Factors Affecting Accuracy of Stereotactic Radioisotope-guided Occult Lesion Localisation for Breast Lesions
ORIGINAL ARTICLE
Factors Affecting Accuracy of Stereotactic Radioisotope-guided Occult Lesion Localisation for Breast Lesions
WL Wong1, LKM Wong2, EPY Fung2, KM Kwok2, WS Mak2, HS Lam2
1 Department of Radiology and Organ Imaging, United Christian Hospital, Hong Kong
2 Department of Diagnostic and Interventional Radiology, Kwong Wah Hospital, Hong Kong
Correspondence: Dr WL Wong, Department of Radiology and Organ Imaging, United Christian Hospital, Hong Kong. Email: jesswong723@gmail.com
Submitted: 28 Aug 2019; Accepted: 11 Nov 2019.
Contributors: All authors designed the study. WLW, LKMW, EPYF, KMK, and WSM were responsible for acquisition of data. All authors
contributed to the analysis of data. WLW wrote the manuscript. All authors made critical revisions of the intellectual content of this article.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: The study was approved by the Hospital Authority of Hong Kong Kowloon Central / Kowloon East Research Ethics Committee (Ref: KC/KE-18-0290/ER-1). The need for patient consent was waived by the ethics committee.
Abstract
Objectives
To assess the surgical success rate of stereotactic radioisotope-guided occult lesion localisation (ROLL)
and to identify factors affecting its accuracy.
Methods
We retrospectively identified all stereotactic ROLL procedures from June 2017 to August 2018 at a
regional hospital in Hong Kong. Demographic data, imaging results, previous biopsy records, surgical records, and
pathology results were reviewed. Independent-sample t tests and Fisher’s exact test were used to assess the association
of multiple factors (including age, breast thickness, depth of lesion, type of target, approach direction, pathology,
operator experience) with localisation accuracy using a 5-mm deviation between the centres of the mammographic
targets and the scintigraphic image as the threshold.
Results
A total of 77 ROLL procedures were identified. Of them, 55 were localisations of nonpalpable lesions and
22 were combined radioisotope-guided sentinel node and occult breast lesion localisations. The overall surgical
success rate for removal of the target lesion was 85.7%, and for excision of malignant nonpalpable breast lesions
with clear margins the success rate was 83.9%. Specimen mammogram and scintigraphic images were available
in 68 cases for subsequent analysis for factors affecting localisation accuracy. A preoperative diagnosis of invasive
carcinoma was associated with poorer target localisation (p = 0.015). Injection of radioisotope via a lateromedial
direction was associated with better target localisation (p = 0.044).
Conclusion
Stereotactic ROLL is effective in localising nonpalpable breast lesions with a high surgical success rate.
There is a significant association between invasive carcinoma with worse localisation. Injection of radioisotope in lateromedial directions is associated with better localisation accuracy.
Key Words: Breast neoplasms; Radioisotopes
中文摘要
影響立體定向放射性同位素引導乳腺隱匿性病變定位準確性的因素
黃慧琳、黃嘉敏、馮寶恩、郭勁明、麥詠詩、林漢城
目的
回顧研究2017年6月至2018年8月期間所有在一所香港地區醫院接受ROLL的患者。
方法
評估ROLL的手術成功率及研究與其定位準確度有關的因素。回顧了人口統計學數據、影像學結果、活檢記錄、手術記錄和病理結果。使用乳房X線照相目標和核素閃爍圖像的中心5毫米偏差作為閾值,通過獨立樣本t檢驗和 Fisher精確檢驗用於評估多個因素(包括年齡、乳房厚度、病變深度、病變類型、手術路徑、病理、操作者經驗)與定位精度的關聯性。
結果
共包括77個ROLL程序。當中,55個是不可觸及病變的定位,22個是結合放射性同位素引導的前哨淋巴結和隱匿性乳腺病變定位。切除目標病灶的總體手術成功率為85.7%,切除邊緣清晰的不可觸及的惡性乳腺病灶的成功率為83.9%。68個病例的樣本乳房X線照片和核素閃爍掃描圖像可供後續分析影響定位精度的因素。浸潤性癌的術前診斷與較差的目標定位相關(p = 0.015)。通過側內側方向注射放射性同位素與更好的目標定位相關(p = 0.044)。
結論
立體定向ROLL可有效定位不可觸及的乳房病變,手術成功率高。浸潤性癌與更差的定位之間存在顯著關聯。在側內側方向注射放射性同位素與更好的定位精度相關。
INTRODUCTION
Breast cancer is the leading cancer affecting women in
Hong Kong, accounting for 26.6% of all new cancer cases
among women in 2016, and has been increasing over the
past decade.[1] As public awareness and the acceptance
of screening for breast cancer are growing, many
breast cancers are detected at a nonpalpable stage. For
nonpalpable breast lesions such as microcalcifications,
distortion, or asymmetric densities detected on screening
mammogram and classified in the Breast Imaging
Reporting and Data System as category ≥4, they are
first evaluated with stereotactic guided percutaneous
core biopsy or vacuum-assisted biopsy. If these lesions
turn out to be malignant or of high risk pathologically,
therapeutic or diagnostic surgical excision is warranted.
