Breast Manifestations in Patients with Systemic Lupus Erythematosus
ORIGINAL ARTICLE
Breast Manifestations in Patients with Systemic Lupus
Erythematosus
DLY Chow1, T Wong2, CM Chau2, RLS Chan2, TS Chan2, DCY Lui2, AWT Yung2, ASL Fung1,
JKF Ma2
1 Department of Radiology and Nuclear Medicine, Tuen Mun Hospital, Hong Kong
2 Department of Radiology, Princess Margaret Hospital, Hong Kong
Correspondence: Dr DLY Chow, Department of Radiology, Tuen Mun Hospital, Hong Kong. Email: deniselychow@gmail.com
Submitted: 10 Mar 2020; Accepted: 4 Jun 2020.
Contributors: DLYC, TW and CMC designed the study and acquired the data. DLYC and TW analysed the data. DLYC drafted the manuscript.
TW, CMC, RLSC, TSC, DCYL, AWTY, ASLF and JKFM critically revised the manuscript for important intellectual content.
Conflicts of Interest: authors have disclosed no conflicts of interest.
Funding/Support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: This research was approved by the Kowloon West Cluster Research Ethics Committee (Ref KW/EX-20-008(143-08)) and New
Territories West Cluster Research Ethics Committee (Ref NTWC/REC/19130). Patient consent was waived by the ethics board (KW/EX-20-008(143-08) and NTWC/REC/19130).
Abstract
Objectives
Breast manifestations in patients with systemic lupus erythematosus (SLE) include primary lupus of the
breast (i.e., lupus mastitis) and secondary manifestations of lupus such as lymphadenopathy or vascular calcifications.
To clarify the spectrum of breast manifestations in patients with SLE, we reviewed the clinical, imaging, and
pathological manifestations of breast diseases in SLE patients.
Methods
We retrospectively reviewed cases of SLE patients with breast imaging performed in five centres from
January 2010 to April 2020. Patient demographics, breast symptoms, imaging, and pathological findings, and their
subsequent management, were reviewed.
Results
A total of 16 cases were included. The mean follow-up period was 61 months. A palpable breast mass was
the most frequent clinical presentation, followed by mastalgia and axillary swelling. A wide range of imaging findings
was encountered on ultrasonography and/or mammography, including extensive calcifications in both breasts, breast
masses with features suspicious for malignancy, fat necrosis, oedema, arterial calcifications, architectural distortion,
and axillary lymphadenopathy. Two cases of lupus mastitis and a case of invasive ductal carcinoma were identified.
Conclusion
No definite distinguishing features between lupus mastitis and breast malignancy were observed
on imaging. Pathological correlation is recommended when imaging features suspicious for malignancy are
demonstrated.
Key Words: Lupus erythematosus, systemic; Magnetic resonance imaging; Mammography; Mastitis; Ultrasonography
中文摘要
系統性紅斑狼瘡患者的乳腺表現
周朗妍、黃婷、周智敏、陳樂詩、陳庭笙、雷彩如、翁維德、馮小玲、馬嘉輝
目的
系統性紅斑狼瘡(SLE)患者的乳腺表現包括乳腺原發性狼瘡(即狼瘡性乳腺炎)和狼瘡的繼發性表現,例如淋巴結腫大或血管鈣化。為了闡明SLE患者的各種乳腺表現,本研究回顧SLE患者乳腺疾病的臨床、影像學和病理學表現。
方法
回顧性分析2010年1月至2020年4月期間在五間醫療中心進行乳腺成像的SLE患者病例。回顧患者的人口統計學、乳腺症狀、影像學和病理學及其後續處理。
結果
共納入16例。平均隨訪期為61個月。最常見的臨床表現是可觸及的乳腺腫塊,其次是乳腺痛和腋窩腫脹。在超聲檢查和/或乳腺X光檢查中發現各種影像學表現,包括雙側乳腺廣泛鈣化、乳腺腫塊具可疑惡性腫瘤特徵、脂肪壞死、水腫、動脈鈣化、結構變形和腋窩淋巴結腫大。病例中有2例狼瘡性乳腺炎和1例浸潤性導管癌。
結論
影像學上未觀察到狼瘡性乳腺炎與乳腺惡性腫瘤之間的明確區分特徵。 當發現可疑惡性腫瘤的影像特徵時,建議進行病理學相關檢查。
INTRODUCTION
Systemic lupus erythematosus (SLE) is a complex
autoimmune disease with multisystem involvement
characterised by inflammation, vasculitis, immune
complex deposition, and vasculopathy.[1] It is substantially
more common in women of childbearing age.[2] Breast
diseases in patients with lupus may be primary
lupus of the breast (i.e., lupus mastitis) or secondary
manifestations of lupus such as lymphadenopathy or
vascular calcifications. These patients are also subject
to breast diseases unrelated to SLE. To our knowledge,
the spectrum of breast manifestations in patients with
SLE has not been described in the literature, with case
reports mainly focusing on lupus mastitis. To clarify the
spectrum of breast manifestations in patients with SLE,
we retrospectively reviewed the clinical, imaging, and
pathological findings in patients with SLE.
METHODS
Cases of SLE with breast imaging (mammography,
ultrasound, or magnetic resonance imaging [MRI])
in five centres from January 2010 to April 2020 were
identified through a search of the Radiology Information
System using the keywords ‘lupus’, ‘SLE’, and ‘systemic
lupus erythematosus’. Cases without breast imaging or
with unavailable imaging were excluded.
Sixteen cases were found; all had available breast imaging studies. Case demographics, breast symptoms,
breast imaging findings, pathological findings (if any)
and subsequent management were reviewed.
RESULTS
Patient Demographics
In total, 16 cases of SLE were identified and included
for analysis. No cases were excluded. The mean age of
presentation of breast symptoms was 44 years (range,
23-71). The mean duration of SLE at time of breast
disease presentation was 11.2 years. Breast symptoms
occurred after diagnosis of SLE in 14 of the 16 cases.
Breast symptoms occurred before diagnosis of SLE in
the remaining two cases, and among them one presented
with a breast mass 2 years before the diagnosis of SLE.
