Cardiovascular Events and Mortality in Patients Undergoing Adjuvant Radiotherapy for Breast Cancer: a Systematic Review
REVIEW ARTICLE CME
Cardiovascular Events and Mortality in Patients Undergoing Adjuvant Radiotherapy for Breast Cancer: a Systematic Review
P Taylor1, S Chan1, AB Wan1, CW Chan2, MM Rodrigues3, H Lam1, E Chow1, FMY Lim2
1 Odette Cancer Centre, Sunnybrook Health Sciences Centre, University of Toronto, Toronto, Ontario, Canada
2 Department of Oncology, Princess Margaret Hospital, Hong Kong
3 Centro Oncológico AZ do Noroeste, Patos de Minas, Minas Gerais, Brazil
Correspondence: Dr FMY Lim, Department of Oncology, Princess Margaret Hospital, Hong Kong. Email: lmy084@ha.org.hk
Submitted: 21 May 2020; Accepted: 15 Jan 2021.
Contributors: PT, SC, ABW, and EC designed the study. PT, ABW, and HL acquired the data. PT, SC, ABW, CWC, MMR, and FMYL
analysed the data. PT and SC drafted the manuscript. All authors critically revised the manuscript for important intellectual content. All authors
had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and
integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: Ethics approval was not required for this review article which does not involve patients / animal or any interventions.
Abstract
Objective
We performed a systematic review to quantify the cardiovascular risk of adjuvant radiotherapy (RT) for breast cancer.
Methods
A literature search was conducted using MEDLINE, Embase, and the Cochrane Central Register of Controlled Trials from inception to July 2020.
Results
The literature search produced 7363 reports, of which 76 met our inclusion criteria. In studies comparing
left-sided RT with right-sided RT, 7 of 35 (20%) studies found increased cardiovascular mortality, and 8 of 28 (29%)
studies found increased cardiovascular events. In studies comparing patients who received RT with those who did
not, 7 of 26 (27%) studies found increased cardiovascular mortality, and 5 of 22 (23%) studies found increased
cardiovascular events.
Conclusion
Most of the studies that found significant associations between laterality and cardiovascular risks included treatment periods that started prior to 1985, suggesting that modern RT techniques have minimised the cardiac exposure in breast cancer patients receiving RT. However, more focused studies must be conducted to investigate the long- term cardiovascular risk associated with modern RT techniques.
Key Words: Breast neoplasms; Heart disease risk factors; Morbidity; Mortality; Radiotherapy, adjuvant
中文摘要
乳腺癌輔助放療患者的心血管疾病和死亡率:系統性文獻回顧
P Taylor、S Chan、AB Wan、陳俊尹、MM Rodrigues、H Lam、E Chow、林美瑩
目的
我們進行系統性文獻回顧,量化乳腺癌術後輔助放療的心血管疾病風險。
方法
使用 MEDLINE、Embase和Cochrane Central Register of Controlled Trials檢索始至2020年7月刊登的文獻。
結果
文獻檢索出7363個結果,其中76個符合我們的納入標準。在比較左側乳腺癌放療與右側乳腺癌放療的研究中,35項研究中有 7 項(20%)發現心血管疾病死亡率增加,28項研究中有8項(29%)發現心血管疾病增加。在比較接受放療的患者與未接受放療的患者的研究中,26項研究中有 7項(27%)發現心血管疾病死亡率增加,22項研究中有 5項(23%)發現心血管疾病增加。
結論
大部份發現單側性乳癌與心血管疾病風險間存在顯著關聯的研究都是1985年或之前,這表明現代放療技術已將接受放療的乳腺癌患者的心臟暴露風險降至最低。然而,必須進行更有針對性的研究以檢視與現代放療技術相關的長期心血管疾病風險。
INTRODUCTION
Breast cancer is the most frequently diagnosed cancer and
the leading cause of cancer death in females worldwide.[1]
It has been shown through randomised trials that
adjuvant radiotherapy (RT) following breast-conserving
surgery substantially reduces breast cancer recurrence
and reduces the absolute breast cancer mortality rate.[2] [3]
RT administered to breast cancer patients usually
exposes the heart, an organ at risk, to some radiation.
Cheng et al[4] conducted a literature review and meta-analysis
on this topic, including studies published prior
to January 2015, and found that breast cancer RT was
associated with an absolute increase of 76.4 cases
of coronary heart disease (95% confidence interval
[CI]=36.8-130.5) and 125.5 cases of cardiac death
(95% CI=98.8-157.9) per 100 000 person-years,
respectively. In order to create optimised and tailored
treatment plans, the current relationship between adjuvant
breast RT and cardiovascular risks must be studied so
that physicians and patients may appropriately consider
the benefits of reduced breast cancer mortality with the
potential long-term cardiovascular risks. We performed
a systematic review to assess the risk of cardiovascular
events (CVEs) and cardiovascular mortality (CVM)
and its correlation with breast/chest wall RT for women
with breast cancer (including breast cancer and ductal
carcinoma in situ) following breast-conserving surgery/mastectomy (for node-positive or involved resection
margin disease), as well as disease laterality. This will
allow radiation oncologists to better inform their patients
about the risks and benefits of adjuvant RT so that
patients may make a more informed decision.
METHODS
Search Strategy
A literature search was completed using MEDLINE,
Embase, and Cochrane Central Register of Controlled Trials from inception through to July 2020. Search
terms for breast cancer included ‘breast cancer’, ‘breast
neoplasm’, ‘breast carcinoma’, and ‘breast tumour or
tumour’ (online supplementary Appendix). RT terms
included ‘radiotherapy’, ‘radiation’, ‘irradiation’, and
‘radiation injury’. CVE terms included ‘heart disease’,
‘heart infarction’, ‘myocardial infarction (MI) or heart
attack’, ‘angina pectoris’, ‘congestive heart failure
(CHF)’, ‘coronary artery disease (CAD)’, ‘coronary
artery obstruction’, ‘heart or cardio or cardiovascular
disease (CVD)’, ‘ischemic heart disease (IHD)’,
‘dosage risk’, ‘cardiovascular risk’, and ‘cardiovascular
death’.
Study Selection
Screening was first done based on the title and abstract
independently by two authors (P Taylor, S Chan), with
discrepancies being resolved through discussion between
the two authors. Then, full-text screening was conducted
independently by the two authors. Inclusion criteria were
reports of the clinical cardiovascular outcomes, including
CVEs and/or CVM as defined above. Specifically,
studies were included if they reported comparisons in
clinical cardiovascular outcomes between patients who
received RT and those who did not receive RT and/or
between patients who received left-sided RT and those
who received right-sided RT. Exclusion criteria included
any studies that investigated cancers other than breast
cancer and the effects of irradiation on organ systems
other than the cardiovascular system. Studies employing
brachytherapy, partial breast irradiation, or boost to the
tumour bed alone were excluded. Full-length papers,
including cohort studies, case-control studies, and
randomised controlled trials published as original papers
written in English, were considered. Any case reports
and non-original articles such as systematic reviews
were excluded.