Localisation of nonpalpable lesions is required to
facilitate surgery.
Hookwire localisation has long been the gold standard in
preoperative localisation of nonpalpable breast lesions.[2] [3]
However, the incidence of margin-positive excision can
be as high as 47%.[4] There are also risks of inaccurate
positioning and wire displacement after positioning.
The first radioisotope-guided occult lesion localisations (ROLL) procedure was performed in 1996.[5] During
the procedure, the nonpalpable lesion was marked by
intratumoural injection of 99mtechnetium (99mTc) labelled
macroaggregate albumin or sulphur colloid under
imaging guidance. The nonpalpable breast lesions were
then localised by a handheld intraoperative gamma
probe to facilitate surgical excision. Multiple studies
have shown that it is a safe and effective procedure
with less radiation dose to the patient as compared with
hookwire localisation technique, since no postprocedural
mammogram is required; yet good margin clearance
rate of 75% to 100% can be achieved.[6] Intraoperative
localisation time for the target is also significantly
shorter for radioisotope-guided localisation than that
for hookwire localisation.[6] Radioisotope-guided
localisation also offers an additional benefit of localising
sentinel lymph nodes, during the same procedure by
injecting 99mTc labelled filtered sulphur colloid (<22 μm)
instead.[4]
The aims of the present study were to assess the
technical success rate of localisation and to investigate
factors affecting the accuracy of ROLL in localisation of
nonpalpable breast lesions.
METHODS
All patients who underwent stereotactic ROLL from
1 June 2017 to 31 August 2018 in the Diagnostic and
Interventional Radiology Department of Kwong Wah
Hospital, a regional hospital in Hong Kong, were
included. Patients were identified and patient data
extracted from the Radiology Information System and
hospital electronic records.
Relevant information such as demographic data, previous
mammogram and imaging reports (experience of the
operator, categories of Breast Imaging Reporting and
Data System, type and location of mammographic target,
injection approach, breast thickness, depth of the target)
and previous biopsy and surgical specimen pathology
results were recorded. Clinical notes were reviewed
for any occurrences of further excision within the same
session, or a second operation. ‘Surgical success’ was
defined as successful removal of the lesion for high-risk
lesions and successful removal with clear margins in the
first operative session for malignant lesions.
In our centre, stereotactic ROLL was performed by
injection of approximately 0.2 mL of saline containing
0.5 mCi (18.5 MBq) 99mTc sulphur colloid using a 22G
spinal needle on a prone table mammographic machine.
For patients with biopsy-proven invasive carcinoma
and high-grade ductal carcinoma in situ (DCIS), 99mTc
labelled filtered sulphur colloid was used for additional
localisation of sentinel nodes. A column of gas was
injected before release of the breast compression and
removal of the needle to push the residual tracer from
the needle into the lesion. An anterior planar image of the patient’s chest and upper abdomen was acquired
30 minutes post-injection to confirm adequate
radioactivity at the injection site and to identify the
sentinel lymph nodes in sentinel node localisation
cases. The patients were operated on 4 to 6 hours after
injection. Surgical excision was guided within the breast
using a handheld gamma probe. Following excision,
the surgical bed was checked for residual radioactivity.
