Management of Type II Endoleaks by Embolisation after Endovascular Abdominal Aortic Aneurysm Repair: Retrospective Review of Patient Data
ORIGINAL ARTICLE
Management of Type II Endoleaks by Embolisation after
Endovascular Abdominal Aortic Aneurysm Repair: Retrospective Review of Patient Data
K Chin, ST Leung, KW Leung, WK Kan
Department of Radiology, Pamela Youde Nethersole Eastern Hospital, Chai Wan, Hong Kong
Correspondence: Dr K Chin, Department of Radiology, Pamela Youde Nethersole Eastern Hospital, Chai Wan, Hong Kong. Email: chinhoikevin@gmail.com
Submitted: 28 Jan 2019; Accepted: 23 Apr 2019.
Contributors: All authors designed the study. KC acquired the data. KC and STL analysed the data. KC drafted the manuscript. All authors
critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the
final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics Approval: The study was approved by Hong Kong East Cluster Research Ethics Committee (Ref No. HKECREC-2021-026). The review
board waived the need for patient consent for this retrospective study.
Abstract
Objective
To review the success and complication rate of transarterial and translumbar embolisation of type II
endoleaks after endovascular aneurysm repair (EVAR) of abdominal aortic aneurysms.
Methods
We conducted a review of post-EVAR type II endoleaks treated by interventional radiology from June
2016 to December 2017.
Results
A total of 17 embolisations for type II endoleaks in 11 patients were identified. Two patients had >1
interventions for recurrent endoleaks. Type IIA endoleaks occurred in seven patients. Three patients had type IIB
endoleaks from the inferior mesenteric artery (IMA) and lumbar artery (LA). The last patient had endoleaks from
multiple LAs. In cases where the IMA was the culprit (n = 6), endovascular access was achieved via the superior
mesenteric artery via the arc of Riolan, followed by embolisation of the IMA, and, in some cases, the aneurysmal sac.
When the LA was responsible (n = 8), it was accessed via the ipsilateral internal iliac artery and iliolumbar artery.
Direct puncture of the aneurysmal sac was performed on five occasions in a single patient with a 13-cm aneurysm
sac. The procedural success rate was 100%. The clinical success rate was 72%, ‘satisfactory’ as defined by stable
sac size. No procedure-related complication was identified.
Conclusion
Transarterial or translumbar embolisation remains an effective treatment option for post-EVAR type II endoleaks.
Key Words: Aorta, abdominal; Aortic aneurysm, abdominal; Blood vessel prosthesis implantation; Embolization,
therapeutic; Mesenteric artery, superior
中文摘要
血管腔內腹主動脈瘤修復後栓塞治療II型內漏:患者數據回顧
錢凱、梁肇庭、梁錦榮、簡偉權
目的
釐回顧血管腔內主動脈瘤修復(EVAR)腹主動脈瘤後行經動脈和經腰椎栓塞術治療II型內漏的成功率和併發症發生率。
方法
回顧2016年6月至2017年12月期間,以介入放射治療EVAR後II型內漏的病例。
結果
11例患者中共有17次II型內漏栓塞。兩名患者因內漏復發進行了超過1次介入治療。7名患者出現IIA型內漏。3名患者的腸繫膜下動脈和腰動脈發生IIB型內漏。最後一名患者多個腰動脈出現內漏。如果內漏因腸繫膜下動脈而起(n = 6),可通過腸繫膜上動脈的Riolan動脈弓行血管內通路,然後栓塞腸繫膜下動脈,而且在某些情況下須栓塞動脈瘤囊。如果內漏因腰動脈返流(n = 8),可通過同側髂內動脈和髂腰動脈行血管內通路。一例有13厘米動脈瘤囊的患者,進行了五次動脈瘤囊直接穿刺。手術成功率為100%。臨床成功率(指囊大小穩定)也令人滿意,達72%。無與手術相關的併發症。
結論
經動脈或經腰椎栓塞術是治療EVAR後II型內漏的有效選擇。
INTRODUCTION
Since its introduction, endovascular aneurysm repair
(EVAR) has evolved rapidly and revolutionised the
treatment of abdominal aortic aneurysm. With the
advancement of technique and stent-graft design, more
and more anatomically challenging abdominal aortic
aneurysms can now be treated with EVAR. Compared
with open repair, studies have shown that EVAR has a
lower perioperative morbidity and mortality rate.[1] [2] [3]
Endoleaks, a complication unique to EVAR, are
unfortunately common. They are estimated to involve
20% to 25% of post-EVAR patients.[4] Endoleak is defined
as evidence of persistent blood flow into the aneurysmal
sac, and is classified as types I to IV (Table 1).[5] Types
I and III need urgent treatment. Type IV is almost
always transient and does not require treatment. The
management of type II endoleaks is variable. Persistent
type II endoleaks can lead to continuous exposure of the
aneurysm sac to arterial pressures and may increase the
risk of delayed rupture of the aneurysm, particularly if
there is associated sac enlargement. Conversely, many
type II endoleaks resolve by themselves, rendering
conservative management an option. Therefore,
patients receiving EVAR require long-term imaging
surveillance.[5]
Table 1. Classification of endoleaks
With increasing experience at many institutions
worldwide, interventional radiology is the modality of choice in managing type II endoleaks, using a variety of
embolic agents, either alone or in combination. It serves
as a versatile and less invasive therapeutic alternative,
compared to open ligation. In this article, we aimed to
review the success and complication rate of transarterial
(TA) and translumbar (TL) embolisations of type II
endoleaks.
