Magnetic Marker Wireless Localisation versus Radioguided Localisation of Nonpalpable Breast Lesions
ORIGINAL ARTICLE CME
Magnetic Marker Wireless Localisation versus Radioguided Localisation of Nonpalpable Breast Lesions
HL Tsui1, EPY Fung2, KM Kwok2, LKM Wong2, LW Lo2, WS Mak2
1 Department of Radiology and Organ Imaging, United Christian Hospital, Hong Kong
2 Department of Diagnostic and Interventional Radiology, Kwong Wah Hospital, Hong Kong
Correspondence: Dr HL Tsui, Department of Radiology and Organ Imaging, United Christian Hospital, Hong Kong. Email: karen.tsuihl@gmail.com
Submitted: 25 Aug 2020; Accepted: 24 Nov 2020.
Contributors: HLT and EPYF designed the study. HLT acquired the data. All authors analysed the data. HLT drafted the manuscript. All authors
critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the
final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: This is a retrospective study approved by the local research ethics committee (Reference number: KC/KE-20-0072/ER-1).
Abstract
Introduction
Precise preoperative localisation is essential for nonpalpable breast lesions undergoing lumpectomy.
Hookwire localisation has been gradually replaced by radioisotope-guided occult lesion localisation (ROLL). We
aimed to evaluate the use of magnetic metal markers (Magseed) as a nonradioactive and wireless alternative.
Methods
We compared cases of Magseed localisation performed between September 2018 and April 2020 with the
same number of ROLL procedures with identical pathology in the same period.
Results
In total, 24 Magseed and 24 ROLL procedures were included. There were no significant differences between
the case groups in terms of target characteristics, operation time, specimen size, pathological diagnosis, margin
clearance, or reoperation rate. Localisation duration was significantly shorter in ROLL procedures (8.7 min) compared
with Magseed localisation (12.9 min, p < 0.0001). No complications were reported. Same-day surgery was performed
in all ROLL and 17 Magseed lesions. The localisation-operation interval for the other seven Magseed lesions were
4 to 14 days. Significantly lower intraoperative re-excision rates (p = 0.006) were observed in the Magseed group
(8.3%) compared with the ROLL group (45.8%). Technical success of the ROLL group was 100%. Twenty-two
(91.7%) Magseed localisations achieved technical success with 11/11 (100%) using ultrasound and 11/13 (84.6%)
using stereotactic guidance. Magseed displacement was up to 4.8 mm in the localisation-operation interval.
Conclusion
Magseed is a safe and effective localisation technique for nonpalpable breast lesions, which allows
decoupling of radiological and surgical schedules.
Key Words: Breast/diagnostic imaging; Breast neoplasms/pathology; Carcinoma
中文摘要
不能觸及乳腺病變的磁性標記物無線定位與放射導向定位的比較
徐愷靈、馮寶恩、郭勁明、黃嘉敏、羅麗雲、麥詠詩
引言
精確的術前定位對於不能觸及乳腺病變切除術至關重要。鈎線定位已逐漸被放射導向隱匿性病灶定位(ROLL)所取代。我們旨在評估乳腺病灶定位標記物Magseed作為無線及非放射性替代品的使用。
方法
比較2018年9月至2020年4月期間進行的Magseed定位病例與相同數目和病理結果的ROLL病例。
結果
納入24例Magseed和24例ROLL。兩組在靶點特徵、手術時間、標本大小、病理診斷、切緣清除率或再手術率等方面均無顯著差異。與Magseed定位相比,ROLL的定位持續時間明顯較短(12.9分鐘比8.7分鐘,p < 0.0001)。兩組均無併發症。所有ROLL和17例Magseed病變均在同一天進行手術,其餘7例Magseed病變的定位日期與手術日期間隔為4至14天。與 ROLL組相比,Magseed組減少術中再切除率(45.8%比8.3%,p = 0.006)。ROLL組的技術成功率為100%。22例Magseed定位取得技術成功(91.7%),其中 11/11(100%)使用超聲,11/13(84.6%)使用立體定向引導。Magseed位移在定位與手術操作間隔中不多於4.8毫米。
結論
Magseed是一種安全有效的定位技術,用於不能觸及的乳腺病變,可以將放射學和手術計劃分開。
INTRODUCTION
The development of screening programmes for breast
cancer has led to a decrease in mortality from breast
cancer in women.[1] [2] Improved screening techniques have
found increasing numbers of nonpalpable breast cancers/high-risk lesions. The smaller lesions and earlier stage
render patients eligible for breast-conserving treatment.
Precise preoperative localisation of nonpalpable breast
lesions is essential to achieve accurate diagnosis in
suspicious lesions and to obtain adequate excision
margins, while avoiding excessive surgical resection
in breast-conserving surgery for cancer.[3] Hookwire
localisation was the gold standard and has been gradually
replaced by radioisotope-guided occult lesion localisation
(ROLL) in recent years.[4] However, ROLL is limited to
centres with a nuclear medicine unit and availability
of same-day surgery. New advances in localisation
techniques for nonpalpable breast lesions have been
introduced, allowing decoupling of localisation and
operation schedules. Nonradioactive magnetic metal
seeds (Magseed, Endomagnetics Inc., Cambridge,
United Kingdom) is one of the new methods. It was
approved by the US Food and Drug Administration in
2016 for breast lesion localisation.[5] [6]
We aimed to evaluate our initial experience with
Magseed localisation of nonpalpable breast lesions with comparison with ROLL, in assessing the feasibility and
effectiveness of this novel localisation technique.
METHODS
This is a retrospective study approved by the local
research ethics committee (Ref: KC/KE-20-0072/ER-1).
