynamically unstable open pelvic fracture
undergo immediate external fixation, preperitoneal
packing, pelvic digital subtraction angiography, and
pelvic embolisation, regardless of whether the bleeding
source is venous or arterial. Therefore, differentiating
pelvic arterial from venous bleeding is not essential
and will not affect patient management in our centre.
(Figure 2).
A study conducted by Stedman et al[27] reported that there
was heterogeneous splenic parenchymal enhancement
in 49% of patients who had been imaged with a split-bolus
protocol WBCT (70 mL + 70 mL, iopamidol
370 mgI/mL). In our study, all cases had homogenous
splenic parenchymal enhancement in the combined
phase. This can be explained by the difference in the split
portion. In the study by Stedman et al,[27] the second bolus
was 50% of the overall contrast dose. Therefore, the
usual heterogeneous arterial phase splenic parenchymal
enhancement was superimposed on the background
uniform venous phase enhancement. In our study, the
second bolus was 33% of the overall contrast dose, so
the arterial phase splenic parenchymal enhancement was
masked by the venous phase enhancement.
CONCLUSION
A single-pass combined arterial and venous phase splitbolus
protocol increased the aortic enhancement to allow
proper assessment and detection of vascular injury of
trauma patients without increasing the radiation dose.
Different split-bolus protocols have been published. Our
study was the one with the lowest contrast dose among
different studies. Further work on the optimal contrast
dose is recommended.
REFERENCES
1. Yoon W, Jeong YY, Kim JK, Seo JJ, Lim HS, Shin SS, et al. CT in blunt liver trauma. Radiographics. 2005;25:87-104. Crossref
2. Novelline RA, Rhea JT, Rao PM, Stuk JL. Helical CT in emergency
radiology. Radiology. 1999;213:321-39. Crossref
3. Willmann JK, Roos JE, Platz A, Pfammatter T, Hilfiker PR,
Marincek B, et al. Multidetector CT: Detection of active
hemorrhage in patients with blunt abdominal trauma. AJR Am J
Roentgenol. 2002;179:437-44. Crossref
4. Shanmuganathan K, Mirvis SE, Sover ER. Value of contrast-enhanced CT in detecting active hemorrhage in patients with blunt
abdominal or pelvic trauma. AJR Am J Roentgenol. 1993;161:65-9. Crossref
5. Melikian R, Goldberg S, Strife BJ, Halvorsen RA. Comparison of
MDCT protocols in trauma patients with suspected splenic injury:
superior results with protocol that includes arterial and portal venous
phase imaging. Diagn Interv Radiol. 2016;22:395-9. Crossref
6. Soto JA, Anderson SW. Multidetector CT of blunt abdominal
trauma. Radiology. 2012;265:678-93. Crossref
7. Boscak AR, Shanmuganathan K, Mirvis SE, Fleiter TR, Miller LA,
Sliker CW, et al. Optimizing trauma multidetector CT protocol for
blunt splenic injury: need for arterial and portal venous phase scans.
Radiology. 2013;268:79-88. Crossref
8. Iacobellis F, Ierardi AM, Mazzei MA, Biasina AM, Carrafiello G,
Nicola R, et al. Dual-phase CT for the assessment of acute vascular
injuries in high-energy blunt trauma: the imaging findings and
management implications. Br J Radiol. 2016;89:20150952. Crossref
9. Corwin MT, Fananapazir G, Lamba R, Salcedo ES, Holmes JF.
Arterial phase CT for the detection of splenic injuries in blunt
trauma: would it improve clinical outcomes? Clin Imaging.
2016;40:212-6. Crossref
10. Uyeda JW, LeBedis CA, Penn DR, Soto JA, Anderson SW. Active
hemorrhage and vascular injuries in splenic trauma: utility of the
arterial phase in multidetector CT. Radiology. 2014;270:99-106. Crossref
11. Leung V, Sastry A, Woo TD, Jones HR. Implementation of a
split-bolus single-pass CT protocol at a UK major trauma centre to reduce excess radiation dose in trauma pan-CT. Clin Radiol.