METHODS
We performed a retrospective review of all post-EVAR
type II endoleaks treated by interventional radiology
at our institution from June 2016 to December 2017,
retrieving patient demographics and clinical data from the
electronic medical record. All endoleaks were diagnosed
on surveillance triphasic computed tomography (CT).
Imaging findings (sac size, type of endoleak, feeding
vessels on both CT and the angiogram), procedural records (approach, angiographic findings, embolic
agent[s] used), complications, follow-up interval,
imaging modalities used on follow-up, and the decision
for re-intervention were reviewed. Either interval sac
enlargement or persistent endoleak (>6 months after
EVAR) was taken as an indication for treatment after
review and consensus between vascular surgeons and
interventional radiologists. We used either a TA or TL/direct sac puncture approach at our institution.
Transarterial Approach
This approach relies on the presence of an anastomosis
between two different vascular territories. It is crucial for
the interventional radiologist to review previous imaging
studies (most commonly a CT angiogram [CTA]) and to
determine the culprit vessel, technical feasibility (presence
and anatomy of the anastomosis) and potential difficulties
of the procedure (e.g., vessel tortuosity or stenosis). We
performed the embolisation in our angiography suite,
mostly utilising a transfemoral, and, occasionally, a
transbrachial approach, based on the vascular anatomy.
A 5-Fr vascular sheath is commonly used for the
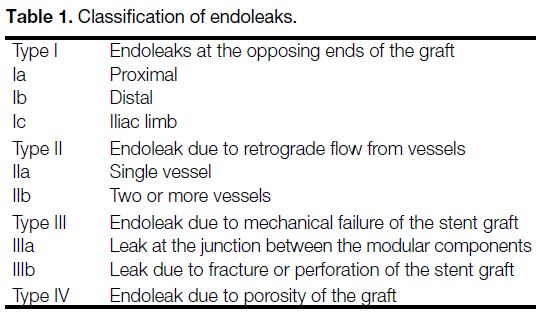
transfemoral approach. A screening aortogram (Figure 1)
would first be performed if the preceding CTA could not
exclude a type I or III endoleak. Then the aneurysmal
sac is navigated, into the culprit feeding vessel using a
combination of catheter, guidewire, microcatheter, and
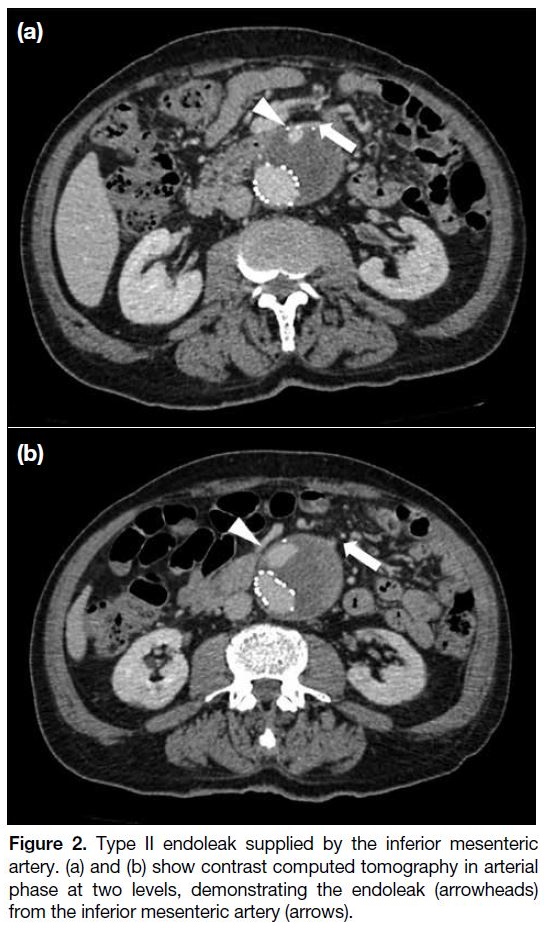
microguidewire. The inferior mesenteric artery (IMA)
can be accessed via the arc of Riolan, if present. It is
a collateral vessel that can be seen (Figures 2 and 3)
arching over the left abdomen between the middle colic
branch of the superior mesenteric artery (SMA) and the
IMA, creating an SMA/IMA anastomosis. A guiding
sheath is first brought into the proximal SMA, followed
by a 4-Fr Cobra catheter (Cordis, the Netherlands) at the
middle colic branch of SMA and a microcatheter into
the IMA and aneurysm sac, establishing triaxial access.