We reviewed all cases of Magseed localisation between
1 September 2018 and 30 April 2020 in a regional
hospital, and matched them with the same number of
ROLL procedures with identical pathology performed
since 1 September 2018. Cases with ROLL with no
specimen scintigraphic images were excluded.
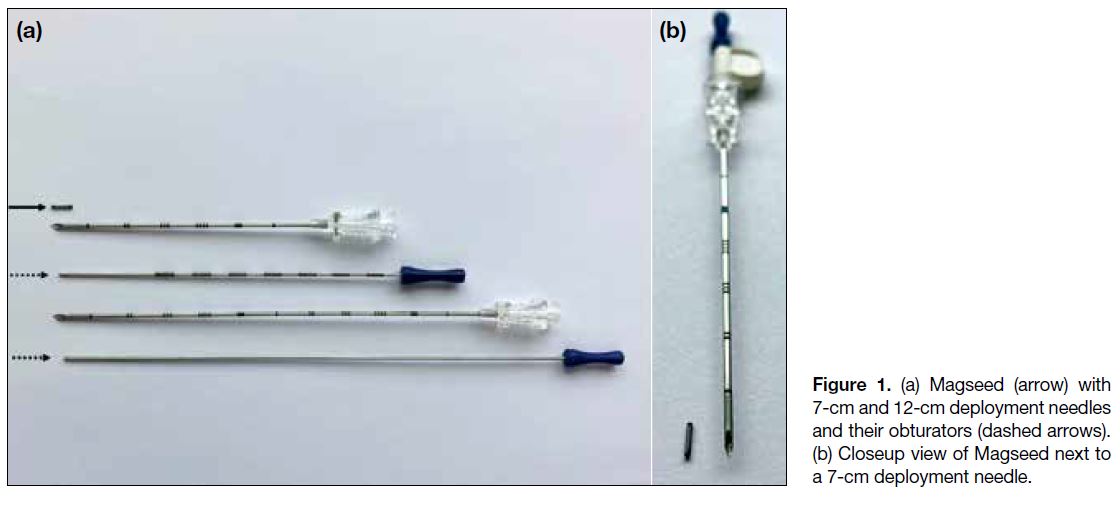
Magseed is a paramagnetic steel and iron oxide seed,
measuring 1 mm in diameter and 5 mm in length
(Figure 1). It can be placed in the breast with either
ultrasound or stereotactic guidance up to 30 days before
surgery as recommended by the vendor (Figures 2 and 3).[7]
It received Food and Drug Administration clearance in
2018 for long-term implantation in the US.[6] The seed
is preloaded into an 18-gauge 7-cm or 12-cm-long steel
needle and is retained by a wax plug. A steel obturator
is used to deploy the seed. The seed is detectable
using a handheld magnetometer (Sentimag probe,
Endomagnetics). The probe generates an alternating
magnetic field to magnetise the iron oxide particles
within the Magseed temporarily. The magnetic signature of the Magseed is then detected by the Sentimag
probe. The Sentimag unit displays a numerical count
and produces an audio tone, related to the strength of the magnetic field, and hence the distance of the seed
from the probe.[5] [8] As ferromagnetic instruments will
interfere with the signal, special nonferromagnetic surgical instruments are necessary. Electrocautery or
other metallic equipment in the operating room can
also interfere with the signal, requiring recalibration
of the probe.[5] The surgical specimen is then sent to the
radiology department for radiography or ultrasound.
Further surgical exploration is performed if all markers
are not identified in the specimen images.
Figure 1. (a) Magseed (arrow) with 7-cm and 12-cm deployment needles and their obturators (dashed arrows). (b) Closeup view of Magseed next to a 7-cm deployment needle.
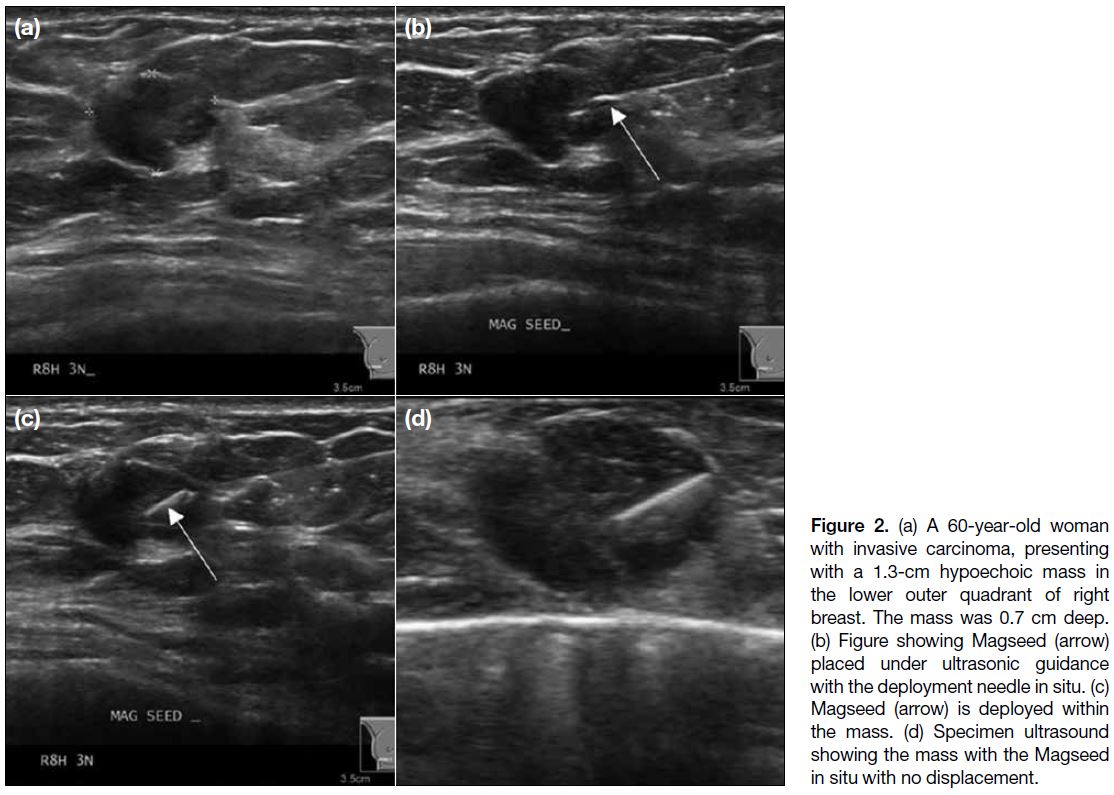
Figure 2. (a) A 60-year-old woman with invasive carcinoma, presenting with a 1.3-cm hypoechoic mass in the lower outer quadrant of right breast. The mass was 0.7 cm deep. (b) Figure showing Magseed (arrow) placed under ultrasonic guidance with the deployment needle in situ. (c) Magseed (arrow) is deployed within the mass. (d) Specimen ultrasound showing the mass with the Magseed in situ with no displacement.