Data Collection and Analysis
Data extraction was conducted independently by two
authors (P Taylor, S Chan). Both authors engaged in
a discussion regarding any discrepancies between the
extracted data and came to a consensus. The following
data were extracted from the papers: publication year,
geographical location, sample size, mean/median
follow-up, number of CVEs, number of cardiovascular
deaths, laterality, hazard ratios (HRs), incidence ratios,
risk ratios (RRs), mortality ratios (MRs), and associated
measures of variance for all categories of outcomes.
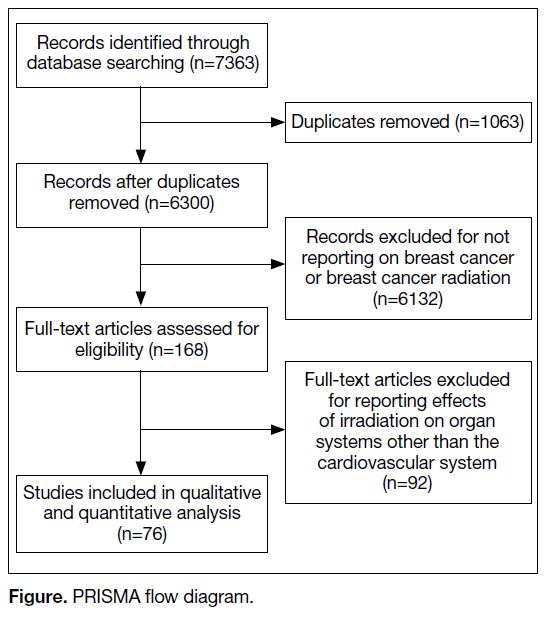
RESULTS
The search identified 7363 publications, of which 1063
were duplicates and excluded (Figure). A further 6132 articles were excluded because they did not meet the
inclusion criteria. The remaining 168 articles underwent
full-text screening. Of them, 92 were excluded for failure
to meet the inclusion criteria, leaving 76 studies that
were analysed in this systematic review.
Figure. PRISMA flow diagram.
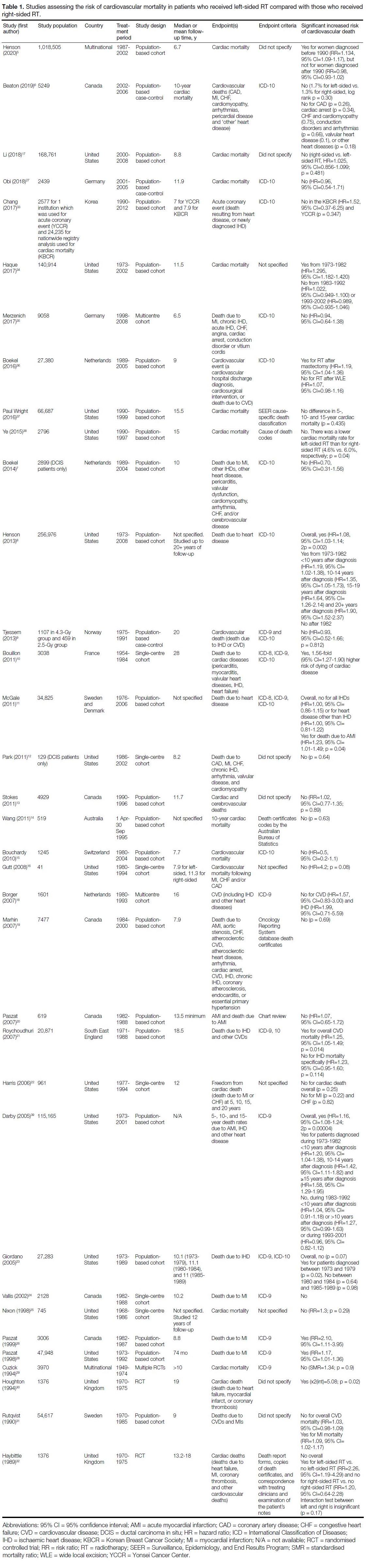
Of the 76 studies, 35 investigated the risk of CVM with
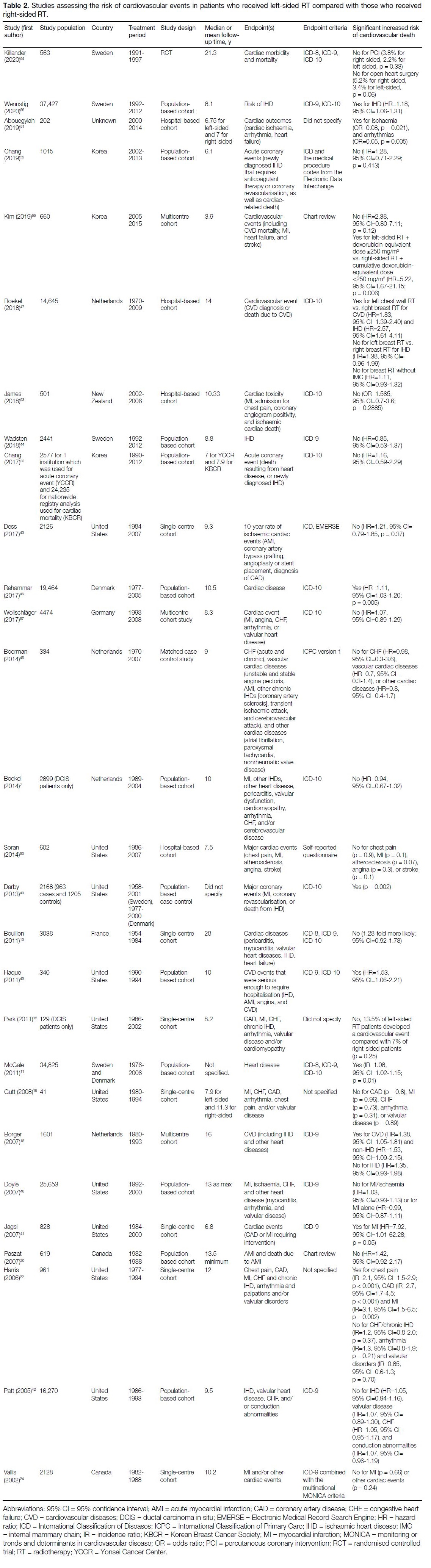
respect to RT laterality,[5] [6] [7] [8] [9] [10] [11] [12] [13] [14] [15] [16] [17] [18] [19] [20] [21] [22] [23] [24] [25] [26] [27] [28] [29] [30] [31] [32] [33] [34] [35] [36] [37] [38] [39] 28 studies investigated the risk
of CVEs with respect to RT laterality,[7] [10] [11] [12][16] [18] [20] [22] [24] [33] [40] [41] [42] [43] [44] [45] [46] [47] [48] [49] [50] [51] [52] [53] [54] [55] [56] [57]
26 studies investigated the risk of CVM with respect to
RT compared with no RT,[5] [7] [10] [14] [21] [27] [29] [30] [32] [38] [54] [58] [59] [60] [61] [62] [63] [64] [65] [66] [67] [68] [69] [70] [71] [72] and
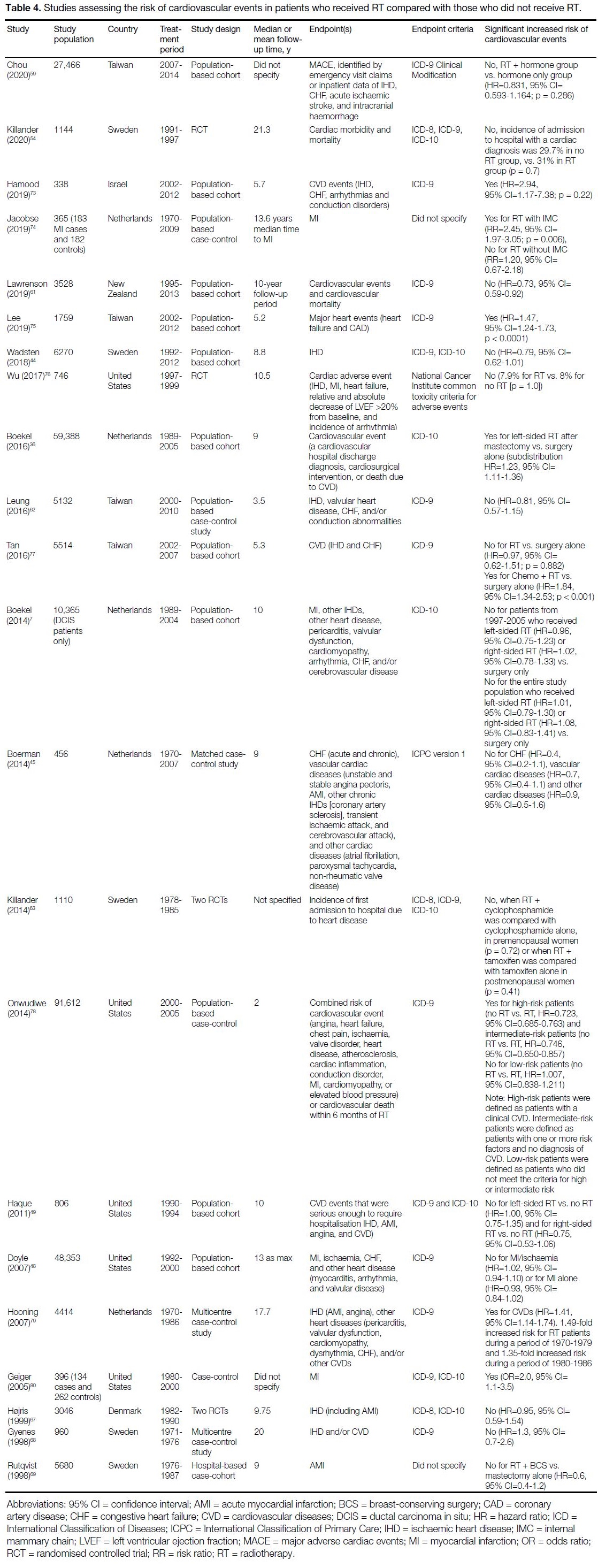
22 studies investigated the risk of CVEs with respect to RT compared with no RT.[7] [36] [44] [45] [48] [49] [54] [59] [61] [62] [63] [67] [68] [69] [73] [74] [75] [76] [77] [78] [79] [80]
Several studies overlapped between the categories and
investigated the risk of CVEs and/or CVM with respect to
RT and/or RT laterality. Results from the largest studies,
based on study population size, will be highlighted for
each of the four categories. The results for all studies are
also reported in Tables 1 2 3 4.
Cardiovascular Mortality in Patients with
Left-sided or Right-sided Radiotherapy