Intra-arterial injection of nitroglycerin or verapamil may
be used to overcome vasospasm during navigation.
Figure 1. Aortogram performed prior to embolisation to exclude
type I and III endoleaks after an inconclusive computed tomography
angiogram. No type I or type III endoleak was identified in this
aortogram.
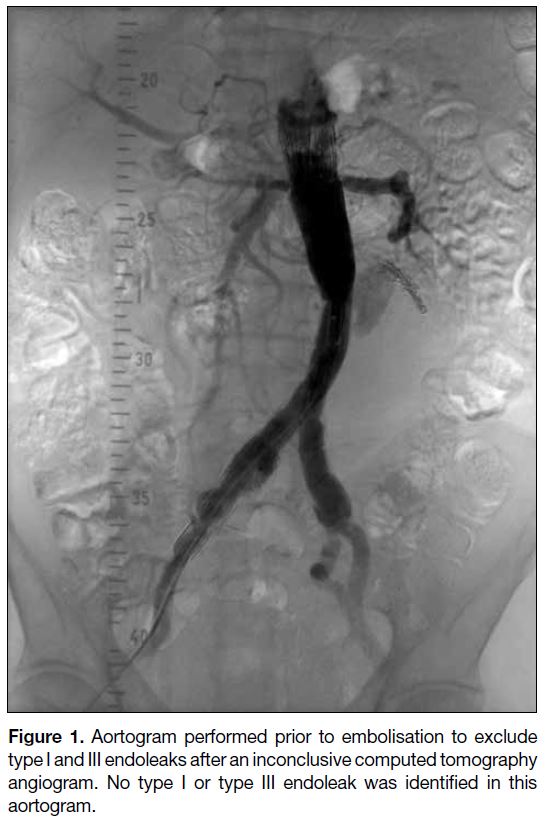
Figure 2. Type II endoleak supplied by the inferior mesenteric
artery. (a) and (b) show contrast computed tomography in arterial
phase at two levels, demonstrating the endoleak (arrowheads)
from the inferior mesenteric artery (arrows).
Figure 3. Arc of Riolan.
(a) Angiogram with microcatheter navigated into the middle colic artery (black arrowhead) of the superior mesenteric artery (black arrow), outlining the arc of Riolan (white arrows in a and b). (b) Angiogram with microcatheter in the arc of Riolan showing endoleak from the inferior mesenteric artery (white arrowheads in a and b).
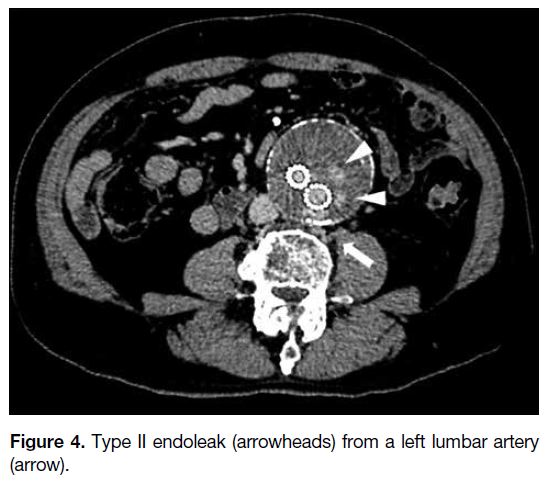
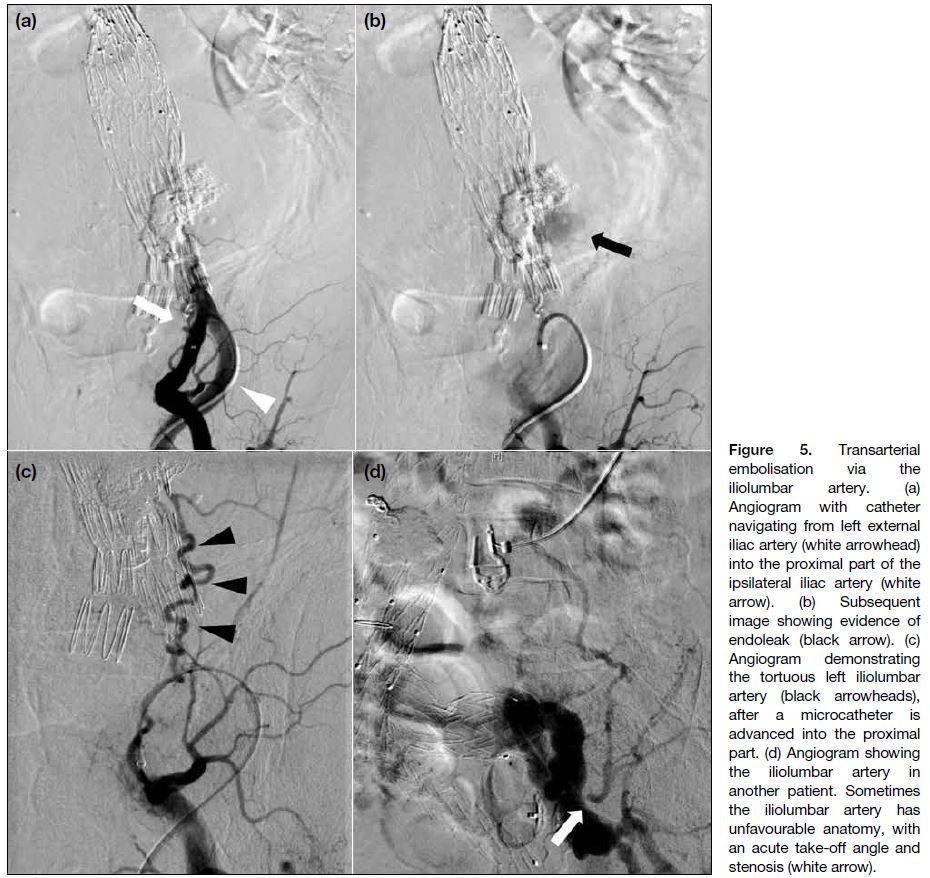
If the culprit vessel is a lumbar artery (LA) [Figure 4],
it might be accessed using an ipsilateral transfemoral
approach. After gaining access, we place a 5-Fr
guiding sheath (Flexor Ansel Guiding Sheath; Cook
Medical [IN], US) in the ipsilateral internal iliac artery
(Figure 5). Oblique projections can help in delineating