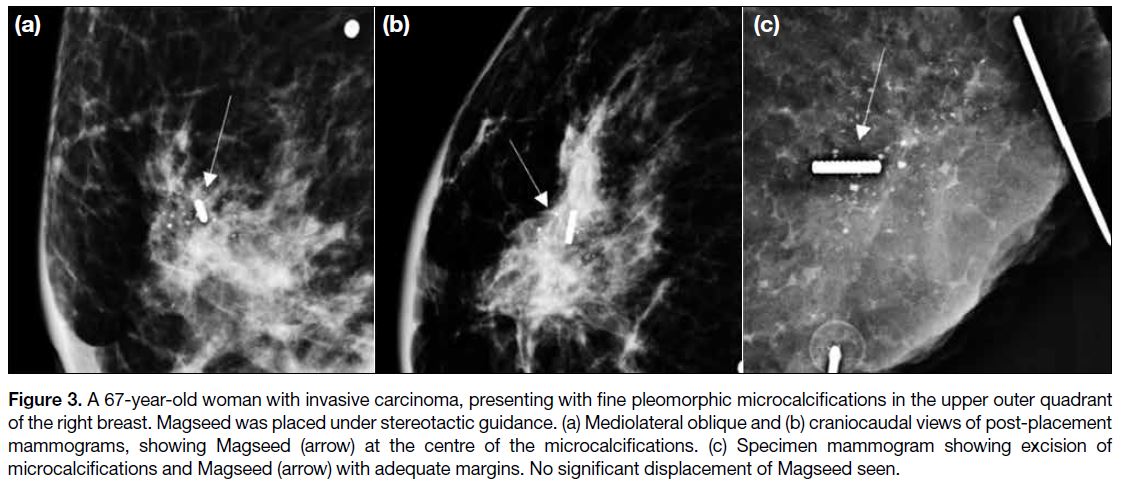
Figure 3. A 67-year-old woman with invasive carcinoma, presenting with fine pleomorphic microcalcifications in the upper outer quadrant
of the right breast. Magseed was placed under stereotactic guidance. (a) Mediolateral oblique and (b) craniocaudal views of post-placement
mammograms, showing Magseed (arrow) at the centre of the microcalcifications. (c) Specimen mammogram showing excision of
microcalcifications and Magseed (arrow) with adequate margins. No significant displacement of Magseed seen.
Radioguided nonpalpable lesion localisation is
performed by intratumoural injection of approximately
0.2 mL of 0.5 mCi (18.5 MBq) technetium-99m (99mTc)
labelled sulphur colloid using a 22G spinal needle
under stereotactic or ultrasound guidance. Filtered
99mTc labelled sulphur colloid, with particle dimension
<100 nm, is used for sentinel node and nonpalpable lesion
localisation in patients with biopsy-proven invasive
carcinoma and high-grade ductal carcinoma in situ. An
anterior planar image of the patient is then acquired at
30 minutes post-injection to confirm adequate
radioactivity at the injection site and at 2 hours post-injection
to identify the sentinel lymph nodes in such
cases if the initial 30-minute scan is negative. The patient
is operated on within 4 to 6 hours. The nonpalpable
breast lesions are detected by a handheld gamma probe.
Following excision, the surgical bed is checked for any
residual radioactivity. The specimen is then sent to the
nuclear medicine department for specimen scintigraphy
and to the radiology department for radiography or
ultrasound (Figure 4). Further surgical exploration is
performed if residual activity remains high in the breast
or if incomplete excision is noted on the specimen
radiograph, ultrasound, or scintigraphic image.[4]
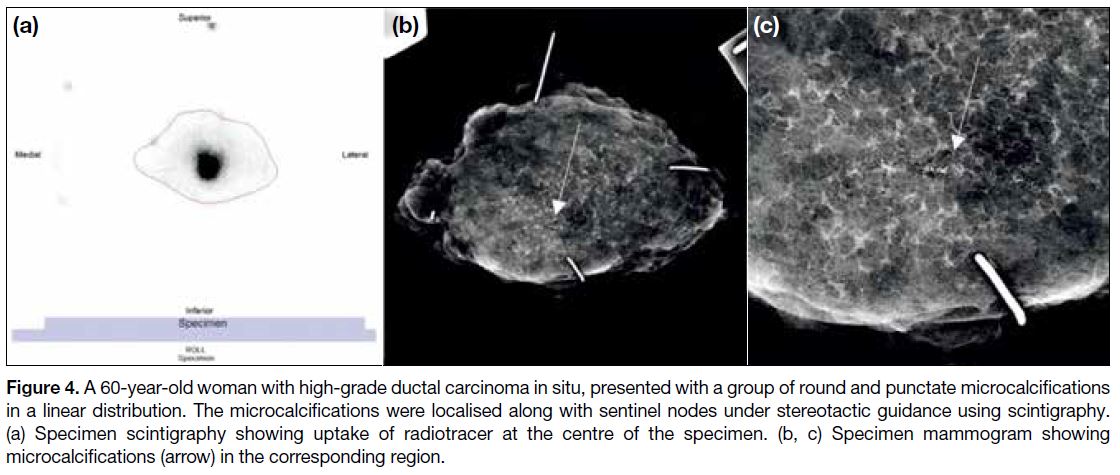
Figure 4. A 60-year-old woman with high grade ductal carcinoma in situ, presented with a group of round and punctate microcalcifications
in a linear distribution. The microcalcifications were localised along with sentinel nodes under stereotactic guidance using scintigraphy.