Of 35 studies investigating the risk of CVM with
respect to RT laterality, seven (20%) found a significant
increased risk of CVM in patients who received left-sided
RT compared with patients who received right-sided
RT, all of which included study periods that started
prior to 1985 (Table 1).[8] [10] [21] [26] [28] [30] [39] An additional six
studies found a significant association between laterality
and CVM only in subgroup analysis.[5] [11] [23] [32] [34] [36] Of these
six studies, three had subgroup analyses based on time
period stratification, where in general older study periods
before 1980-1990 were significant while more recent
time periods were not (Table 1).[5] [23] [34]
Table 1. Studies assessing the risk of cardiovascular mortality in patients who received left-sided RT compared with those who received right-sided RT.
Cardiovascular Events in Patients with
Left-sided Radiotherapy or Right-sided Radiotherapy
Of 28 studies investigating the risk of CVEs with
respect to RT laterality, eight (29%) found a significant
increased risk of CVEs in patients who received
left-sided RT compared with patients who received
right-sided RT (Table 2).[11] [18] [40] [41] [46] [49] [51] [56] Of these eight
studies, five included study periods that started prior
to 1985.[11] [18] [40] [41] [46] An additional three studies found a
significant association between laterality and CVEs only
in subgroup analysis.[22] [47] [55] These subgroup analyses
were based on different treatment types and differences
in types of CVEs.
Table 2. Studies assessing the risk of cardiovascular events in patients who received left-sided RT compared with those who received right-sided RT.
Cardiovascular Mortality in Patients with
Radiotherapy or without Radiotherapy
Of 26 studies investigating the risk of CVM for
patients that received RT compared with those who
did not receive RT, seven (27%) found a significant
increase in CVM (Table 3).[29] [30] [32] [63] [64] [71] [72] Of these seven
studies, six included study periods that started prior
to 1985. An additional five studies found a significant
association between RT and CVM only in subgroup
analysis.[5] [10] [21] [68] [70] These subgroup analyses were based
on different treatment types and differences in specific
causes of CVM, such as death from cardiac diseases compared with death from vascular diseases.