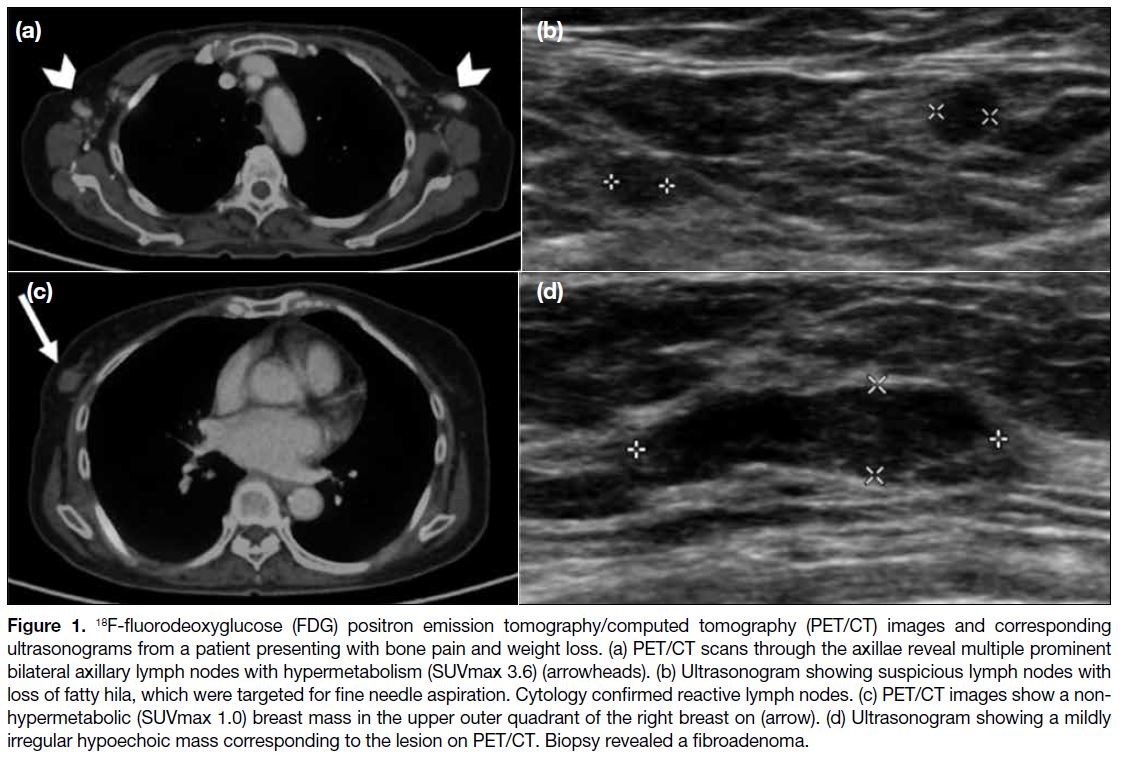
One patient presented with bone pain and weight loss,
and breast imaging was performed at the same time as
part of the systemic investigation for SLE (Figure 1).
The mean follow-up period was 61 months.
Figure 1. 18F-fluorodeoxyglucose (FDG) positron emission tomography/computed tomography (PET/CT) images and corresponding
ultrasonograms from a patient presenting with bone pain and weight loss. (a) PET/CT scans through the axillae reveal multiple prominent
bilateral axillary lymph nodes with hypermetabolism (SUVmax 3.6) (arrowheads). (b) Ultrasonogram showing suspicious lymph nodes with
loss of fatty hila, which were targeted for fine needle aspiration. Cytology confirmed reactive lymph nodes. (c) PET/CT images show a non-hypermetabolic
(SUVmax 1.0) breast mass in the upper outer quadrant of the right breast on (arrow). (d) Ultrasonogram showing a mildly
irregular hypoechoic mass corresponding to the lesion on PET/CT. Biopsy revealed a fibroadenoma.
Clinical Presentation
Among the 16 patients, palpable breast mass was the
most common clinical presentation in nine (56%)
patients, followed by mastalgia in three (19%) patients
and axillary swelling in two (13%) patients. One (6%)
patient presented with a palpable breast mass and axillary
swelling, and one (6%) patient presented with bone pain
and weight loss.
Investigations
One (6%) patient underwent mammography,
ultrasonography, and MRI scans, eight (50%) patients
underwent mammography and ultrasonography, six
(38%) patients underwent ultrasonography only, and the
remaining one (6%) patient underwent mammography
only. In total, ultrasonography of the breasts was
performed in 15 (94%) of the 16 cases.
Imaging Findings
Imaging findings included breast mass, calcifications,
fat necrosis, architectural distortion, breast oedema, and
axillary lymphadenopathy (Table).
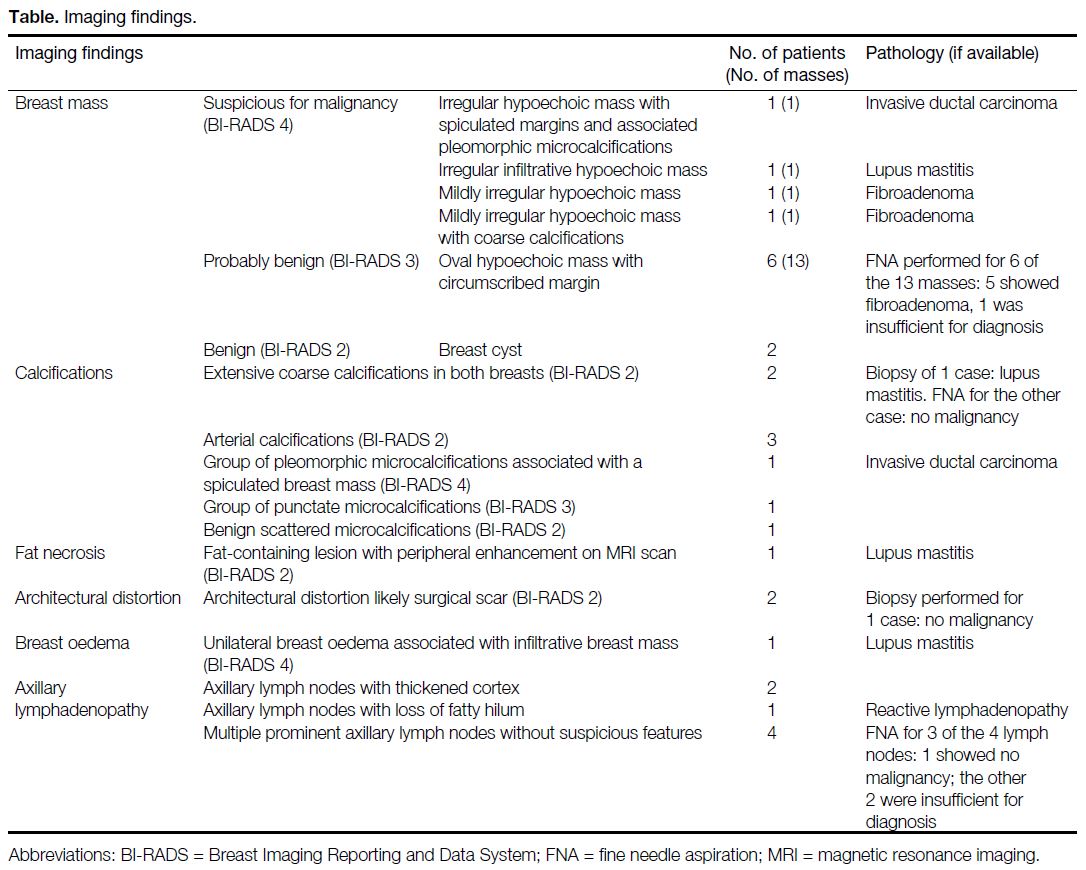
Table. Imaging findings.