(a) Specimen scintigraphy showing uptake of radiotracer at the centre of the specimen. (b, c) Specimen mammogram showing
microcalcifications (arrow) in the corresponding region.
Patients’ demographics, localisation indications,
localisation-operation intervals, localisation procedures
(image modality, approach in stereotactic guided
localisation, localisation duration, lesion type, depth
of lesion and size of lesion), complications, pathology
reports (specimen and tumour size, margin status),
operation records (operation time and intra-operative
re-excision) and reoperation records were reviewed via
electronic patient records. Pre-localisation mammograms
(if available), specimen mammograms, ultrasounds, and
scintigraphic images were also reviewed.
Technical success of the Magseed method was defined as
the seed being ≤1 cm from the target in post-placement
image. Magseed is considered placed within the target if
there is no distance gap between the two. Displacement
of the seed relative to the target during the localisation-operation
interval was determined by the differences in
distance between the centre of the seed and that of the
target in post-placement and specimen images.
Specimen and tumour volumes were calculated
by multiplying the three dimensions reported by
the pathologist. To determine the breast volume,
mammographic measurements were recorded and breast
volume was calculated using the formula for an elliptical
cone (V=1/3π × rCC × rMLO × hMLO).9
Statistical Analysis
Simple descriptive summary statistics of the main
parameters were derived. Percentages for categorical
variables, means, medians and range values for quantitative factors were calculated as appropriate.
Continuous variables were analysed using independent
t tests for parametric data and the Mann-Whitney U
test for nonparametric data. Categorical variables were
analysed using Pearson’s Chi-square test or Fisher’s
exact test where appropriate. Statistical analysis was
performed using commercial software (SPSS Windows
version 23.0; IBM Corp, Armonk [NY], US). p Values
were calculated, with p < 0.05 defined as significant.
RESULTS
A total of 24 lesions were localised with Magseed in
23 patients. One patient had two Magseeds placed for
two lesions in the same breast that were 2.1 cm apart.
Twenty-four ROLL lesions with matched pathology
were included for comparison.
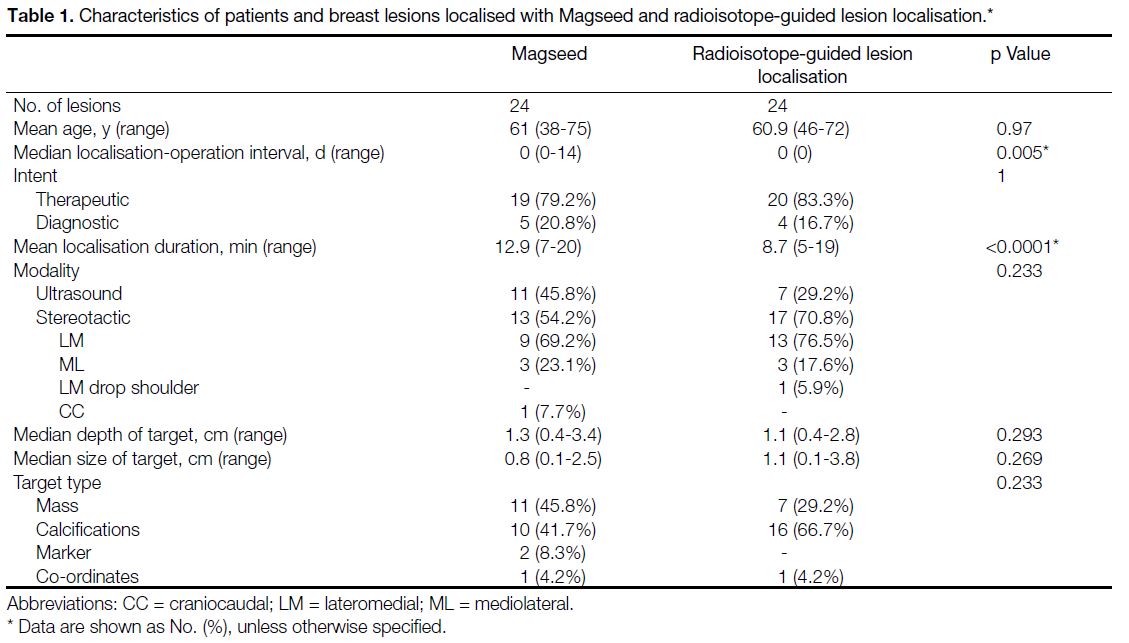
Demographics, target characteristics, and localisation
techniques are shown in Table 1. Most lesions in
both groups were targeted for therapeutic intent. No complications related to Magseed insertion or ROLL
injection were reported. Same-day surgery was performed
in all lesions localised with radioisotope guidance and in
17 Magseed-localised lesions. The localisation-operation
interval for the other seven Magseed lesions was 4 to
14 days.