Table 3. Studies assessing the risk of cardiovascular mortality in patients who received RT compared with those who did not receive RT.
Cardiovascular Events in Patients with
Radiotherapy or without Radiotherapy
Of 22 studies investigating the risk of CVEs for patients
that received RT compared with those who did not
receive RT, five (23%) found a significant association
between RT and CVEs (Table 4).[36] [73] [75] [79] [80] An additional
three studies found a significant association between
RT and CVEs only in subgroup analysis.[74] [77] [78] These
subgroup analyses were based on different treatment
types and the presence of pre-existing cardiovascular
risk factors.
Table 4. Studies assessing the risk of cardiovascular events in patients who received RT compared with those who did not receive RT.
DISCUSSION
This review summarises the cardiovascular morbidity
and mortality risk associated with breast adjuvant RT
and the laterality of the RT. When comparing patients
who received left-sided RT with those who received
right-sided RT, we found that 7 of 35 studies found
a significant increase in the risk of CVM and 8 of 28
studies found a significantly increased risk of CVEs.
For patients who received RT compared with those who
did not receive RT, 7 of 26 studies found a significantly
increased risk of CVM and 5 of 22 studies found a
significant increased risk of CVEs.
A previous meta-analysis conducted by Cheng et al[4]
examined studies of breast cancer patients from 1966 to 2015. The authors[4] found that patients who received
RT had an increased risk of coronary heart disease
(RR=1.30, 95% CI=1.13-1.49) and cardiac mortality
(RR=1.38, 95% CI=1.18-1.62) compared with patients
who did not receive RT. They also found that patients
who received left-sided RT experienced an increased
risk of developing coronary heart disease compared
with patients receiving right-sided RT (RR=1.29,
95% CI=1.13-1.48).[4] Patients receiving left-sided RT also
experienced an increased risk of cardiac death compared
with patients receiving right-sided RT (RR=1.22,
95% CI=1.08-1.37).[4] In contrast to Cheng et al,[4] in the
present review, we found newer studies in which there
was no significant increased risk of CVEs or CVM in
patients who received RT compared with patients who
did not receive RT. We also found more studies in which
patients who received left-sided RT had no significant
increased risk of CVEs or CVM compared with patients
who received right-sided RT. These differences likely
reflect the fact that the present review includes many
new studies since 2015 in which the study populations
received modern RT techniques. However, because we
did not conduct a meta-analysis in the present review,
it remains unclear whether our findings represent a
significantly different association between breast cancer
RT and cardiovascular risk compared with that reported
by Cheng et al.[4]
A systematic review conducted by Drost et al[81] found
that the mean heart dose steadily decreased from 4.6 Gy
in 2014 to 2.6 Gy in 2017 (p = 0.003). Combining this
with the dose-dependent relationship between major
cardiac events and mean dose to the heart by Darby et al,[40]
it is likely that the decrease in mean heart dose owing
to improved contemporary RT techniques has led to
improved outcomes in breast cancer patients in recent
years. Our findings are also in support of this hypothesis
since all studies that found a significant association
between RT laterality and CVM included treatment
groups that started prior to 1985. This is consistent with
the systematic review by Cheng et al,[4] which found an
increased risk of cardiovascular death and coronary
heart disease associated with RT among studies in
which the breast cancer patients were diagnosed and
irradiated before 1980 (RR=1.45, 95% CI=1.14-1.89) compared with women diagnosed and irradiated after
1980 (RR=1.15, 95% CI=0.92-1.44; p = 0.04). Similarly,
Giordano et al[23] found that in 1979, the HR for ischaemic
heart disease mortality in left-sided compared with right-sided
disease was 1.50 (95% CI=1.19-1.87), but this HR
declined by 6% with each succeeding year between 1979
and 1988 (HR=0.94; 95% CI=0.91-0.98).
As such, newer research has started to evaluate whether
the use of modern linear accelerator machines instead
of 60Co fields and various contemporary radiation
techniques reduces the cardiac radiation dose and
subsequent cardiac toxicities. One example is intensity-modulated
RT, which allows a more conformal target
coverage without exposing organs at risk to as much
radiation.[82] Other notable advancements in cardiac
sparing techniques include the use of deep inspiration breath hold, enhanced patient positioning, and heart
blocking.[83] Deep inspiration breath hold and respiratory
gating rely on the principle that during inspiration, the
diaphragm flattens, and the lungs expand, causing the
heart to be pulled away from the chest wall and thus
decrease the radiation dose to the heart and the left
anterior descending artery.[84] A 2019 study conducted
by Simonetto et al[85] found that the use of deep
inspiration breath hold reduced the risk of estimated
mean heart dose by 35%, compared with free breathing.
Furthermore, to reduce the heart dose in breast cancer
patients, prone positioning can be used to increase the
planning target volume to heart distance by displacing
cardiac structures and substructures out of irradiated
volumes.[86] [87] [88] Other common methods include multileaf
collimator modification during RT planning.[89] [90]
However, an important pitfall is that it may shield part
of the breast tissue, which needs to be irradiated; thus, a
balance must be achieved in order to maximise the heart
shielding while minimising the target volume missed.[89]
In addition to these RT techniques, the omission of
internal mammary chain lymph node irradiation and rib
inclusion for chest wall RT has been utilised in early-stage
breast cancer patients to reduce the dose to the
normal tissue. However, long-term studies are needed
to investigate the effect of contemporary RT planning
techniques in minimising radiation exposure to nearby
normal tissue and the heart.
In addition to the use of modern RT techniques,
considerations must be made in terms of whether
RT is being combined with chemotherapeutic agents
as common chemotherapeutic agents have known
cardiotoxic effects.[91] [92] [93] [94] A 10-year cohort study of
breast cancer patients receiving concomitant RT and
chemotherapy found that there was no significant
association between CVEs and RT laterality (HR=2.38,
95% CI=0.80-7.11; p = 0.12).[55] However, there was
a significant increase in CVEs for patients receiving
left-sided RT with a doxorubicin-equivalent dose
≥250 mg/m2 compared with patients receiving right-sided
RT with a cumulative doxorubicin-equivalent
dose <250 mg/m2 (HR=5.22, 95% CI=1.67-21.15;
p = 0.006).[55] Similarly, a 2016 study found that there was
no significant increase in the risk of CVEs for patients
who received RT compared with those who received
surgery alone (HR=0.97, 95% CI=0.62-1.51; p = 0.882).[77]
However, there was a significant increase in CVEs in
patients who received RT and chemotherapy compared
with those who received surgery only (HR=1.84;
95% CI=1.34-2.53; p < 0.001).[77]
There are several limitations to this systematic review.