The specimen(s) would then be sent to our department;
specimen radiography and scintigraphic images were
obtained. Further surgical exploration was needed if
residual activity remained high in the breast or if the
specimen radiograph/scintigraphic image suggested
incomplete excision. Cases with no specimen radiographs
or scintigraphic images were excluded for subsequent
analysis on factors affecting localisation accuracy.
To quantify localisation accuracy, specimen radiographs
and scintigraphic images were reviewed to assess the
distance between the centre of the mammographic target
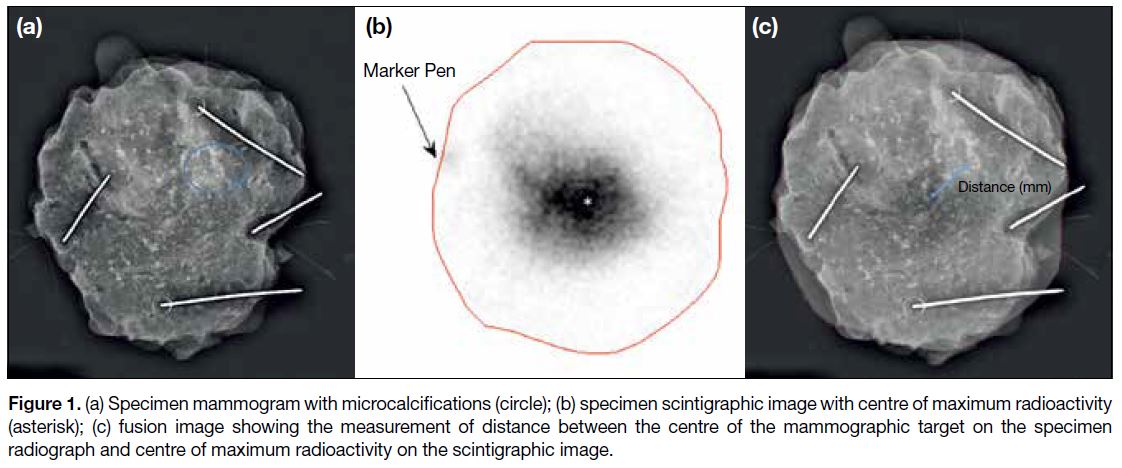
and the centre of maximum radioactivity (Figure 1).
Patients with ≤5 mm between the centres were
categorised as ‘good’, those with 5 to 15 mm between the
centres were categorised as ‘fair’, and those with ≥15 mm
between the centres were categorised as ‘suboptimal’.
The patient demographics, imaging results, and
pathological information were compared among these
groups to identify factors affecting localisation accuracy.
Statistical analysis was performed with SPSS (Windows
version 23.0; IBM Corp, Armonk [NY], United States).
Age, breast thickness, and depth of target were compared
with independent t tests. Pathology, injection approach,
appearance on the 30-minute scintigraphic and specimen images, nature of mammographic target, and experience
of operator were compared with Fisher’s exact test. A
p value of <0.05 was taken as statistically significant.
Figure 1. (a) Specimen mammogram with microcalcifications (circle); (b) specimen scintigraphic image with centre of maximum radioactivity
(asterisk); (c) fusion image showing the measurement of distance between the centre of the mammographic target on the specimen
radiograph and centre of maximum radioactivity on the scintigraphic image.
RESULTS
During the study period, a total of 77 stereotactic
ROLL procedures were performed in 77 patients. Table 1 shows the demographics and pathologies. The
dose of radioisotope injected ranged from 0.16 mCi
to 0.44 mCi (5.9 MBq to 16.3 MBq) (mean=0.34 mCi/12.6 MBq). The mammographic targets were
microcalcifications in 59 cases, focal asymmetry in four
cases, and metallic markers in 10 cases. In four patients,
the microcalcifications were too faint after biopsy and
difficult to be visualised on preprocedural mammogram
on the day of the procedure. The geometric coordinates
from their previous stereotactic guided biopsy were used
to assist in localisation of the lesion. Evidence of previous
biopsy and concordant pathology were identified in the
surgical specimens of these cases.
Table 1. Demographics of the patient undergoing the localisation procedure (n = 77).