the vascular anatomy and better visualisation of the
iliolumbar artery. The iliolumbar artery is subsequently
catheterised using a microcatheter with the aid of a
microguidewire (1.5-2.7-Fr microcatheter, 0.008-0.018-inch microguidewire).
Figure 4. Type II endoleak (arrowheads) from a left lumbar artery (arrow).
Figure 5. Transarterial
embolisation via the iliolumbar artery. (a) Angiogram with catheter navigating from left external iliac artery (white arrowhead) into the proximal part of the ipsilateral iliac artery (white arrow). (b) Subsequent image showing evidence of endoleak (black arrow). (c) Angiogram demonstrating the tortuous left iliolumbar artery (black arrowheads), after a microcatheter is advanced into the proximal part. (d) Angiogram showing the iliolumbar artery in another patient. Sometimes the iliolumbar artery has unfavourable anatomy, with an acute take-off angle and stenosis (white arrow).
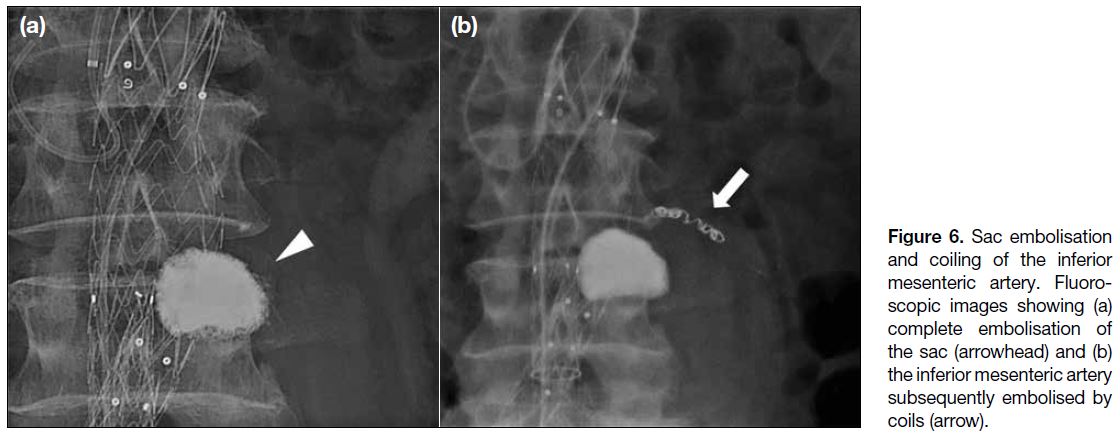
Once advanced into the aneurysm sac, contrast is injected
via the microcatheter (saccogram), to confirm the tip
position of the microcatheter and evaluate the intra-sac
flow dynamics (e.g., net flow rate, outflow vessels, and
filling defects). The aneurysm sac is then embolised,
followed by the feeding vessel(s), and confirmation of
stasis (Figure 6).