Table 1. Characteristics of patients and breast lesions localised with Magseed and radioisotope-guided lesion localisation
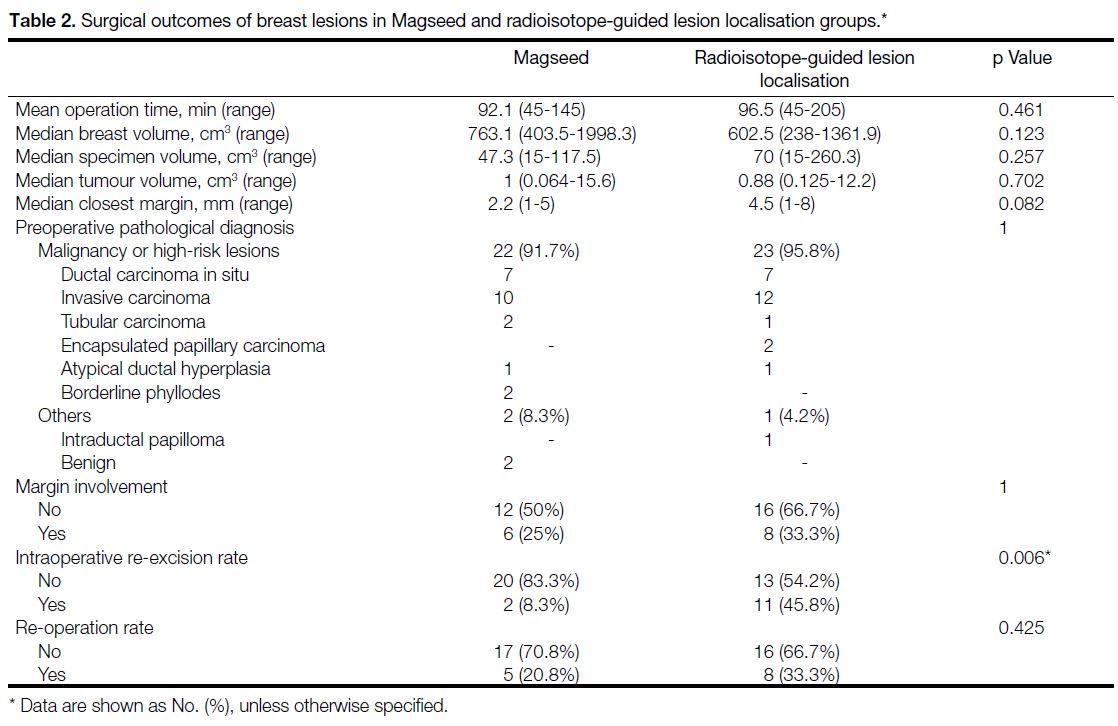
Surgical outcomes are listed in Table 2. All nonpalpable
breast lesions were successfully identified and excised
using the predetermined localisation technique except
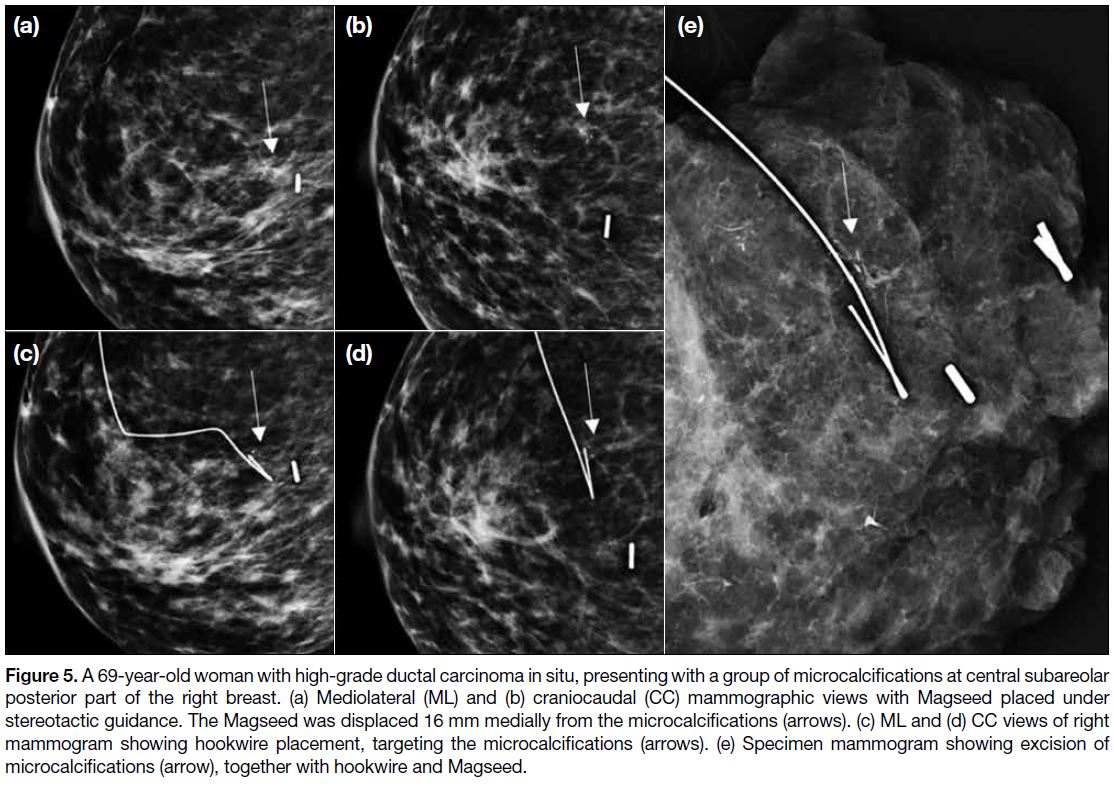
for two Magseed lesions. Both cases were planned for
same-day surgery, but the Magseeds were displaced
from the target after placement. One was displaced
1.6 cm and the other 1.7 cm. A hookwire was placed
to target the lesions in both instances after discussion
with surgeons. The target, hookwire and Magseed were
successfully retrieved during the surgeries (Figure 5).