Lupus Mastitis
There were two cases of lupus mastitis with pathological
confirmation. Both patients presented with a palpable
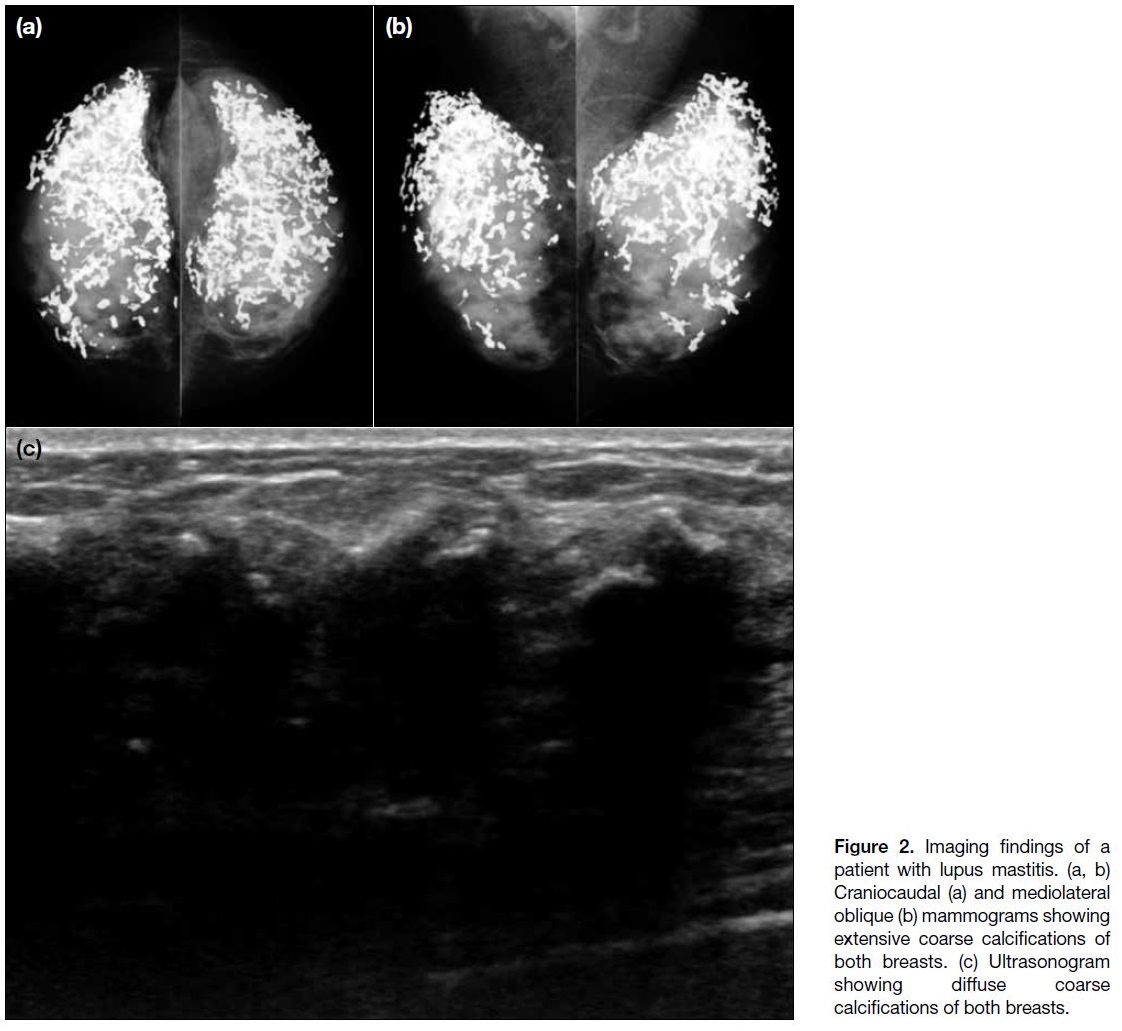
breast mass. In the first case, extensive calcifications
were seen both on mammography and ultrasound and
the patient subsequently underwent core biopsy (Figure 2). Pathology showed hyaline fat necrosis, nodular
perilobular and perivascular inflammatory infiltrate
comprising lymphocytes and plasma cells, and stromal fibrosis with microcalcifications, compatible with lupus
mastitis. The patient was treated with systemic steroids
and immunosuppressants as part of her therapy for
SLE. During >6 years of clinical and imaging follow-up
examinations, the calcifications remained stable and
the patient did not have recurrence of lupus mastitis
symptoms. No further biopsy was performed.
Figure 2. Imaging findings of a
patient with lupus mastitis. (a, b)
Craniocaudal (a) and mediolateral
oblique (b) mammograms showing
extensive coarse calcifications of
both breasts. (c) Ultrasonogram
showing diffuse coarse
calcifications of both breasts.
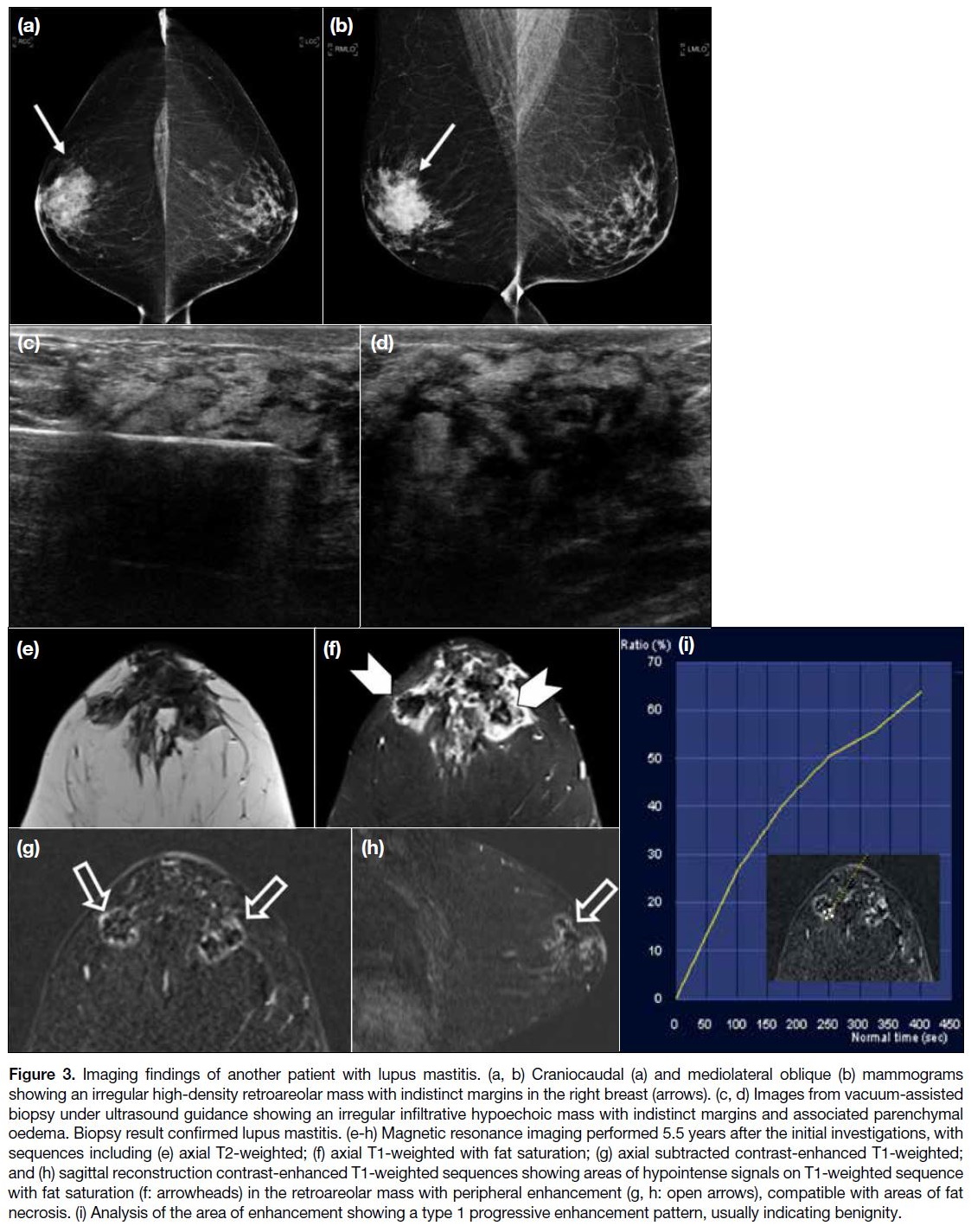
In the second case (Figure 3), an irregular high-density
retroareolar mass with indistinct margins was
present in the right breast on mammography, with
ultrasound showing a corresponding irregular infiltrative
hypoechoic mass with associated parenchymal oedema.