2015;70:1110-5. Crossref
12. Yaniv G, Portnoy O, Simon D, Bader S, Konen E, Guranda L.
Revised protocol for whole-body CT for multi-trauma patients
applying triphasic injection followed by a single-pass scan on a
64-MDCT. Clin Radiol. 2013;68:668-75. Crossref
13. Jeavons C, Hacking C, Beenen LF, Gunn ML. A review of split-bolus
single-pass CT in the assessment of trauma patients. Emerg
Radiol. 2018;25:367-74. Crossref
14. Stengel D, Ottersbach C, Matthes G, Weigeldt M, Grundei S,
Rademacher G, et al. Accuracy of single-pass whole-body
computed tomography for detection of injuries in patients with
major blunt trauma. CMAJ. 2012;184:869-76. Crossref
15. Loupatatzis C, Schindera S, Gralla J, Hoppe H, Bittner J, Schröder R,
et al. Whole-body computed tomography for multiple traumas
using a triphasic injection protocol. Eur Radiol. 2008;18:1206-14. Crossref
16. Scialpi M, Schiavone R. Split-bolus single-pass in trauma pan-CT:
how to ensure reproducibility and diagnostic efficacy. Clin Radiol.
2016;71:497-8. Crossref
17. Hakim W, Kamanahalli R, Dick E, Bharwani N, Fetherston S,
Kashef E. Trauma whole-body MDCT: an assessment of image
quality in conventional dual-phase and modified biphasic injection.
Br J Radiol. 2016;89:20160160. Crossref
18. Nguyen D, Platon A, Shanmuganathan K, Mirvis SE, Becker CD,
Poletti PA. Evaluation of a single-pass continuous whole-body
16-MDCT protocol for patients with polytrauma. AJR Am J
Roentgenol. 2009;192:3-10. Crossref
19. Alagic Z, Eriksson A, Drageryd E, Motamed SR, Wick MC. A
new low-dose multi-phase trauma CT protocol and its impact on
diagnostic assessment and radiation dose in multi-trauma patients.
Emerg Radiol. 2017;24:509-18. Crossref
20. Standards of Practice and Guidance for Trauma Radiology in
Severely Injured Patients, second edition. Faculty of Clinical Radiology. The Royal College of Radiologists; 2015. BFCR(15)5.
21. Christner JA, Kofler JM, McCollough CH. Estimating effective
dose for CT using dose-length product compared with using organ
doses: consequences of adopting International Commission on
Radiological Protection publication 103 or dual-energy scanning.
AJR Am J Roentgenol. 2010;194:881-9. Crossref
22. Platt JF, Reige KA, Ellis JH. Aortic enhancement during abdominal
CT angiography: Correlation with test injections, flow rates, and
patient demographics. AJR Am J Roentgenol. 1999;172:53-6. Crossref
23. Baliyan V, Verdini D, Meyersohn NM. Noninvasive aortic imaging.
Cardiovasc Diagn Ther. 2018;8(Suppl 1):S3-18. Crossref
24. Yamashita Y, Komohara Y, Takahashi M, Uchida M, Hayabuchi N,
Shimizu T, et al. Abdominal helical CT: evaluation of optimal doses
of intravenous contrast material — A prospective randomized study.
Radiology. 2000;216:718-23. Crossref
25. Bae KT. Intravenous contrast medium administration and
scan timing at CT: considerations and approaches. Radiology.
2010;256:32-61. Crossref
26. Kim SH, Kamaya A, Willmann JK. CT perfusion of the liver: Principles and applications in oncology. Radiology. 2014;272:322-44. Crossref
27. Stedman JM, Franklin JM, Nicholl H, Anderson EM, Moore NR.
Splenic parenchymal heterogeneity at dual-bolus single-acquisition
CT in polytrauma patients — 6-months experience from Oxford,
UK. Emerg Radiol. 2014;21:257-60. Crossref
28. Anderson SW, Soto JA, Lucey BC, Burke PA, Hirsch EF, Rhea JT.
Blunt trauma: feasibility and clinical utility of pelvic CT
angiography performed with 64-detector row CT. Radiology.
2008;246:410-9. Crossref
29. Cheng M, Lee KY, Chang AM, Ho HF, Chan LP, Lee KB, et al.
Three-in-one protocol reduces mortality of patients with
haemodynamically unstable pelvic fractures — a five year multi-centred
review in Hong Kong. Int Orthop. 2018;42:2459-66. Crossref