First, the heterogeneity of the data made it difficult to
make direct comparisons among studies. Many of
the studies did not report data on the individual types
of CVEs or the specific cause of CVM, and so we
used a composite outcome of CVE and CVM, which
included myocardial infarction, coronary artery disease,
conduction abnormalities, congestive heart failure, and
other cardiovascular diseases. Heterogeneity also exists
because of the lack of detail on RT techniques, RT
volume, dose, and fractionation. In addition, variability
in morbidity and mortality assessment, as well as in
the follow-up time of the studies, is another source of
heterogeneity. Second, the dose-dependent relationship
of cardiovascular risk cannot be evaluated since radiation
doses were not available for all studies. Lastly, because
this was a systematic review, and a meta-analysis was
not conducted, studies were not weighted based on
the number of patients. In the future, a meta-analysis
would aid in determining whether there is a significant
increase in the risk of CVEs and CVM associated with
the use of RT and/or RT laterality. Other confounding
variables may also be investigated and stratified, such as
menopausal status, types of adjuvant chemotherapy and
hormonal agents, which may impact cardiotoxicity.
CONCLUSION
Although modern RT techniques seem to have minimised
the cardiac exposure in breast cancer patients receiving
RT, more comprehensive studies with longer follow-up
periods must be conducted to investigate any associated
cardiovascular risk.
REFERENCES
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer
J Clin. 2018;68:394-424. Crossref
2. EBCTCG (Early Breast Cancer Trialists’ Collaborative Group); McGale P, Taylor C, Correa C, Cutter D, Duane F, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year
recurrence and 20-year breast cancer mortality: meta-analysis of
individual patient data for 8135 women in 22 randomised trials.
Lancet. 2014;383:2127-35. Crossref
3. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG),
Darby S, McGale P, Correa C, Taylor C, Arriagada R, et al.
Effect of radiotherapy after breast-conserving surgery on 10-year
recurrence and 15-year breast cancer death: meta-analysis of
individual patient data for 10,801 women in 17 randomised trials.
Lancet. 2011;378:1707-16. Crossref
4. Cheng YJ, Nie XY, Ji CC, Lin XX, Liu LJ, Chen XM, et al. Long-term
cardiovascular risk after radiotherapy in women with breast
cancer. J Am Heart Assoc. 2017;6:e005633. Crossref
5. Henson KE, McGale P, Darby SC, Parkin M, Wang Y, Taylor CW.
Cardiac mortality after radiotherapy, chemotherapy and endocrine therapy for breast cancer: cohort study of 2 million women from 57
cancer registries in 22 countries. Int J Cancer. 2020;147:1437-49. Crossref
6. Beaton L, Bergman A, Nichol A, Aparicio M, Wong G, Gondara L,
et al. Cardiac death after breast radiotherapy and the QUANTEC
cardiac guidelines. Clin Transl Radiat Oncol. 2019;19:39-45. Crossref
7. Boekel NB, Schaapveld M, Gietema JA, Rutgers EJ, Versteegh MI, Visser O, et al. Cardiovascular morbidity and mortality after
treatment for ductal carcinoma in situ of the breast. J Natl Cancer
Inst. 2014;106:dju156. Crossref
8. Henson KE, McGale P, Taylor C, Darby SC. Radiation-related mortality from heart disease and lung cancer more than 20 years after radiotherapy for breast cancer. Br J Cancer. 2013;108:179-82. Crossref
9. Tjessem KH, Johansen S, Malinen E, Reinertsen KV, Danielsen T,
Fosså SD, et al. Long-term cardiac mortality after hypofractionated
radiation therapy in breast cancer. Int J Radiat Oncol Biol Phys.
2013;87:337-43. Crossref
10. Bouillon K, Haddy N, Delaloge S, Garbay JR, Garsi JP, Brindel P, et al. Long-term cardiovascular mortality after radiotherapy for breast cancer. J Am Coll Cardiol. 2011;57:445-52. Crossref
11. McGale P, Darby SC, Hall P, Adolfsson J, Bengtsson NO,
Bennet AM, et al. Incidence of heart disease in 35,000 women
treated with radiotherapy for breast cancer in Denmark and Sweden.
Radiother Oncol. 2011;100:167-75. Crossref
12. Park CK, Li X, Starr J, Harris EE. Cardiac morbidity and mortality
in women with ductal carcinoma in situ of the breast treated with
breast conservation therapy. Breast J. 2011;17:470-6. Crossref
13. Stokes EL, Tyldesley S, Woods R, Wai E, Olivotto IA. Effect of
nodal irradiation and fraction size on cardiac and cerebrovascular
mortality in women with breast cancer treated with local
and locoregional radiotherapy. Int J Radiat Oncol Biol Phys.
2011;80:403-9. Crossref
14. Wang W, O’Connell D, Stuart K, Boyages J. Analysis of 10-year
cause-specific mortality of patients with breast cancer treated
in New South Wales in 1995. J Med Imaging Radiat Oncol.
2011;55:516-25. Crossref
15. Bouchardy C, Rapiti E, Usel M, Majno SB, Vlastos G, Benhamou S,
et al. Excess of cardiovascular mortality among node-negative
breast cancer patients irradiated for inner-quadrant tumors. Ann
Oncol. 2010;21:459-65. Crossref
16. Gutt R, Correa CR, Hwang WT, Solin LJ, Litt HI, Ferrari VA, et al.
Cardiac morbidity and mortality after breast conservation treatment
in patients with early-stage breast cancer and preexisting cardiac
disease. Clin. Breast Cancer. 2008;8:443-8. Crossref
17. Li WH, Zhang ZG, Huang ZR, Zhang W, Li ZB, Qi ZQ. No association between tumor laterality and cardiac-related mortality
in breast cancer patients after radiotherapy: a population-based
study. Cancer Manag Res. 2018;10:3649-56. Crossref
18. Borger JH, Hooning MJ, Boersma LJ, Snijders-Keilholz A, Aleman BM, Lintzen E, et al. Cardiotoxic effects of tangential breast
irradiation in early breast cancer patients: the role of irradiated heart
volume. Int J Radiat Oncol Biol Phys. 2007;69:1131-8. Crossref
19. Marhin W, Wai E, Tyldesley S. Impact of fraction size on cardiac mortality in women treated with tangential radiotherapy for localized breast cancer. Int J Radiat Oncol Biol Phys. 2007;69:483-9. Crossref
20. Paszat LF, Vallis KA, Benk VM, Groome PA, Mackillop WJ,
Wielgosz A. A population-based case-cohort study of the risk of
myocardial infarction following radiation therapy for breast cancer.