It was classified as highly suspicious for malignancy.
Vacuum-assisted biopsy was performed in this case with
pathology confirming lupus mastitis.
Figure 3. Imaging findings of another patient with lupus mastitis. (a, b) Craniocaudal (a) and mediolateral oblique (b) mammograms
showing an irregular high-density retroareolar mass with indistinct margins in the right breast (arrows). (c, d) Images from vacuum-assisted
biopsy under ultrasound guidance showing an irregular infiltrative hypoechoic mass with indistinct margins and associated parenchymal
oedema. Biopsy result confirmed lupus mastitis. (e-h) Magnetic resonance imaging performed 5.5 years after the initial investigations, with
sequences including (e) axial T2-weighted; (f) axial T1-weighted with fat saturation; (g) axial subtracted contrast-enhanced T1-weighted;
and (h) sagittal reconstruction contrast-enhanced T1-weighted sequences showing areas of hypointense signals on T1-weighted sequence
with fat saturation (f: arrowheads) in the retroareolar mass with peripheral enhancement (g, h: open arrows), compatible with areas of fat
necrosis. (i) Analysis of the area of enhancement showing a type 1 progressive enhancement pattern, usually indicating benignity.
Ultrasound and MRI scans were performed 5.5 years
later in view of persistent breast symptoms. MRI
scans showed a heterogeneous retroareolar mass on
T2-weighted images with associated skin thickening
and mild breast oedema, which was proven to be lupus
mastitis on previous biopsy. Areas of signal suppression with peripheral enhancement within the mass on the T1-weighted fat-saturated sequence were suggestive of fat
necrosis, compatible with the pathological process of
lupus panniculitis. The peripheral enhancement showed
a progressive enhancement pattern suggestive of a type
1 kinetic curve.[3] Ultrasound findings were static, and the
patient remained stable with medical therapy of SLE.
Breast Mass with Suspicious Imaging Features for
Malignancy
Apart from the previously mentioned cases of
pathologically confirmed lupus mastitis, there were three
other breast masses with suspicious imaging features
for malignancy in our patient cohort. One of them was
proven to be invasive ductal carcinoma, while the other
two were fibroadenomas.
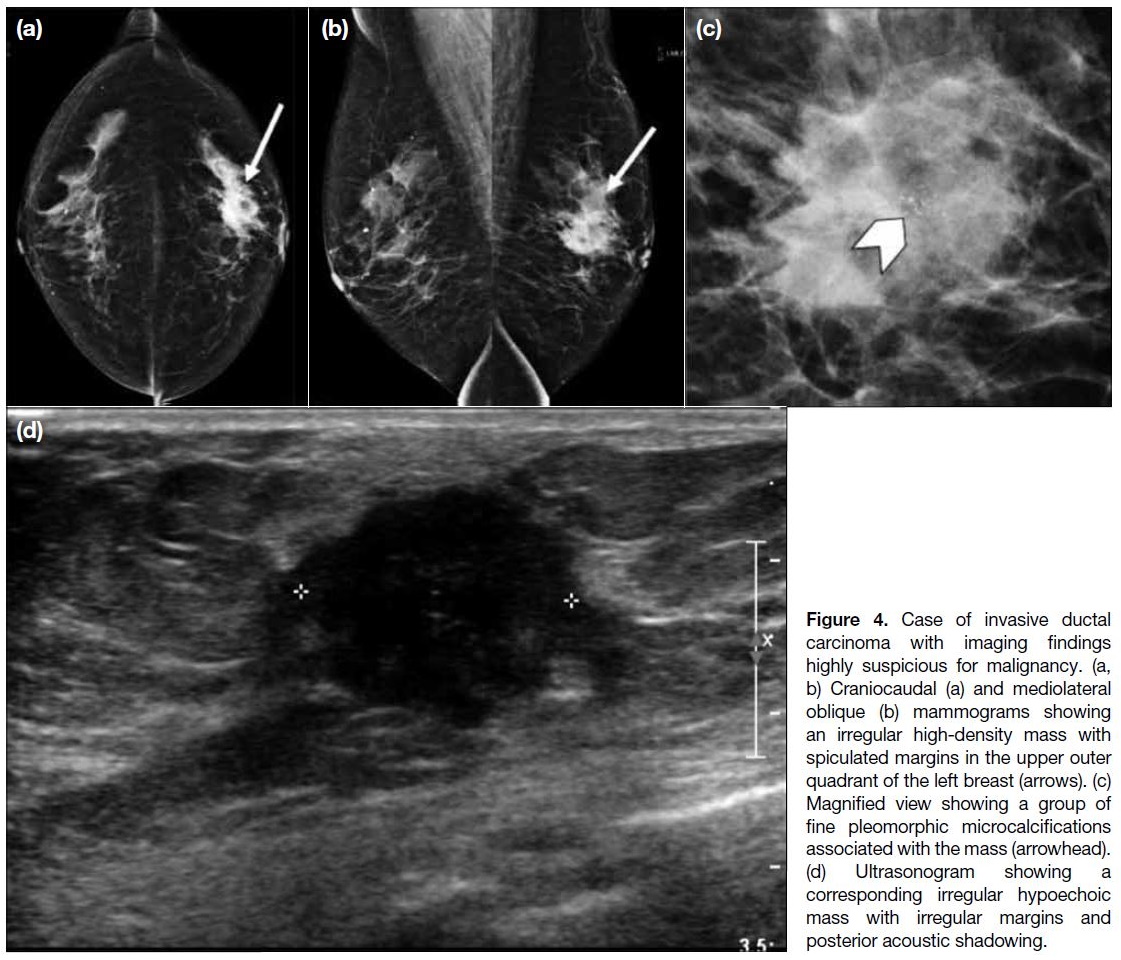
The invasive ductal carcinoma (Figure 4) was highly suspicious for malignancy (Breast Imaging Reporting
and Data System [BI-RADS] 4C) on imaging. On
mammography, there was a spiculated mass with
associated fine pleomorphic microcalcifications. On
ultrasonography, a corresponding irregular hypoechoic
mass with spiculated margin and posterior acoustic
shadowing was detected. Core biopsy was performed
and yielded invasive ductal carcinoma. The patient
subsequently underwent mastectomy and axillary
dissection with adjuvant chemotherapy.
Figure 4. Case of invasive ductal
carcinoma with imaging findings
highly suspicious for malignancy. (a,
b) Craniocaudal (a) and mediolateral
oblique (b) mammograms showing
an irregular high-density mass with
spiculated margins in the upper outer
quadrant of the left breast (arrows). (c)
Magnified view showing a group of
fine pleomorphic microcalcifications
associated with the mass (arrowhead).