Additional excision within the same operation session
after review of specimen radiographs was performed
in 37 patients (48%). Of the additional excisions, 29
(78.4%) were required because of narrow resection
margin around the mammographic target or the presence
of scattered microcalcifications near the resection
margin.
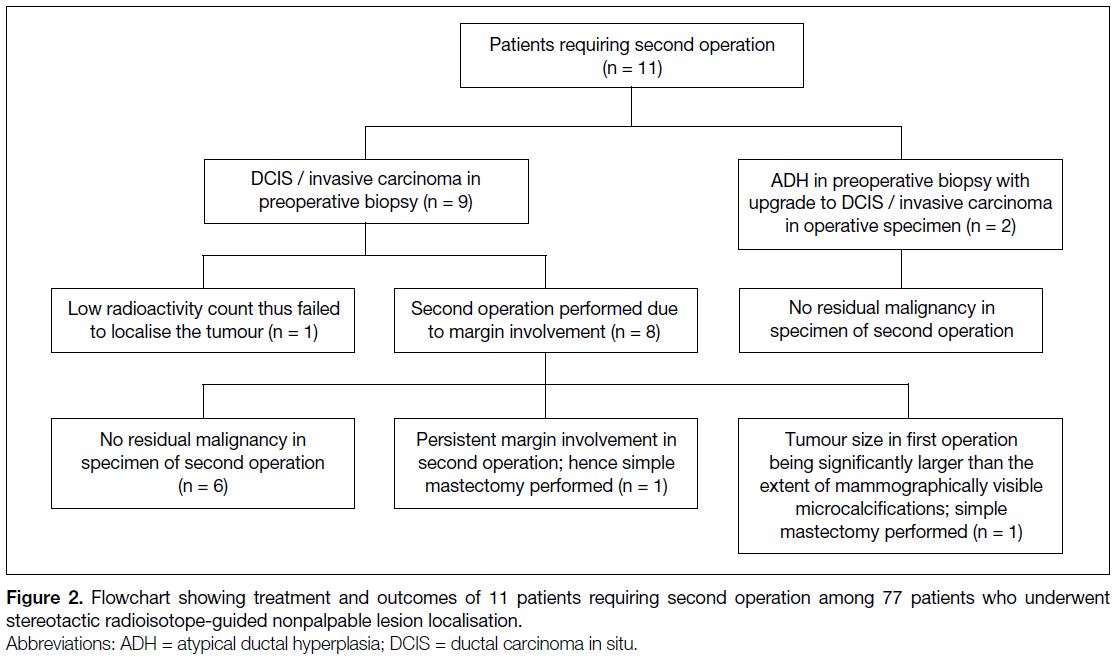
Among the 77 cases, 11 (14.3%) required a second
operation (Figure 2). Two of them had a preoperative
diagnosis of atypical ductal hyperplasia but were
subsequently upgraded to DCIS and invasive ductal
carcinoma on the basis of the pathology in the surgical specimens. The other nine cases with preoperatively
diagnosed DCIS or invasive carcinoma required second
operations. Eight of these nine patients were reoperated
for margin involvement. In the remaining one patient,
30-minute post-injection scintigraphy showed faint
radioactivity; excision was performed according to
the location with maximum radioactivity but failed
to remove the tumour. Among these 11 patients with
second operations, eight of them had had re-excision in
the first operation.
Figure 2. Flowchart showing treatment and outcomes of 11 patients requiring second operation among 77 patients who underwent stereotactic radioisotope-guided nonpalpable lesion localisation.