Since these lesions were localised with hookwire rather
than Magseed, these lesions were excluded from the
tumour analysis in Magseed group.
Table 2. Surgical outcomes of breast lesions in Magseed and radioisotope-guided lesion localisation groups
Figure 5. A 69-year-old woman with high-grade ductal carcinoma in situ, presenting with a group of microcalcifications at central subareolar
posterior part of the right breast. (a) Mediolateral (ML) and (b) craniocaudal (CC) mammographic views with Magseed placed under
stereotactic guidance. The Magseed was displaced 16 mm medially from the microcalcifications (arrows). (c) ML and (d) CC views of right
mammogram showing hookwire placement, targeting the microcalcifications (arrows). (e) Specimen mammogram showing excision of
microcalcifications (arrow), together with hookwire and Magseed.
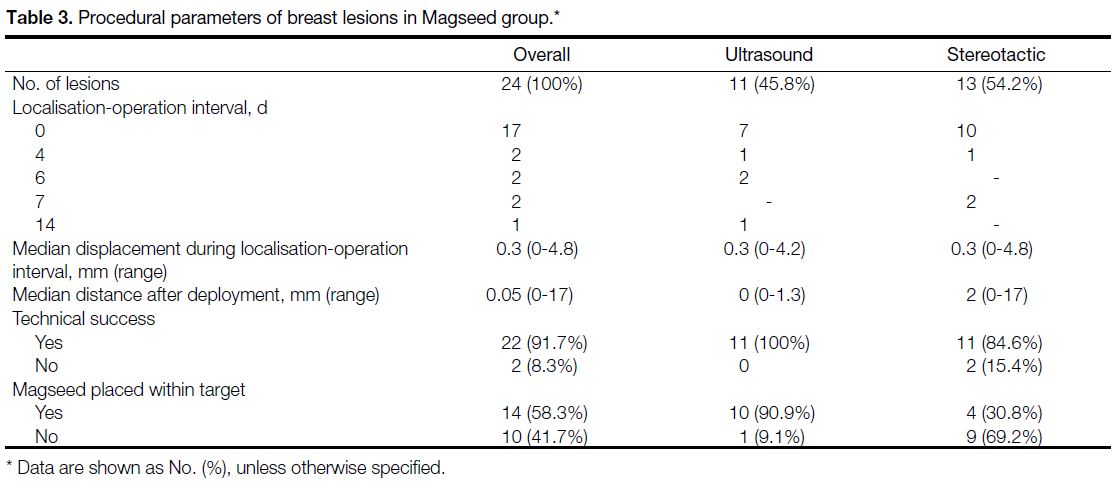
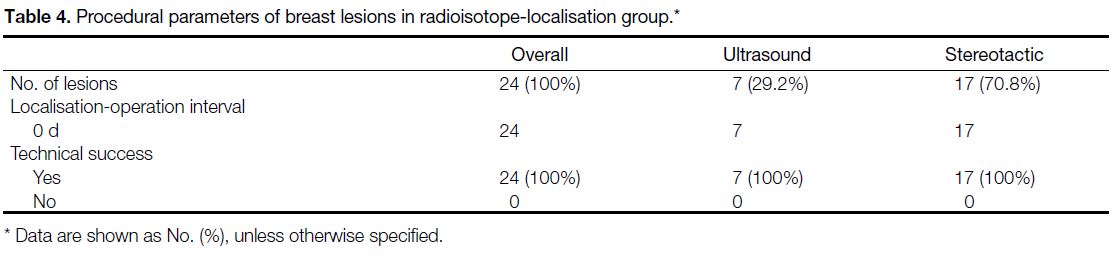
Magseed and ROLL procedural parameters are listed
in Tables 3 and 4, respectively. Minimal displacement
of Magseeds was observed in two cases during the localisation-operation interval. Both cases were operated
on the same day. In the rest of the cases that were operated
on 4 to 14 days post-placement, the displacement was
up to 2.7 mm. Four Magseeds were dislodged from the
specimen either during surgery or during transfer to the
radiology department. Therefore, the distance between
the target and Magseed could not be measured and they
were excluded from the analysis.
Table 3. Procedural parameters of breast lesions in Magseed group
Table 4. Procedural parameters of breast lesions in radioisotope-localisation group
The mean operation times were comparable in both
groups, being 92.1 min in Magseed group and 96.5 min
in the ROLL group (p = 0.461). The intraoperative re-excision
rate was significantly lower in the Magseed
group (8.3%) compared with the ROLL group (45.8%,
p = 0.006) The re-excision rate was 20.8% in the
Magseed group and 33.3% in ROLL group (p = 0.425).
Five Magseed and two ROLL lesions had no
preoperative mammograms performed in our hospital
as they were performed in other hospitals or outside
facilities. They were thus excluded from the breast
volume analysis. Table 2 shows the breast, specimen,
and tumour volumes were not significantly different
between the two groups.