(d) Ultrasonogram showing a
corresponding irregular hypoechoic
mass with irregular margins and
posterior acoustic shadowing.
The fibroadenomas were slightly irregular masses with
and without internal coarse calcifications.
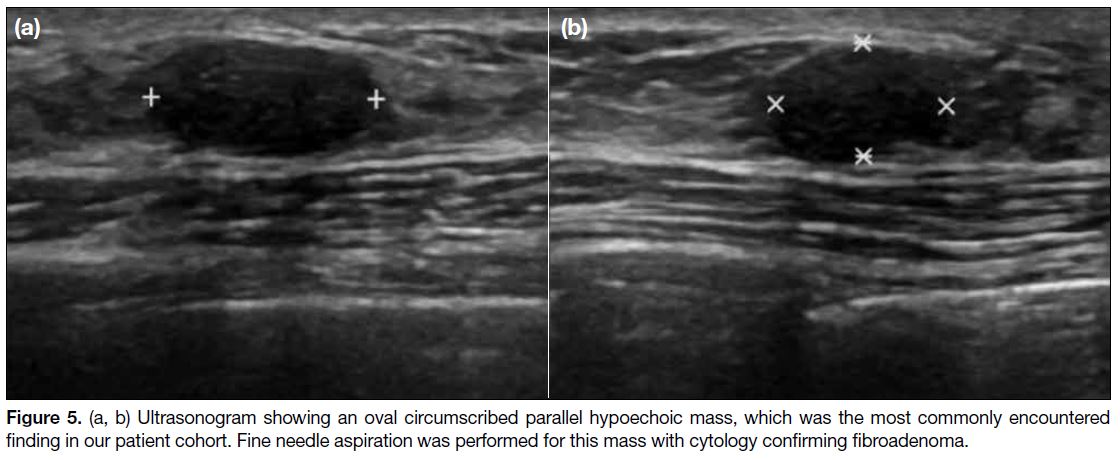
Probably Benign Oval Circumscribed Hypoechoic
Breast Masses
Oval circumscribed hypoechoic masses on
ultrasonography, which were classified as probably benign (BI-RADS 3), were the most commonly
encountered breast manifestation in our cases, with
13 masses observed in six of the patients (Figure 5). Fine
needle aspiration (FNA) was performed for six of these
13 masses, yielding fibroadenoma for five of the cases.
One of these masses showed mild interval enlargement
on new ultrasonography at 6 months; results of a new core
biopsy showed no malignancy. The remaining one case
with FNA performed showed inconclusive results as the
FNA sample was insufficient for diagnosis; this patient
was followed up clinically and with breast ultrasound
for >3 years, showing stability of the oval hypoechoic masses. All of the 13 oval circumscribed hypoechoic
masses were followed up with breast imaging, with the
exception of the aforementioned mass that showed mild
interval enlargement, the other 12 masses all showed
stability with range of follow-up period from 3.5 years to
7 years, thus classified as benign.
Figure 5. (a, b) Ultrasonogram showing an oval circumscribed parallel hypoechoic mass, which was the most commonly encountered
finding in our patient cohort. Fine needle aspiration was performed for this mass with cytology confirming fibroadenoma.
Calcifications
We encountered two cases of extensive calcifications in
both breasts; one was the case of lupus mastitis (Figure 2)
described above, while the other case showed no
malignancy by FNA.
Arterial calcifications were observed in three patients.
All three patients had a history of lupus nephritis.
Two of them did not have traditional risk factors for
atherosclerosis, namely hypertension, diabetes mellitus,
hyperlipidaemia and obesity, while the other patient had
hyperlipidaemia but not known of diabetes mellitus or
hypertension.
Other calcifications observed in our patients included
groups of fine pleomorphic microcalcifications in
association with a spiculated breast mass in the case of
invasive ductal carcinoma (Figure 4), a group of punctate
microcalcifications that was stable on follow-up, and
benign scattered microcalcifications.
Architectural Distortion
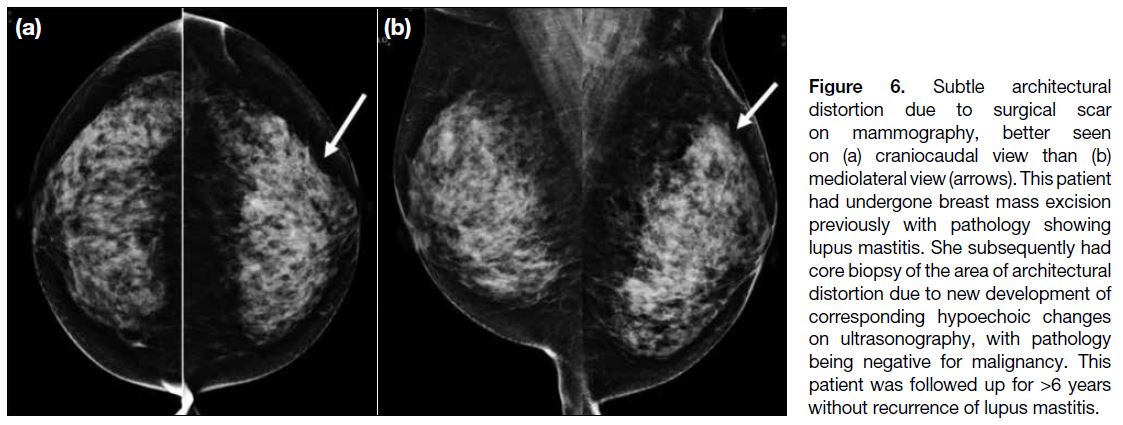
Architectural distortion was observed in two of the cases
and was likely due to scar related to previous breast
surgery. One of the patients had a history of excision of
phyllodes tumour and fibroadenoma. The other patient
(Figure 6) had undergone previous breast mass excision
with pathology showing lupus mastitis. This patient
subsequently had core biopsy due to new development
of subtle hypoechoic changes on ultrasonography, which
corresponded to the site of architectural distortion, with
pathology being negative for malignancy. This patient
was followed up for 6 years with stable imaging results
and without recurrence of breast symptoms suggestive
of lupus mastitis.
Figure 6. Subtle architectural
distortion due to surgical scar
on mammography, better seen
on (a) craniocaudal view than (b)
mediolateral view (arrows). This patient
had undergone breast mass excision
previously with pathology showing
lupus mastitis. She subsequently had
core biopsy of the area of architectural
distortion due to new development of
corresponding hypoechoic changes
on ultrasonography, with pathology
being negative for malignancy. This
patient was followed up for >6 years
without recurrence of lupus mastitis.