Figure 6. Sac embolisation
and coiling of the inferior mesenteric artery. Fluoroscopic images showing (a) complete embolisation of the sac (arrowhead) and (b) the inferior mesenteric artery subsequently embolised by coils (arrow).
Translumbar or Direct Sac Puncture Approach
The retroperitoneal aneurysm sac can also be assessed
with the patient lying prone, under either fluoroscopy,
cone-beam CT, or multidetector CT guidance. At
our centre, we use a combination of these methods,
with additional reference to prior CTA findings. The
radiopaque stent-graft, adjacent bony structures, and
any prior radiopaque embolisation material offer good
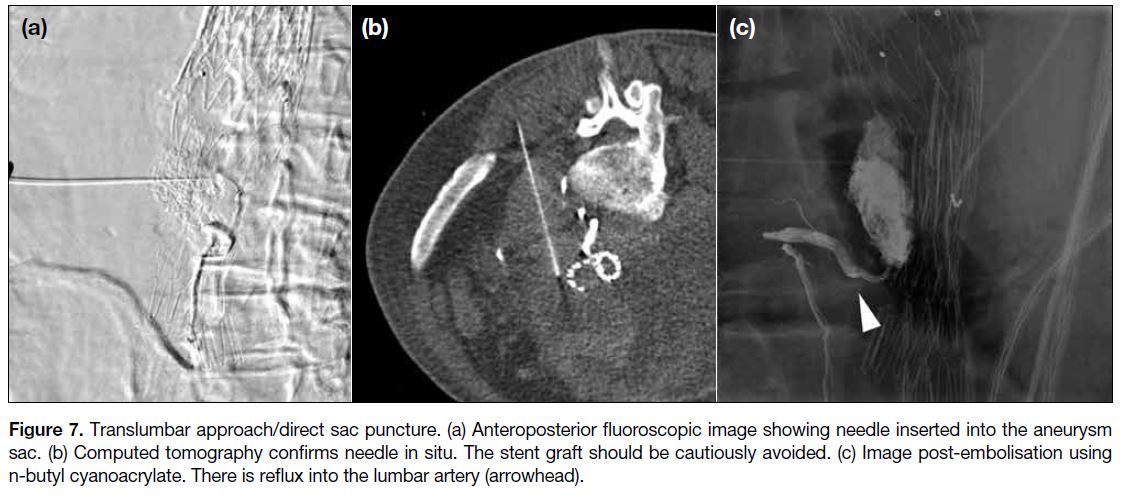
radiological landmarks on fluoroscopy. An 18-gauge
trocar-type needle is used for puncturing the aneurysm
sac, at the side where the aneurysm sac is most readily accessible (usually the left) through the flank region,
and is angled anteromedially (Figure 7). The needle is
advanced under fluoroscopic guidance in orthogonal
planes, or under CT guidance, until back bleeding is
encountered. Contrast is then injected into the sac to
confirm needle tip position, followed by embolisation
and a saccogram for confirming stasis. Some reports
suggest measuring the pressure within the aneurysm
sac and documenting the loss of the arterial waveform
within the sac as additional evidence of successful
embolisation.[4] [6] Such measures were not adopted at our
centre.
Figure 7. Translumbar approach/direct sac puncture. (a) Anteroposterior fluoroscopic image showing needle inserted into the aneurysm
sac. (b) Computed tomography confirms needle in situ. The stent graft should be cautiously avoided. (c) Image post-embolisation using
n-butyl cyanoacrylate. There is reflux into the lumbar artery (arrowhead).
Embolic Agents
We commonly use liquid embolic agents (n-butyl
cyanoacrylate [NBCA], 13/17 of our cases), or ethylene
vinyl alcohol copolymer (Onyx; ev3, Irvine [CA], US)
[2/17]. In one case we used bovine-based thrombin
(THROMBIN-JMI; Pfizer Inc, New York [NY], US).
Ideally, the aim is for complete, permanent sac exclusion
and controlled reflux into the most distal segment of the
feeding vessels, without causing non-target embolisation
into the IMA or LA. In practice, we considered a
substantial reduction of intra-sac flow acceptable as
a procedural endpoint, particularly in aneurysm sacs with high net outflow rates, after weighing the risks of
non-target embolisation. Additional coils were usually
placed at the feeding vessel(s). We mixed NBCA with
ethiodized oil (Lipiodol; Laboratoire Guerbet, Aulnay-Sous-Bois, France) in the range of 12.5% to 25%. The
addition of ethiodized oil increases the radiodensity of
the mixture, allowing its visualisation during procedures.