In all, 22 out of 24 Magseed lesions were malignant
or high-risk lesions in the preoperative core biopsy
pathology report. A patient whose two ultrasounds
detected Breast Imaging-Reporting and Data System
4A lesions had core biopsy result of benignity but
she underwent excisional biopsy due to radiological-clinical
discordance. Two Magseeds were placed 2.1 cm
apart. Both lesions were successfully resected with the
Magseed. Final pathology came back benign. In another
Magseed case, a preoperative diagnosis of invasive ductal
carcinoma was made with stereotactic-guided biopsy of
microcalcifications. The patient underwent neoadjuvant
chemotherapy and subsequent Magseed localisation and
breast-conserving surgery. The microcalcifications were
successfully localised with the Magseed 4.2 mm from
the target. The pathological report showed no residual
tumour within the specimen. In another Magseed case,
a preoperative diagnosis of tubular carcinoma was
made with stereotactic biopsy of microcalcifications.
The microcalcifications were successfully localised
with a Magseed 2 mm from the target. The pathological report, however, showed no malignancy. Therefore,
margin clearance of these lesions was not included in
the analysis. In total, 23 out of 24 lesions identified with
ROLL showed malignant or high-risk lesions. Margin
clearance of lesions was 66.7% in both Magseed and
ROLL groups.
DISCUSSION
Successful management of nonpalpable breast lesions
depends on accurate preoperative localisation. Various
previous studies have shown the effectiveness of
Magseed as a convenient method for nonpalpable breast
lesion localisation.[8] [10] [11] [12] However, to our knowledge, no
published studies have compared Magseed with ROLL.
Our study is the first study to show comparable results
between Magseed and ROLL in terms of operation
time, surgical specimen size, margin clearance and re-operation
rate. Magseed is superior with a significantly
reduced intraoperative re-excision rate as well as
allowing decoupling of localisation and operation
schedules. Although the majority of the Magseed cases were operated on the same day, 29.2% (7/24) cases
were operated 4 to 14 days post-Magseed localisation
and no significant displacement of the Magseed was
observed during the localisation-operation interval. This
is more efficient in workflow and allows flexibility in
appointment arrangement. The localisation procedure can
be performed days to weeks before surgery, eliminating
the need for coordination between radiology, nuclear
medicine departments, and operating theatres and thus
reducing any possible delays in surgery due to difficult
localisation or waste of resources due to cancelled cases.
In addition, Magseed has no radiation. The results of
this study suggest that Magseed can be used to replace
ROLL.
In our study, 20.8% Magseed cases require reoperation
due to positive margins. It is similar to previous published
studies. In a study by Lamb et al,[10] 21.9% tumour-positive
or close surgical margins requiring re-excision.
In another study by Price et al,[11] 17.2% malignant cases
had positive or close margin.
The localisation duration was shorter for the ROLL
technique in our study. As Magseed is a new localisation
technique, we postulate there is a learning curve for
radiologists to adapt to this new method. Deploying
Magseed is similar to marker placement. With more
practice, the localisation duration will likely be
shortened. In addition, patients undergoing ROLL need
to be transferred to the nuclear medicine department
for scintigraphy, whose time needed for transfer and
scanning were not included in the calculation. Magseed
patients, on the other hand, do not need to be transferred
to the nuclear medicine unit for scintigraphy, which
contributes to a shorter overall preoperative localisation
duration.
In our study, we achieved 100% technical success rate
in Magseed placement under ultrasound guidance.
Technical success of Magseed was lower in stereotactic
placement (84.6%) with two cases showing significant
Magseed displacement, namely 16 mm and 17 mm
from the target. These were observed in our 5th and 8th stereotactically guided cases. We believe this
occurred due to the accordion effect, which is also
not uncommonly seen in marker clip placement
after stereotactic biopsy.[13] [14] [15] [16] [17] [18] In subsequent cases, we
attempted to maintain manual breast compression over
the Magseed insertion site upon releasing the paddle
compression. The manual breast compression was then
slowly released. Technical success was achieved in the
remaining five stereotactic cases using this manoeuvre.
Minimal displacement of a Magseed up to 4.8 mm was
observed during the localisation-operation interval.
No previous published studies related to Magseed had
documented any significant or late migration of Magseed
during the localisation-operation interval. Movement of
Magseed could occur while it was in the breast or after
it was excised. It could be related to inconsequential
movement of Magseed within the breast after placement,
or manipulation by surgeons during or after operation,
when the architectural support from surrounding breast
tissues is lost. Furthermore, displacement of Magseed
was insignificant and did not affect the accuracy of
localisation during operation, excision of the nonpalpable
breast lesions or the overall performance.
There was one case in each Magseed and ROLL
groups where the microcalcifications were too faint to
be identified on the prone biopsy table. Therefore, the
previous biopsy scar, stereotactic biopsy position, breast
compression thickness, and coordinates in previous
biopsy were used as reference. Both cases resulted in
successful localisation and excision with the surgical
specimen pathology matching the one biopsied. We
suggest placement of a marker clip after biopsy if the
microcalcifications are too faint to be identified during
and after biopsy, in order to aid further localisation if
needed.
Constraints with ROLL have been previously
documented, such as inaccurate injections of isotopes
in a compressed breast (especially thin ones) ductal
migration of isotopes, the need of a nuclear medicine
department and timely transfer to operation theatres.[4]
Magseed also has its limitations. Non-magnetic surgical
equipment and retractors need to be used to eliminate
any possible interference with the detection probe, which
can be a limitation. Economical concern is also a part of
considerations for some institutions as Magseed is more
expensive than ROLL.