Radiother Oncol. 2007;82:294-300. Crossref
21. Roychoudhuri R, Robinson D, Putcha V, Cuzick J, Darby S,
Møller H. Increased cardiovascular mortality more than fifteen
years after radiotherapy for breast cancer: a population-based study.
BMC Cancer. 2007;7:9. Crossref
22. Harris EE, Correa C, Hwang WT, Liao J, Litt HI, Ferrari VA,
et al. Late cardiac mortality and morbidity in early-stage breast
cancer patients after breast-conservation treatment. J Clin Oncol.
2006;24:4100-6. Crossref
23. Giordano SH, Kuo YF, Freeman JL, Buchholz TA, Hortobagyi GN,
Goodwin JS. Risk of cardiac death after adjuvant radiotherapy for
breast cancer. J Natl Cancer Inst. 2005;97:419-24. Crossref
24. Vallis KA, Pintilie M, Chong N, Holowaty E, Douglas PS,
Kirkbride P, et al. Assessment of coronary heart disease morbidity
and mortality after radiation therapy for early breast cancer. J Clin
Oncol. 2002;20:1036-42. Crossref
25. Nixon AJ, Manola J, Gelman R, Bornstein B, Abner A, Hetelekidis S,
et al. No long-term increase in cardiac-related mortality after breast-conserving
surgery and radiation therapy using modern techniques.
J Clin Oncol. 1998;16:1374-9. Crossref
26. Paszat LF, Mackillop WJ, Groome PA, Schulze K, Holowaty E.
Mortality from myocardial infarction following postlumpectomy
radiotherapy for breast cancer: A population-based study in Ontario,
Canada. Int J Radiat Oncol Biol Phys. 1999;43:755-62. Crossref
27. Obi N, Eulenburg C, Seibold P, Eilber U, Thöne K, Behrens S, et al.
Associations between adjuvant radiotherapy and different causes
of death in a German breast cancer cohort. Breast. 2018;38:75-80. Crossref
28. Paszat LF, Mackillop WJ, Groome PA, Boyd C, Schulze K,
Holowaty E. Mortality from myocardial infarction after adjuvant
radiotherapy for breast cancer in the surveillance, epidemiology,
and end-results cancer registries. J Clin Oncol. 1998;16:2625-31. Crossref
29. Cuzick J, Stewart H, Rutqvist L, Honghton J, Edwards R,
Redmond C, et al. Cause-specific mortality in long-term survivors
of breast cancer who participated in trials of radiotherapy. J Clin
Oncol. 1994;12:447-53. Crossref
30. Houghton J, Baum M, Haybittle JL. Role of radiotherapy following
total mastectomy in patients with early breast cancer. World J Surg.
1994;18:117-22. Crossref
31. Rutqvist LE, Johansson H. Mortality by laterality of the primary
tumour among 55,000 breast cancer patients from the Swedish
cancer registry. Br J Cancer. 1990;61:866-8. Crossref
32. Haybittle JL, Brinkley D, Houghton J, A’Hern RP, Baum M.
Postoperative radiotherapy and late mortality: evidence from the
Cancer Research Campaign trial for early breast cancer. BMJ.
1989;298:1611-4. Crossref
33. Chang JS, Ko BK, Bae JH, Yu JH, Park MH, Jung Y, et al.
Radiation-related heart disease after breast cancer radiation therapy
in Korean women. Breast Cancer Res Treat. 2017;166:249-57. Crossref
34. Haque W, Verma V, Haque A, Butler EB, Teh BS. Trends in cardiac
mortality in women with ductal carcinoma in situ. Breast Cancer
Res Treat. 2017;161:345-51. Crossref
35. Merzenich H, Bartkowiak D, Schmidberger H, Schmidt M,
Schwentner L, Wiegel T, et al. 3D conformal radiotherapy is not
associated with the long-term cardiac mortality in breast cancer
patients: a retrospective cohort study in Germany (PASSOS-Heart
Study). Breast Cancer Res Treat. 2017;161:143-52. Crossref
36. Boekel NB, Schaapveld M, Gietema JA, Russell NS, Poortmans P,
Theuws JC, et al. Cardiovascular disease risk in a large, population-based
cohort of breast cancer survivors. Int J Radiat Oncol Biol
Phys. 2016;94:1061-72. Crossref
37. Paul Wright G, Drinane JJ, Sobel HL, Chung MH. Left-sided breast
irradiation does not result in increased long-term cardiac-related
mortality among women treated with breast-conserving surgery.
Ann Surg Oncol. 2016;23:1117-22. Crossref
38. Ye JC, Yan W, Christos P, Nori D, Chao KS, Ravi A. Second
cancer, breast cancer, and cardiac mortality in stage T1aN0 breast
cancer patients with or without external beam radiation therapy: a
national registry study. Clin Breast Cancer. 2015;15:54-9. Crossref
39. Darby SC, McGale P, Taylor CW, Peto R. Long-term mortality
from heart disease and lung cancer after radiotherapy for early
breast cancer: prospective cohort study of about 300 000 women
in US SEER cancer registries. Lancet Oncol. 2005;6:557-65. Crossref
40. Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U,
Brønnum D, et al. Risk of ischemic heart disease in women after
radiotherapy for breast cancer. N Engl J Med. 2013;368:987-98. Crossref
41. Jagsi R, Griffith KA, Koelling T, Roberts R, Pierce LJ. Rates of
myocardial infarction and coronary artery disease and risk factors
in patients treated with radiation therapy for early-stage breast
cancer. Cancer. 2007;109:650-7. Crossref
42. Patt DA, Goodwin JS, Kuo YF, Freeman JL, Zhang DD,
Buchholz TA, et al. Cardiac morbidity of adjuvant radiotherapy
for breast cancer. J Clin Oncol. 2005;23:7475-82. Crossref
43. Dess RT, Liss AL, Griffith KA, Marsh RB, Moran JM, Mayo C,
et al. Ischemic cardiac events following treatment of the internal
mammary nodal region using contemporary radiation planning
techniques. Int J Radiat Oncol Biol Phys. 2017;99:1146-53. Crossref
44. Wadsten C, Wennstig AK, Garmo H, Nilsson G, Blomqvist C,
Holmberg L, et al. Risk of ischemic heart disease after radiotherapy
for ductal carcinoma in situ. Breast Cancer Res Treat. 2018;171:95-101. Crossref
45. Boerman LM, Berendsen AJ, van der Meer P, Maduro JH,
Berger MY, de Bock GH. Long-term follow-up for cardiovascular
disease after chemotherapy and/or radiotherapy for breast cancer in
an unselected population. Support Care Cancer. 2014;22:1949-58. Crossref
46. Rehammar JC, Jensen MB, McGale P, Lorenzen EL, Taylor C,
Darby SC, et al. Risk of heart disease in relation to radiotherapy
and chemotherapy with anthracyclines among 19,464 breast cancer
patients in Denmark, 1977-2005. Radiother Oncol. 2017;123:299-305. Crossref
47. Boekel NB, Jacobse JN, Schaapveld M, Hooning MJ, Gietema JA,
Duane FK, et al. Cardiovascular disease incidence after internal
mammary chain irradiation and anthracycline-based chemotherapy
for breast cancer. Br J Cancer. 2018;119:408-18. Crossref
48. Doyle JJ, Neugut AI, Jacobson JS, Wang J, McBride R, Grann A,
et al. Radiation therapy, cardiac risk factors, and cardiac toxicity
in early-stage breast cancer patients. Int J Radiat Oncol Biol Phys.