Axillary Lymphadenopathy
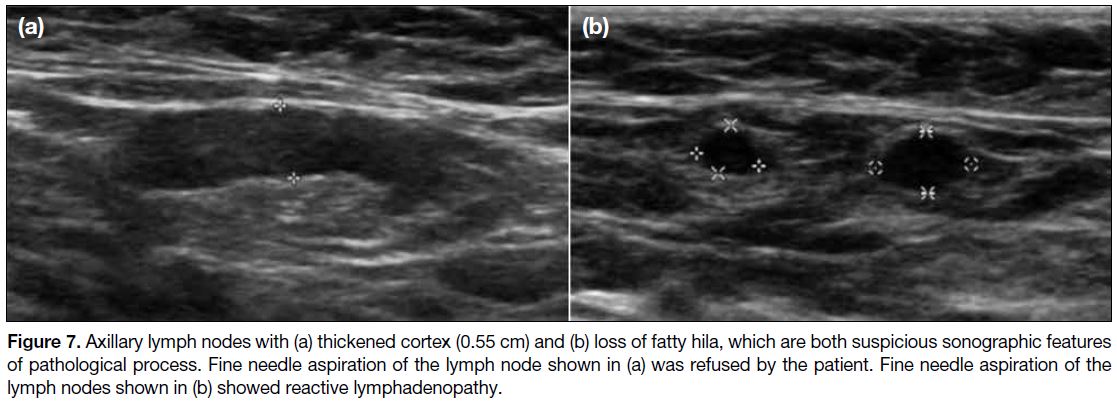
Axillary lymph nodes with suspicious features such as
thickened cortex and/or loss of fatty hila were present in
three patients (Figure 7). FNA was performed for one
patient with cytology being reactive lymphadenopathy.
FNA was not performed for the other two cases with
suspicious axillary lymph nodes as the patient refused
for one case and clinical features were not suspicious for
the other case. Multiple axillary lymph nodes without
suspicious imaging features were observed in four
patients. Three patients underwent FNA of lymph nodes
with one of them showing no evidence for malignancy,
while the other two samples were insufficient for
diagnosis. All of the cases, including those with suspicious features, showed stability on follow-up
ultrasound with follow-up period of 15 months to 8
years.
Figure 7. Axillary lymph nodes with (a) thickened cortex (0.55 cm) and (b) loss of fatty hila, which are both suspicious sonographic features
of pathological process. Fine needle aspiration of the lymph node shown in (a) was refused by the patient. Fine needle aspiration of the
lymph nodes shown in (b) showed reactive lymphadenopathy.
DISCUSSION
Our review showed that a wide spectrum of generally
nonspecific sonographic and mammographic findings
can be present in patients with SLE. Some are due to
SLE, including primary lupus of the breast (i.e., lupus
mastitis) and secondary manifestations of SLE such
as lymphadenopathy or vascular calcifications. Other
findings, such as breast cancer, are not known to be
lupus-related and can occur in women unaffected by
lupus.
Lupus Mastitis
Lupus mastitis is a subset of lupus panniculitis that is
localised to the breast and is a rare manifestation of
SLE. Lupus panniculitis is a rare chronic inflammatory
reaction of the subcutaneous fat that can occur in 2% to
3% of patients with SLE, usually between age 20 and
50 years, and more common in women than in men.[4] [5] [6]
Clinically, lupus mastitis can occur in patients with an
established diagnosis of SLE or can rarely be the first
manifestation of the disease.[5] In both cases of lupus
mastitis in our cohort, SLE was established before the
onset of breast symptoms. The most common presentation
is a palpable lesion, often associated with pain,[7] as in
both of our cases. The overlying skin may be normal
but cutaneous changes such as erythema, hyperkeratosis,
lipoatrophy or even ulceration can be evident.[5] Diffuse
breast enlargement and palpable axillary lymph nodes
are less common.[7] The clinical course of lupus mastitis
is often chronic with flares and remissions.[6]
For histopathological diagnosis, the major criteria are fat
hyaline necrosis, lymphocytic infiltration with lymphoid
nodules surrounding the necrosis, periseptal or lobular
panniculitis, and microcalcifications. Minor criteria are
changes of discoid lupus erythematosus in the overlying
skin, lymphocytic vasculitis, mucin deposition, and
hyalinisation of subepidermal papillary zones. The
combination of four major and four minor criteria is
virtually diagnostic and permits differentiation from
other forms of panniculitis.[8]
In our two cases of confirmed lupus mastitis, one patient had extensive macrocalcifications in both breasts on
imaging, while the other had an infiltrative irregular
breast mass. Mammography, ultrasonography, and
MRI scans are used as imaging investigations for lupus
mastitis, as for other breast diseases. Various imaging
findings of lupus mastitis have been described,[4] [7] [9] [10] [11] [12] [13] [14]
depending on the stages of fat necrosis.
On mammography, large, dystrophic calcifications are
the most commonly encountered findings, as in one of
our cases.[7] Early mammographic findings include thin
curvilinear calcifications that, on subsequent images,
progressively enlarge and coarsen, mirroring the
pathologic evolution of focal panniculitis to maturing
subcutaneous fat necrosis.[9] Early calcifications frequently
simulate malignancy and present in a ductal distribution
or appear fine linear-branching in morphology. As
fat necrosis progresses, the calcifications increase in
size and become coarse and benign in appearance and
also can often be seen on ultrasound and MRI scans.[10]
Lupus mastitis can also present as a mass (often illdefined)
or asymmetry (focal or global) and may mimic
carcinoma.[7] Interval increase in mammographic density
and decreasing breast size over time can be present due
to underlying fibrosis.[11]
Sonographic findings of lupus mastitis also depend on
and reflect stages of fat necrosis. The most commonly
seen findings on ultrasonography are a hypoechoic ill-defined
mass, areas of architectural distortion, or changes
in echotexture of the breast that are more hyperechoic
due to infiltration of the subcutaneous fat and/or breast
parenchyma.[7] In patients who present with a discrete mass, non-specific features such as solid, irregular,
echogenic lesions with ill-defined margins have been
described.[5] [6] [13] Cutaneous involvement has also been
described and it is postulated to result from increased
vascularity in the subcutaneous plane, which may also
affect deeper planes than in the dermis.[4] [7] These findings
may mimic those of advanced breast carcinoma with
skin involvement. Calcifications with marked posterior
acoustic shadowing can be seen on ultrasonography when
dystrophic calcifications due to fat necrosis are present.
MRI is infrequently used for the evaluation of lupus
mastitis. The MRI features of lupus mastitis are
nonspecific and MRI can be helpful for showing
the extent of the disease and in demonstrating skin
involvement as in our case.[4] [7] [10] Some of the reported
MRI findings of lupus mastitis include skin thickening
with marked fat stranding, large coarse calcifications
seen as low-signal lesions, irregular masses (which
can demonstrate fat content) with rim enhancement
and a variable enhancement curve. The morphological
and kinetic features can be indistinguishable from
malignancy.[12] [13] [14] High signal intensity within the lesion
on pre-contrast scan with fat suppression may be hints
for underlying fat necrosis,[12] [13] [14] which was also seen in
our case of biopsy-proven lupus mastitis. A type 1 kinetic
curve was observed in our case, a finding that is usually
associated with benignity. As the enhancement pattern
for lupus mastitis can be variable, the morphology of
lesions on MRI scans is often more informative than
the kinetic curve alone. Overall MRI findings were
indistinguishable from malignancy, which emphasises
that MRI should not be performed to differentiate
between lupus mastitis and malignancy, but rather to
delineate the extent of known lupus mastitis.