By varying its proportion, it also enables the adjustment
of polymerisation time of NBCA in an inverse fashion.
The total volume of NBCA used varied substantially
depending on the sac size, ranging from 0.5 to 52 mL
in our review. Some suggest putting coils into the aneurysm sac before injection of liquid agent, with the
intention of slowing the flow within the aneurysm sac
and reducing the risk of non-target embolisation into
the inflow vessels. The type of embolic agents used
has bearing on subsequent imaging surveillance. In
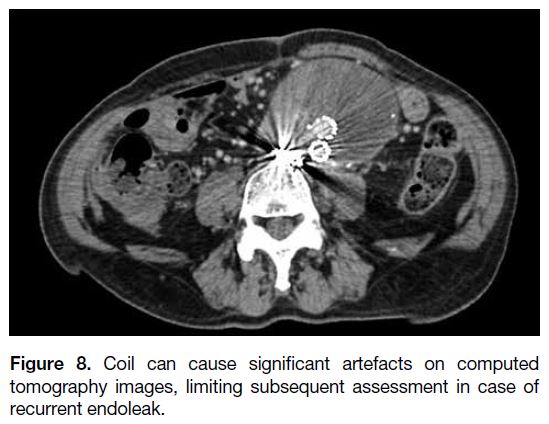
our experience, coils cause significant artefact on CT
(Figure 8), which might affect surveillance or assessment
in the case of recurrent endoleak.
Figure 8. Coil can cause significant artefacts on computed
tomography images, limiting subsequent assessment in case of
recurrent endoleak.
RESULTS
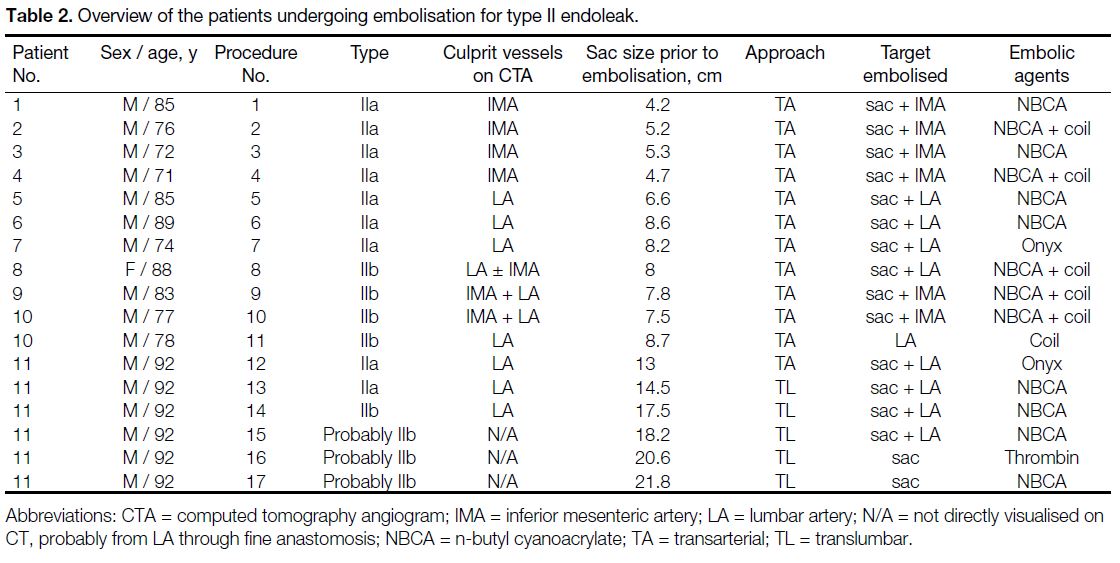
A total of 17 embolisations for type II endoleaks on
11 patients (10 men, 1 woman) with median age 85 years,
(range, 71-92 years) were identified (Table 2). The median
aneurysmal sac size was 8.2 cm (range, 4.2-21.8 cm).
Table 2. Overview of the patients undergoing embolisation for type II endoleak.
Type IIa endoleaks were seen in seven patients: four of
them with IMA supply and three with LA supply. Two
patients had more than one intervention for recurrent
endoleaks. Three patients with type IIb endoleaks from
the IMA and the LA had embolisations performed on the
IMA alone (n = 1), LA alone (n = 1), or both IMA and
LA (n = 1). The last patient had endoleaks supplied by
varying numbers of LAs over time.
Direct puncture of the aneurysmal sac (TL approach)
was performed on five occasions for a single patient
(initial sac size 13 cm). Either NBCA (12.5% to 25%
concentration) or thrombin (a total of 8000 units, in a
dilution of 1000 U/mL) was injected.