Just as precise localisation of nonpalpable breast lesions
is essential, accurate localisation of sentinel lymph nodes is equally important. In a prospective, multicentre
and multinational study by Thill et al,[19] Sienna dye, a
magnetic tracer with superparamagnetic iron oxide
compound and particle dimensions of 60 nm, was used
for sentinel lymph node localisation and biopsy. It was
compared with 99mTc filtered sulphur colloid, which
is the traditional standard to localise sentinel lymph
node. The study included 150 patients in each group
and showed comparable result between Sienna dye
and radioisotope.[19] The Sienna dye can be injected by
the surgeons in the operation room at least 20 minutes
before sentinel lymph node biopsy and is detected with
the Sentimag probe. Therefore, in Magseed cases, it is
possible to perform sentinel lymph node mapping with
the same probe in the same session with the use of Sienna
dye during surgery.
There are several limitations to our study. This was a
retrospective review with a small patient sample size
from a single institution. It revealed our early experience
with a new technique, and both the radiologists and
surgeons involved were new to this method. The decision
of Magseed localisation or ROLL was determined
during combined clinical and radiological meetings with
surgeons. The preference of surgeons might impose a
selection bias. Larger multi-institutional prospective
randomised studies would be necessary to fully compare
Magseed with ROLL.
CONCLUSION
We have shown that Magseed is a safe and effective
localisation technique for breast lesion localisation, and
superior to ROLL as it is non-radioactive and allows
decoupling of radiological and surgical schedules. This
study showed results in operation time, margin clearance,
and reoperation rate comparable to those of ROLL.
REFERENCES
1. Lui CY, Lam HS, Chan LK, Tam KF, Chan CM, Leung TY, et al. Opportunistic breast cancer screening in Hong Kong; a revisit of the Kwong Wah Hospital experience. Hong Kong Med J. 2007;13:106-
13.
2. Sitt JC, Lui C, Sinn LH, Fong JC. Understanding breast cancer
screening — past, present, and future. Hong Kong Med J.
2018;24:166-74. Crossref
3. Corsi F, Sorrentino L, Bossi D, Sartani A, Foschi D. Preoperative
localization and surgical margins in conservative breast surgery.
Int J Surg Oncol. 2013;2013:793819. Crossref
4. Chu TY, Lui CY, Hung WK, Kei SK, Choi CL, Lam HS.
Localisation of occult breast lesion: a comparative analysis
of hookwire and radioguided procedures. Hong Kong Med J.
2010;16:367-72.
5. Jeffries DO, Dossett LA, Jorns JM. Localization for breast surgery:
the next generation. Arch Pathol Lab Med. 2017;141:1324-9. Crossref
6. Magseed magnetic marker receives FDA clearance for long-term
and soft tissue implantation [Internet]. Available from: https://www.mammotome.com/wp-content/uploads/2018/03/Magseed-Indication-.... Accessed 14 Jun 2020.
7. Endomagnetics Ltd (Endomag). Indications for use. Available from: https://www.endomag.com/indications-for-use/. Accessed
9 Nov 2020.
8. Harvey JR, Lim Y, Murphy J, Howe M, Morris J, Goyal A, et al.
Safety and feasibility of breast lesion localization using magnetic
seeds (Magseed): a multi-centre, open-label cohort study. Breast
Cancer Res Treat. 2018;169:531-6. Crossref
9. Fung JT, Chan SW, Chiu AN, Cheung PS, Lam SH. Mammographic determination of breast volume by elliptical cone estimation. World J Surg. 2009;34:1442-5. Crossref
10. Lamb LR, Bahl M, Specht MC, D’Alessandro HA, Lehman CD. Evaluation of a nonradioactive magnetic marker wireless
localization program. AJR Am J Roentgenol. 2018;211:940-5. Crossref
11. Price ER, Khoury AL, Esserman LJ, Joe BN, Alvarado MD. Initial
clinical experience with an inducible magnetic seed system for
preoperative breast lesion localization. AJR Am J Roentgenol.
2018;210:913-7. Crossref
12. Zacharioudakis K, Down S, Bholah Z, Lee S, Khan T, Maxwell AJ,
et al. Is the future magnetic? Magseed localisation for non-palpable
breast cancer. A multi-centre non randomised control study. Eur J Surg Oncol. 2019;45:2016-21. Crossref
13. Burbank F, Forcier N. Tissue marking clip for stereotactic
breast biopsy: initial placement accuracy, long-term stability,
and usefulness as a guide for wire localization. Radiology.
1997;205:407-15. Crossref
14. Liberman L, Dershaw DD, Morris EA, Abramson AF, Thornton CM,
Rosen PP. Clip placement after stereotactic vacuum-assisted breast
biopsy. Radiology. 1997;205:417-22. Crossref
15. Burnside ES, Sohlich RE, Sickles EA. Movement of a biopsy-site
marker clip after completion of stereotactic directional vacuum-assisted
breast biopsy: case report. Radiology. 2001;221:504-7. Crossref
16. Rosen EL, Vo TT. Metallic clip deployment during stereotactic breast biopsy: retrospective analysis. Radiology. 2001;218:510-6. Crossref
17. Philpotts LE, Lee CH. Clip migration after 11-gauge vacuum-assisted stereotactic biopsy: case report. Radiology. 2002;222:794-6. Crossref
18. Esserman LE, Cura MA, DaCosta D. Recognizing pitfalls in early
and late migration of clip markers after imaging-guided directional
vacuum-assisted biopsy. Radiographics. 2004;24:147-56. Crossref
19. Thill M, Kurylcio A, Welter R, van Haasteren V, Grosse B,
Berclaz G, et al. The Central-European SentiMag study: Sentinel
lymph node biopsy with superparamagnetic iron oxide (SPIO) vs.
radioisotope. Breast. 2014;23:175-9. Crossref