The second case of lupus mastitis displayed
extensive coarse calcifications in both breasts on both
ultrasonography and mammography. Freehand FNA of
both breasts was performed by surgeons before imaging
with results showing no malignancy, thus further biopsy
was not performed after imaging after discussion with the
patient. Therefore, in cases of suspected lupus mastitis
where extensive calcifications are present, we suggest
core biopsy to allow histological analysis rather than
FNA. In addition, the history of SLE should be clearly
stated in the request form as this is important to allow
pathologists to accurately identify lupus mastitis.
Breast carcinoma, in particular inflammatory breast
carcinoma, can show clinical and radiological features similar to those of lupus mastitis, especially in patients
who present with a rapidly enlarging breast mass with
skin involvement.[7] Owing to the small number of cases
of lupus mastitis and breast malignancy in our cases,
we did not observe any specific imaging features to
distinguish between these two entities in our review.
The concept of lupus panniculitis being exacerbated by
localised trauma, such as biopsy, has been described.[8]
However, due to the similarities of clinical presentation
and imaging findings between lupus mastitis and breast
malignancy, we advocate biopsy for histopathological
correlation when suspicious imaging features are present.
The differential diagnosis of the findings seen in
lupus mastitis include diabetic mastopathy, idiopathic
granulomatous mastitis, and lymphoma. Clinical history
is crucial in distinguishing diabetic mastopathy from
lupus mastitis, while histopathological correlation
to rule out idiopathic granulomatous mastitis (which
lacks lymphocytic vasculitis), and immunohistological
chemical staining for lymphoma may be needed to
distinguish among these entities.
The clinical course of lupus mastitis is often chronic with
flares and remissions.[6] Both patients with lupus mastitis
had subjective breast mass during the follow-up periods
while they also had mild flares of other clinical aspects
of SLE. This suggests that the course of lupus mastitis
may be associated with the overall disease course and
process, though more research is needed to establish the
potential association.
Breast Mass
Apart from lupus mastitis, breast masses that affect
patients without lupus can also be present in patients with
SLE. Probably benign (BI-RADS 3) oval circumscribed
hypoechoic masses were the most frequently encountered
imaging findings in our patient cohort, as these are also
commonly seen in women without SLE. In our review,
all of the cases either showed benign pathological results
or were stable on imaging follow-up over the period of
3.5 to 7 years. These breast masses can be managed as
per those in patients who are unaffected by SLE.
Previous literature has shown that SLE is associated
with an increased risk of cancers overall, but is not more
significantly associated with breast cancer.[15] [16] Since the
radiological features of lupus mastitis, tumour and other
benign entities can overlap, as for patients unaffected
by SLE, histological correlation is warranted when
suspicious clinical and imaging features are present.
Calcifications
SLE causing lupus panniculitis is one of a few systemic
diseases that can cause stromal calcifications of the
breasts. Other systemic diseases which can cause diffuse
dystrophic calcifications of the subcutaneous fat include
scleroderma and dermatomyositis.[17] In lupus mastitis,
thin curvilinear calcifications seen in the early phase of
the disease progressively enlarge and coarsen to form
dystrophic calcifications due to the evolution of fat
necrosis as described earlier.
Other breast calcifications that are not lupus-related
can also be present in patients in SLE, for example a
suspicious group of fine pleomorphic microcalcifications
in the case of invasive ductal carcinoma. Biopsy is
warranted when suspicious microcalcifications are
present, as for patients without SLE.
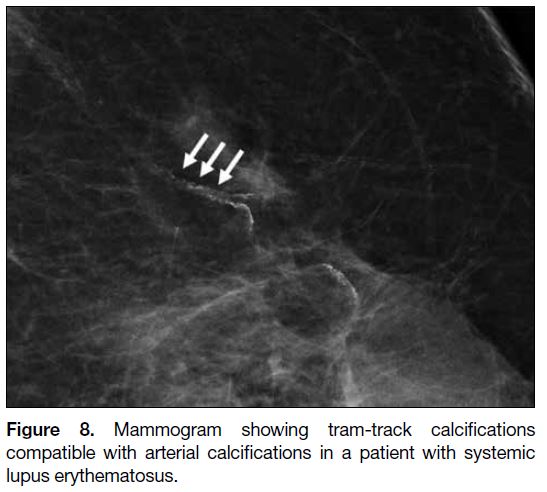
Vascular Manifestations
Arterial calcifications observed as tram-track
calcifications on mammogram were present in three
of our patients (Figure 8). Patients with SLE have
been shown to have significantly higher prevalence
and extent of systemic arterial calcifications compared
with age- and sex-matched controls.[18] This is probably
multifactorial in nature, as lupus-related renal disease,
corticosteroid-induced dyslipoproteinaemia, and
secondary hypertension from renal disease all contribute
to accelerated atherosclerosis.[19] In our three cases with
arterial calcifications, two of them did not have history
of traditional risk factors for atherosclerosis, thus the arterial calcifications may be secondary consequence of
SLE, given their known history of lupus nephritis. On
mammography, arterial calcifications are regarded as
incidental findings with no specific treatment indicated.
Figure 8. Mammogram showing tram-track calcifications
compatible with arterial calcifications in a patient with systemic
lupus erythematosus.
Mondor’s disease is a rare entity characterised by
thrombophlebitis of superficial veins, usually of the
chest wall or breast.[20] Many possible aetiologies have
been described, including trauma, local inflammation,
malignancy, rheumatologic diseases including SLE, and
hypercoagulable states, which can be also associated
with SLE.[21] The presentation can be painful or painless,
classically with sudden appearance of a subcutaneous
cord. The course of the disease is benign and self-limiting,
lasting between 4 to 8 weeks.[22] Mammography
should be performed to exclude underlying malignancy,
which is one of the potential causes of Mondor’s
disease.[23] On mammography, the thrombosed, inflamed
vein is seen as a tubular density in the region of pain or
a palpable mass, which may be mistaken for a dilated
duct.[24] Ultrasound correlation is helpful, with findings
including an enlarged superficial vessel with absent
Doppler flow with or without intraluminal thrombus. A
thrombosed vein tends to be longer than a duct and has a
beaded appearance.[24]
Breast Oedema
On imaging, breast oedema is seen as breast enlargement,
increased parenchymal density, trabecular thickening,
increased interstitial markings, and skin thickening.[23] [25]
In patients with SLE, breast oedema can be secondary
to lupus-related chronic renal failure or congestive heart
failure, which usually affects both breasts but can be
unilateral with lateralisation to the dependent breast in
cases where patient is immobile. In patients with an upper
limb arteriovenous fistula for dialysis, unilateral breast
oedema can also occur secondary to complications of
arteriovenous fistula such as thrombosis.[9] Occasionally,
breast oedema can be associated with lupus mastitis, as in
the case in our patient cohort (Figure 3). As with patients
without SLE, breast oedema can also be caused by
venous obstruction, inflammatory breast cancer, mastitis,
post-irradiation changes, or lymphatic obstruction.
Axillary Lymphadenopathy
Many autoimmune diseases are associated with
lymphadenopathy, including rheumatoid arthritis,
Sjögren’s syndrome and SLE. Lymphadenopathy has
been reported to affect 23% to 34% of patients with
SLE. Seven of our 16 patients (43.8%) were reported
to have enlarged axillary lymph nodes, slightly higher than the reported percentage. In general, lymph nodes
related to SLE are soft, non-tender and vary in size and
there may be fluctuation of lymphadenopathy with SLE
disease exacerbations. Lymph node pathology in this
case generally showed diffuse hyperplasia with scarce
follicles.[26] [27]
In patients with SLE, axillary lymphadenopathy can
be due to the disease itself or occasionally related to
lupus mastitis. SLE has been shown to be associated
with an increased risk of non-Hodgkin lymphoma
and Hodgkin lymphoma, which can also present
with lymphadenopathy.[15] Other causes of axillary
lymphadenopathy not related to SLE include metastasis
(most commonly from breast cancer), infection,
inflammatory causes, or granulomatous diseases.