2007;68:82-93. Crossref
49. Haque R, Yood MU, Geiger AM, Kamineni A, Avila CC, Shi J,
et al. Long-term safety of radiotherapy and breast cancer
laterality in older survivors. Cancer Epidemiol Biomarkers Prev.
2011;20:2120-6. Crossref
50. Soran O, Vargo JA, Polat AV, Soran A, Sumkin J, Beriwal S. No
association between left-breast radiation therapy or breast arterial
calcification and long-term cardiac events in patients with breast
cancer. J Womens Health (Larchmt). 2014;23:1005-11. Crossref
51. Abouegylah M, Braunstein LZ, Alm El-Din MA, Niemierko A,
Salama L, Elebrashi M, et al. Evaluation of radiation-induced
cardiac toxicity in breast cancer patients treated with trastuzumab-based
chemotherapy. Breast Cancer Res Treat. 2019;174:179-85. Crossref
52. Chang JS, Shin J, Park EC, Kim YB. Risk of cardiac disease after
adjuvant radiation therapy among breast cancer survivors. Breast.
2019;43:48-54. Crossref
53. James M, Swadi S, Yi M, Johansson L, Robinson B, Dixit A.
Ischaemic heart disease following conventional and hypofractionated
radiation treatment in a contemporary breast cancer series. J Med
Imaging Radiat Oncol. 2018;62:425-31. Crossref
54. Killander F, Wieslander E, Karlsson P, Holmberg E, Lundstedt D,
Holmberg L, et al. No increased cardiac mortality or morbidity of
radiation therapy in breast cancer patients after breast-conserving
surgery: 20-year follow-up of the randomized SweBCGRT trial.
Int J Radiat Oncol Biol Phys. 2020;107:701-9. Crossref
55. Kim DY, Youn JC, Park MS, Lee S, Choi SW, Ryu KH, et al.
Cardiovascular outcome of breast cancer patients with concomitant
radiotherapy and chemotherapy: A 10-year multicenter cohort
study. J Cardiol. 2019;74:175-81. Crossref
56. Wennstig AK, Wadsten C, Garmo H, Fredriksson I, Blomqvist C,
Holmberg L, et al. Long-term risk of ischemic heart disease
after adjuvant radiotherapy in breast cancer: results from a large
population-based cohort. Breast Cancer Res. 2020;22:10. Crossref
57. Wollschläger D, Merzenich H, Schwentner L, Janni W, Wiegel T,
Bartkowiak D, et al. Self-reported long-term cardiac morbidity in
breast cancer patients: a retrospective cohort study in Germany
(PASSOS Heart Study). Breast Cancer Res Treat. 2017;163:595-604. Crossref
58. Boekel NB, Duane FK, Jacobse JN, Hauptmann M, Schaapveld M,
Sonke GS, et al. Heart failure after treatment for breast cancer. Eur
J Heart Fail. 2020;22:366-74. Crossref
59. Chou YH, Huang JY, Kornelius E, Chiou JY, Huang CN. Major
adverse cardiovascular events after treatment in early-stage breast
cancer patients receiving hormone therapy. Sci Rep. 2020;10:1408. Crossref
60. Lee J, Hur H, Lee JW, Youn HJ, Han K, Kim NW, et al. Long-term
risk of congestive heart failure in younger breast cancer
survivors: A nationwide study by the SMARTSHIP group. Cancer.
2020;126:181-8. Crossref
61. Lawrenson R, Lao C, Ali A, Campbell I. Impact of radiotherapy on
cardiovascular health of women with breast cancer. J Med Imaging
Radiat Oncol. 2019;63:250-6. Crossref
62. Leung HW, Chan AL, Muo CH. Late cardiac morbidity of adjuvant
radiotherapy for early breast cancer — a population-based study. J
Cardiol. 2016;67:567-71. Crossref
63. Killander F, Anderson H, Kjellén E, Malmström P. Increased cardio
and cerebrovascular mortality in breast cancer patients treated with
postmastectomy radiotherapy — 25 year follow-up of a randomised
trial from the South Sweden Breast Cancer Group. Eur J Cancer.
2014;50:2201-10. Crossref
64. Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans V,
et al. Effects of radiotherapy and of differences in the extent
of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet.
2005;366:2087-106. Crossref
65. Ragaz J, Olivotto IA, Spinelli JJ, Phillips N, Jackson SM,
Wilson KS, et al. Locoregional radiation therapy in patients with
high-risk breast cancer receiving adjuvant chemotherapy: 20-year
results of the British Columbia randomized trial. J Natl Cancer
Inst. 2005;97:116-26. Crossref
66. Woodward WA, Strom EA, McNeese MD, Perkins GH, Outlaw EL,
Hortobagyi GN, et al. Cardiovascular death and second non-breast
cancer malignancy after postmastectomy radiation and doxorubicin-based
chemotherapy. Int J Radiat Oncol Biol Phys. 2003;57:327-35. Crossref
67. Højris I, Overgaard M, Christensen JJ, Overgaard J. Morbidity
and mortality of ischaemic heart disease in high-risk breast-cancer
patients after adjuvant postmastectomy systemic treatment with or
without radiotherapy: analysis of DBCG 82b and 82c randomised
trials. Radiotherapy Committee of the Danish Breast Cancer
Cooperative Group. Lancet. 1999;354:1425-30. Crossref
68. Gyenes G, Rutqvist LE, Liedberg A, Fornander T. Long-term
cardiac morbidity and mortality in a randomized trial of pre- and
postoperative radiation therapy versus surgery alone in primary
breast cancer. Radiother Oncol. 1998;48:185-90. Crossref
69. Rutqvist LE, Liedberg A, Hammar N, Dalberg K. Myocardial
infarction among women with early-stage breast cancer treated
with conservative surgery and breast irradiation. Int J Radiat Oncol
Biol Phys. 1998;40:359-63. Crossref
70. Rutqvist LE, Lax I, Fornander T, Johansson H. Cardiovascular mortality in a randomized trial of adjuvant radiation therapy versus
surgery alone in primary breast cancer. Int J Radiat Oncol Biol
Phys. 1992;22:887-96. Crossref
71. Jones JM, Ribeiro GG. Mortality patterns over 34 years of breast
cancer patients in a clinical trial of post-operative radiotherapy.