The median fluoroscopic time and dose-area product
were 30.2 minutes (range, 14.3-66.3 minutes) and 237.0 Gy·cm2
(range, 27.4-772.6 Gy·cm2), respectively.
The procedural success rate was 100%, with angiographic
evidence of haemostasis or substantial flow reduction
within the aneurysm sac achieved in all 11 patients. The
clinical success rate was satisfactory (72%), as eight
patients had stable sac size during a median follow-up
period of 9.5 months (range, 1-27 months). The results in this
group of patients were as follows: reduced or completely
resolved endoleak in six patients (75%) and persistent
endoleak in two patients (25%). Two patients had an
increase in sac size (6-mm and 12-mm increase) during a mean follow-up period of 6.3 months (range, 4-8.5 months);
one patient received a second embolisation, resulting in a
stable sac size but a new endoleak with an indeterminate
culprit vessel on CTA while the other was managed
conservatively. The eleventh patient with a 13-cm
initial sac size had multiple embolisations performed
over 5 months because of persistent endoleaks and sac
enlargement. No procedural-related complications were
noted.
DISCUSSION
The clinical course for type II endoleaks can be quite
variable. It is usually divided into early (within 6 months
after EVAR) and late (>6 months after EVAR). A
substantial number of early type II endoleaks will
resolve spontaneously, with prevalence at 6 months
approximately 10% to 15%.[5] Some patients develop
‘de novo’ delayed type II endoleaks. For patients with
persistent type II endoleaks, the clinical course is also
variable: the majority of the patients (50%-70%) have
a stable sac size, 25% have a decrease in sac size,
and the remaining 25% have an increase.[5] It has been
shown that persistent endoleaks result in persistent
arterial pressurisation of the aneurysm sac, which could
theoretically increase the risk of delayed sac rupture.
Whether to adopt conservative management with
continuous imaging surveillance or early treatment
for these patients is still up to debate and likely varies
among local practices,[7] [8] but most agree that a significant increase in sac size (defined as >5 mm in diameter)[5] on
interval imaging surveillance should be regarded as an
indication for expeditious treatment, with other factors
taken into consideration (e.g., pretreatment sac size,
presence of culprit vessel[s] on imaging, symptoms, and
patient’s co-morbidities and preferences).
Different feeding vessels contribute to the formation of
type II endoleaks, either alone (type IIa) or in combinations
(type IIb). Examples include the IMA (Figure 2), the
lower LA, and, less frequently, the median sacral artery
or even an accessory renal artery.[9] Risk factors for a
type II endoleak includes a patent IMA, number and
diameter of patent LAs, and continued angicoagulation.[5]
One study showed that the cross-sectional area of the
contrast-enhanced aortic lumen at the level of the IMA
is positively associated with the development of a type II
endoleak.[10]
Previous studies have shown that durable embolisation
of type II endoleaks requires embolisation of both
the aneurysm sac and the feeding vessels. This
approach is analogous to the approach to arteriovenous
malformations in the brain: embolisation of the feeding
arteries alone without embolising the nidus will lead
to persistence or recurrence of the underlying vascular
lesion.[11] [12] Similarly, embolisation of the aortic aneurysm
sac alone has a higher rate of endoleak recurrence.[11] [12]
The most common approach for treating endoleaks
includes TA and TL routes, as adopted at our institution.
The technical challenge in the TA approach mainly lies
in the vascular anatomy and the significant length of
the vessels that need to be traversed before reaching the
aneurysm sac. Vasospasm may occur with prolonged
procedure. Embolising a culprit LA is usually more
challenging, as the vessel tends to be more tortuous,
and it is not uncommon for the iliolumbar artery to have
an unfavourable take-off angle from the internal iliac
artery (Figure 5d). The transcaval approach is reported
in the literature but rarely utilised due to the potentially
serious consequences of the complications, such as
aortocaval fistula, pulmonary embolism from non-target
embolisation, and retroperitoneal bleeding.[4]
Complications of TA and TL embolisations can be
categorised mainly into embolic agent-related or
approach-related. Overall complication rate ranges
from 0% to 12%.[11] [12] [13] [14] Nontarget embolisation into
proximal parts of the IMA and LA when using a liquid
embolic agent is the main concern. Ischaemic sciatic neuropathy after TA embolisation using NBCA and
non-target embolisation of IMA branches has been
reported.[12] Approach-related complications specific to
TL embolisation include: inadvertent injury to the LA
and nerve root as the needle traverses the abdominal
wall; accidental puncture of the stent graft, resulting
in type III endoleak; and retroperitoneal bleeding and
haematoma.[11] [12] [13] [14] Still, the TL approach is considered a
safe approach for managing type II endoleak.