On breast imaging, axillary lymph nodes are most
commonly seen on the mediolateral oblique view
mammogram or ultrasound. There can be considerable
overlap in morphological appearances of benign and
pathological lymph nodes on imaging. Features that
are suspicious for pathological lymph nodes include
loss of the normal fatty hilum, loss of the normal oval
or reniform shape, poorly circumscribed margins, and
increased size and opacity compared with findings on
prior images.[9] It is important to not assume that nodal
enlargement is reactive until malignancy has been ruled
out, and pathological correlation is warranted if clinical
suspicion is present.
In conclusion, our review showed a wide spectrum of
breast manifestations and mostly nonspecific imaging
findings that are primary or secondary to SLE. It is
important for clinicians and radiologists to be aware
of these SLE-related breast manifestations as they may
have an impact on the management plan. No recognised
imaging features distinguishing between lupus mastitis
and breast malignancy have been identified, and
pathological correlation is advocated in cases where
suspicious imaging features are demonstrated.
REFERENCES
1. Mok CC, Lau CS. Pathogenesis of systemic lupus erythematosus. J Clin Pathol. 2003;56:481-90. Crossref
2. Murphy G, Isenberg D. Effect of gender on clinical presentation
in systemic lupus erythematosus. Rheumatology (Oxford).
2013;52:2108-15. Crossref
3. Macura KJ, Ouwerkerk R, Jacobs MA, Bluemke DA. Patterns of
enhancement on breast MR images: interpretation and imaging
pitfalls. Radiographics. 2006;26:1719-34. Crossref
4. Cho CC, Chu WC, Tang AP. Lupus Panniculitis of the breast—mammographic and sonographic features of a rare manifestation of systemic lupus. Hong Kong J Radiol. 2008;11:41-3.
5. Rosa M, Mohammadi A. Lupus mastitis: a review. Ann Diagn Pathol. 2013;17:230-3. Crossref
6. Georgian-Smith D, Lawton TJ, Moe RE, Couser WG. Lupus mastitis: radiologic and pathologic features. AJR Am J Roentgenol.
2002;178:1233-5. Crossref
7. Voizard B, Lalonde L, Sanchez LM, Richard-Chesnay J, David J, Labelle M, et al. Lupus mastitis as a first manifestation of systemic disease: about two cases with a review of the literature. Eur J Radiol. 2017;92:124-31. Crossref
8. De Bandt M, Meyer O, Grossin M, Kahn MF. Lupus mastitis
heralding systemic lupus erythematosus with antiphospholipid
syndrome. J Rheumatol. 1993;20:1217-20.
9. Cao MM, Hoyt AC, Bassett LW. Mammographic signs of systemic
disease. Radiographics. 2011;31:1085-100. Crossref
10. Mosier AD, Boldt B, Keylock J, Smith DV, Graham J. Serial MR
findings and comprehensive review of bilateral lupus mastitis with
an additional case report. J Radiol Case Rep. 2013;7:48-58. Crossref
11. Lee SJ, Saidian L, Wahab RA, Khan S, Mahoney MC. The evolving
imaging features of lupus mastitis. Breast J. 2019;25:753-4. Crossref
12. Thapa A, Parakh A, Arora J, Goel RK. Lupus mastitis of the male
breast. BJR Case Rep. 2015;2:20150290. Crossref
13. Sabaté JM, Gómez A, Torrubia S, Salinas T, Clotet M, Lerma E.
Lupus panniculitis involving the breast. Eur Radiol. 2006;16:53-6. Crossref
14. Pinho MC, Souza F, Endo E, Chala LF, Carvalho FM, de Barros N.
Nonnecrotizing systemic granulomatous panniculitis involving the
breast: imaging correlation of a breast cancer mimicker. AJR Am
J Roentgenol. 2007;188:1573-6. Crossref
15. Song L, Wang Y, Zhang J, Song N, Xu X, Lu Y. The risks of
cancer development in systemic lupus erythematosus (SLE)
patients: a systematic review and meta-analysis. Arthritis Res Ther.
2018;20:270. Crossref
16. Rezaieyazdi Z, Tabaei S, Ravanshad Y, Akhtari J, Mehrad-Majd H.
No association between the risk of breast cancer and systemic lupus
erythematosus: evidence from a meta-analysis. Clin Rheumatol.
2018;37:1511-9. Crossref
17. Łukasiewicz E, Ziemiecka A, Jakubowski W, Vojinovic J,
Bogucevska M, Dobruch-Sobczak K. Fine-needle versus core-needle
biopsy—which one to choose in preoperative assessment
of focal lesions in the breasts? Literature review. J Ultrason.
2017;17:267-74. Crossref
18. Yiu KH, Wang S, Mok MY, Ooi GC, Khong PL, Mak KF, et al.
Pattern of arterial calcification in patients with systemic lupus
erythematosus. J Rheumatol. 2009;36:2212-7. Crossref
19. Lalani TA, Kanne JP, Hatfield GA, Chen P. Imaging findings in
systemic lupus erythematosus. Radiographics. 2004;24:1069-86. Crossref
20. Amano M, Shimizu T. Mondor’s disease: a review of the literature. Intern Med. 2018;57:2607-12. Crossref
21. Raviv B, Israelit SH. Mondor’s disease of the chest wall—a forgotten
cause of chest pain: clinical approach and treatment. J Gen Pract.
2014;2:3. Available from: https://www.hilarispublisher.com/open-access/
mondors-disease-of-the-chest-wall-a-forgotten-cause-of-chest-pain-clinical-approach-and-treatment-2329-9126.1000157.pdf. Accessed 29 Oct 2019.
22. Crisan D, Badea R, Crisan M. Thrombophlebitis of the lateral
chest wall (Mondor’s disease). Indian J Dermatol Venereol Leprol.
2014;80:96. Crossref
23. Dilaveri C, Mac Bride MB, Sandhu NP, Neal L, Ghosh K,
Wahner-Roedler DL. Breast manifestations of systemic diseases.
Int J Womens Health. 2012;4:35-43. Crossref
24. Shetty MK, Watson AB. Mondor’s disease of the breast:
sonographic and mammographic findings. AJR Am J Roentgenol. 2001;177:893-6. Crossref
25. Kwak JY, Kim EK, Chung SY, You JK, Oh KK, Lee YH, et al.
Unilateral breast edema: spectrum of etiologies and imaging
appearances. Yonsei Med J. 2005;46:1-7. Crossref
26. Shapira Y, Weinberger A, Wysenbeek AJ. Lymphadenopathy in systemic lupus erythematosus. Prevalence and relation to disease
manifestations. Clin Rheumatol. 1996;15:335-8. Crossref
27. Melikoglu MA, Melikoglu M. The clinical importance of lymphadenopathy in systemic lupus erythematosus. Acta Reumatol
Port. 2008;33:402-6. Crossref