Clin Radiol. 1989;40:204-8. Crossref
72. Høst H, Brennhovd IO, Loeb M. Postoperative radiotherapy in breast cancer — long-term results from the Oslo study. Int J Radiat
Oncol Biol Phys. 1986;12:727-32. Crossref
73. Hamood R, Hamood H, Merhasin I, Keinan-Boker L. Risk of cardiovascular disease after radiotherapy in survivors of breast
cancer: A case-cohort study. J Cardiol. 2019;73:280-91. Crossref
74. Jacobse JN, Duane FK, Boekel NB, Schaapveld M, Hauptmann M,
Hooning MJ, et al. Radiation dose-response for risk of myocardial
infarction in breast cancer survivors. Int J Radiat Oncol Biol Phys.
2019;103:595-604. Crossref
75. Lee CH, Zhang JF, Yuan KS, Wu AT, Wu SY. Risk of cardiotoxicity
induced by adjuvant anthracycline-based chemotherapy and
radiotherapy in young and old Asian women with breast cancer.
Strahlenther Onkol. 2019;195:629-39. Crossref
76. Wu SP, Tam M, Vega RM, Perez CA, Gerber NK. Effect of breast
irradiation on cardiac disease in women enrolled in BCIRG-001 at
10-year follow-up. Int J Radiat Oncol Biol Phys. 2017;99:541-8. Crossref
77. Tan CH, Chao TT, Liu JC, Lin CH, Huang YS, Chang CM, et al.
Breast cancer therapy and age difference in cardiovascular disease
risks: a population-based cohort study in Taiwan. Taiwan J Obstet
Gynecol. 2016;55:98-103. Crossref
78. Onwudiwe NC, Kwok Y, Onukwugha E, Sorkin JD, Zuckerman IH,
Shaya FT, et al. Cardiovascular event-free survival after
adjuvant radiation therapy in breast cancer patients stratified by
cardiovascular risk. Cancer Med. 2014;3:1342-52. Crossref
79. Hooning MJ, Botma A, Aleman BM, Baaijens MH, Bartelink H, Klijn JG, et al. Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. J Natl Cancer Inst. 2007;99:365-75. Crossref
80. Geiger AM, Chen W, Bernstein L. Myocardial infarction risk and tamoxifen therapy for breast cancer. Br J Cancer. 2005;92:1614-20. Crossref
81. Drost L, Yee C, Lam H, Zhang L, Wronski M, McCann C, et al. A systematic review of heart dose in breast radiotherapy. Clin Breast Cancer. 2018;18:e819-24. Crossref
82. Hong TS, Ritter MA, Tomé WA, Harari PM. Intensity-modulated radiation therapy: Emerging cancer treatment technology. Br J Cancer. 2005;92:1819-24. Crossref
83. Yeboa DN, Evans SB. Contemporary breast radiotherapy and cardiac toxicity. Semin Radiat Oncol. 2016;26:71-8. Crossref
84. Bergom C, Currey A, Desai N, Tai A, Strauss JB. Deep inspiration
breath hold: Techniques and advantages for cardiac sparing during
breast cancer irradiation. Front Oncol. 2018;8:87. Crossref
85. Simonetto C, Eidemüller M, Gaasch A, Pazos M, Schönecker S,
Reitz D. Does deep inspiration breath-hold prolong life? Individual
risk estimates of ischaemic heart disease after breast cancer
radiotherapy. Radiother Oncol. 2019;131:202-7. Crossref
86. Duma MN, Baumann R, Budach W, Dunst J, Feyer P, Fietkau R,
et al. Heart-sparing radiotherapy techniques in breast cancer
patients: a recommendation of the breast cancer expert panel of the
German Society of Radiation Oncology (DEGRO). Strahlenther
Onkol. 2019;195:861-71. Crossref
87. Mulliez T, Veldeman L, Speleers B, Mahjoubi K, Remouchamps V,
Van Greveling A, et al. Heart dose reduction by prone deep
inspiration breath hold in left-sided breast irradiation. Radiother
Oncol. 2015;114:79-84. Crossref
88. Formenti SC, Gidea-Addeo D, Goldberg JD, Roses DF, Guth A,
Rosenstein BS, et al. Phase I-II trial of prone accelerated intensity
modulated radiation therapy to the breast to optimally spare normal
tissue. J Clin Oncol. 2007;25:2236-42. Crossref
89. Yue NJ, Goyal S, Park JH, Jones S, Xu X, Khan A, et al.
Optimization of heart block in the left-sided whole breast radiation
treatments. Front Oncol. 2014;4:342. Crossref
90. Welsh B, Chao M, Foroudi F. Reducing cardiac doses: a novel
multi-leaf collimator modification technique to reduce left anterior
descending coronary artery dose in patients with left-sided breast
cancer. J Med Radiat Sci. 2017;64:114-9. Crossref
91. Brana I, Zamora E, Oristrell G, Tabernero J. Cardiotoxicity. In:
Dicato MA, Van Cutsem E, editors. Side Effects of Medical Cancer
Therapy: Prevention and Treatment. 2nd ed. London: Springer
International Publishing; 2018: p 367-406. Crossref
92. Jones LW, Haykowsky MJ, Swartz JJ, Douglas PS, Mackey JR.
Early breast cancer therapy and cardiovascular injury. J Am Coll
Cardiol. 2007;50:1435-41. Crossref
93. Jones RL, Ewer MS. Cardiac and cardiovascular toxicity of
nonanthracycline anticancer drugs. Expert Rev Anticancer Ther.
2006;6:1249-69. Crossref
94. Chu TF, Rupnick MA, Kerkela R, Dallabrida SM, Zurakowski D, Nguyen L, et al. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet. 2007;370:2011-9. Crossref