Studies have shown that both TA and TL approaches have
comparable clinical success rates, with no significant
differences in aneurysm sac growth, persistent endoleak,
or complications. TL approach may result in shorter
fluoroscopy time and total procedural time.[4] [12] [14]
There has been no universal definition of ‘clinical
success’. Some define it as cessation of type II endoleak.
In this retrospective study, we defined clinical success as
absence of a substantial increase in sac size. Our results
are comparable to those in a relatively large retrospective
study conducted by Stavropoulos et al.[12]
Limitations of our study include its retrospective nature,
the small sample size, and the relatively short and wide
range of follow-up times. All TL embolisations were
performed in a single patient presenting with a 13-cm
aneurysm sac. This limits further statistical analysis and
comparison between the TA and TL approaches.
CONCLUSION
In the era of EVAR, both TA and TL embolisations are
effective treatment options for type II endoleaks.
REFERENCES
1. Malas M, Arhuidese I, Qaiz U, Black J, Perler B, Freischlag JA.
Perioperative mortality following repair of abdominal aortic
aneurysms: application of a randomized clinical trial to real-world
practice using a validated nationwide data set. JAMA Surg.
2014;149:1260-5. Crossref
2. Jackson RS, Chang DC, Freischlag JA. Comparison of long-term
survival after open vs endovascular repair of intact abdominal aortic
aneurysm among Medicare beneficiaries. JAMA. 2012;307:1621-8. Crossref
3. Speicher PJ, Barbas AS, Mureebe L. Open versus endovascular
repair of ruptured abdominal aortic aneurysms. Ann Vasc Surg.
2014;28:1249-57. Crossref
4. Bryce Y, Schiro B, Cooper K, Ganguli S, Khayat M, Lam CK, et al.
Type II endoleaks: diagnosis and treatment algorithm. Cardiovasc
Diagn Ther. 2018;8(Suppl 1):S131-7. Crossref
5. Chaikof EL, Dalman RL, Eskandari MK, Jackson BM, Lee WA,
Mansour MA, et al. The Society for Vascular Surgery practice
guidelines on the care of patients with an abdominal aortic
aneurysm. J Vasc Surg. 2018;67:2-77.e2. Crossref
6. Baum RA, Carpenter JP, Cope C, Golden MA, Velazquez OC,
Neschis DG, et al. Aneurysm sac pressure measurements after endovascular repair of abdominal aortic aneurysms. J Vasc Surg.
2001;33:32-41. Crossref
7. Gelfand DV, White GH, Wilson SE. Clinical significance of type II endoleak after endovascular repair of abdominal aortic aneurysm. Ann Vasc Surg. 2006;20:69-74. Crossref
8. Karthikesalingam A, Thrumurthy SG, Jackson D, Choke E,
Sayers RD, Loftus IM, et al. Current evidence is insufficient to
define an optimal threshold for intervention in isolated type II
endoleak after endovascular aneurysm repair. J Endovasc Ther.
2012;19:200-8. Crossref
9. Müller-Wille R, Schötz S, Zeman F, Uller W, Güntner O, Pfister K,
et al. CT features of early type II endoleak after endovascular
repair of abdominal aortic aneurysms help predict aneurysm sac
enlargement. Radiology. 2015;254:906-16. Crossref
10. Güntner O, Zeman F, Wohlgemuth WA, Heiss P, Jung EM,
Wiggermann P, et al. Inferior mesenteric arterial type II endoleaks
after endovascular repair of abdominal aortic aneurysm: are they predictable? Radiology. 2014;270:910-9. Crossref
11. Baum RA, Carpenter JP, Golden MA, Velaquez OC, Clark TW,
Stavropoulos SW, et al. Treatment of type 2 endoleaks after
endovascular repair of aortic aneurysms: comparison of transarterial
and translumbar techniques. J Vasc Surg. 2002;35:23-9. Crossref
12. Stavropoulos SW, Park J, Fairman R, Carpenter J. Type 2
endoleak embolization comparison: translumbar embolization
versus modified transarterial embolization. J Vasc Interv Radiol.
2009;20:1299-302. Crossref
13. Stavropoulos SW, Kim H, Clark TW, Fairman RM, Velazquez O,
Carpenter JP. Embolization of type 2 endoleaks after endovascular
repair of abdominal aortic aneurysms with use of cyanoacrylate
with or without coils. J Vasc Interv Radiol. 2005;16:857-61. Crossref
14. Yang RY, Tan KT, Beecroft JR, Rajan DK, Jaskolka JD.
Direct sac puncture versus transarterial embolization of type II
endoleaks: an evaluation and comparison of outcomes. Vascular.
2017;25:227-33. Crossref