Among the 77 cases, scintigraphic images were not
available in five cases and specimen mammograms
were unavailable in four cases; a total of 68 cases
remained for analysis for factors affecting localisation
accuracy. Of them, the distance between the centre of
the mammographic target and the centre of maximum
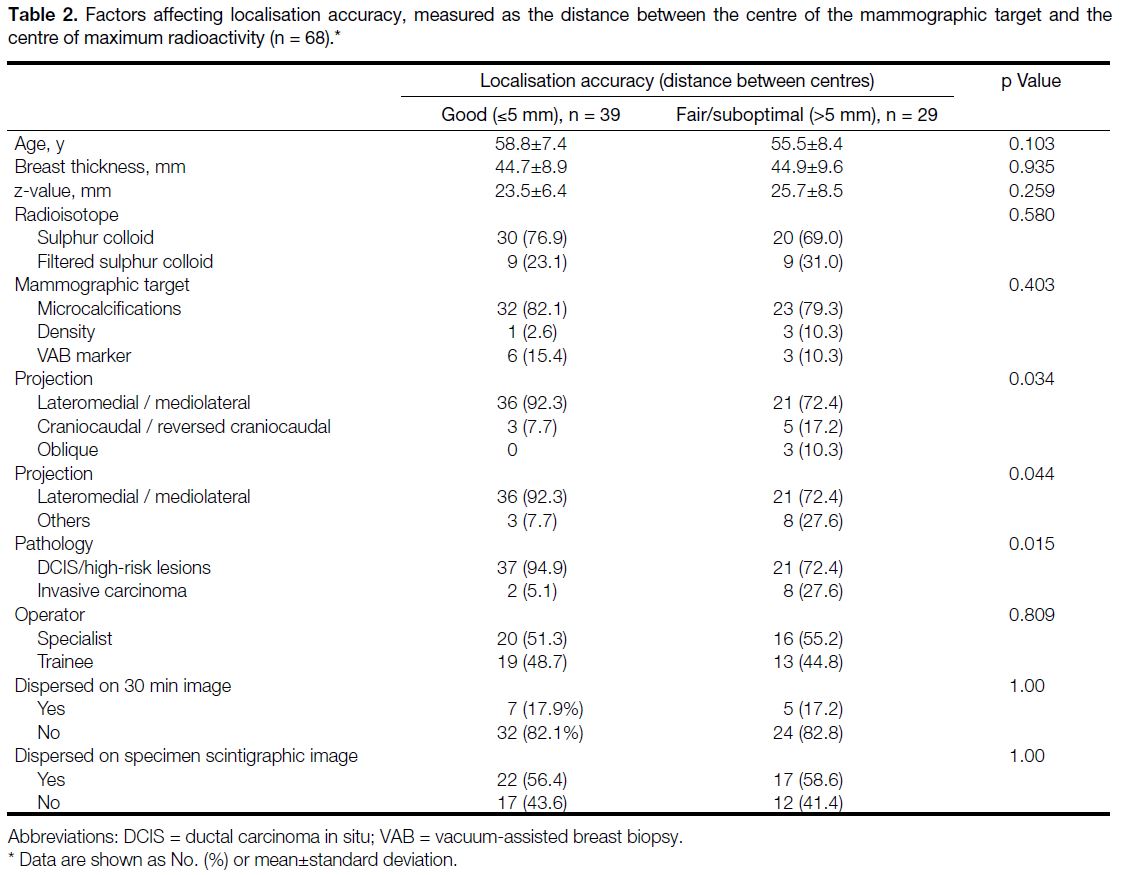
radioactivity was ≤5 mm in 39 (57.4%) patients and
>5 mm in 29 (42.6%) patients. Of the cases, 39 (57.4%)
were categorised as good, 17 (25.0%) as fair, and
12 (17.6%) as suboptimal. There were no significant
differences in demographics between the patients of
the good target and fair/suboptimal groups. Inaccurate
lesion localisation was more frequently observed in
patients with invasive cancer than in patients with DCIS
or high-risk breast lesions significance (p = 0.015).
There was a significant difference in the approach used
for performing the injection of radioisotope between
the two groups (p = 0.034). Significantly better lesion
localisation was achieved by approaching the lesion
in the lateromedial direction (i.e., in lateromedial or
mediolateral projections) than in the craniocaudal (i.e.,
craniocaudal or reversed craniocaudal projections) or
lateromedial oblique directions (p = 0.044).
The mammographic targets used for localisation,
being vacuum-assisted breast biopsy markers,
microcalcifications, or abnormal densities, did not
affect the accuracy in localisation significantly. Whether
the injection site appeared dispersed on the 30-minute
scintigraphic image or on the specimen scintigraphic
image did not cause significant difference. There was
no statistically significant difference in the experience of
the operators in the two groups. Detailed figures of the
comparison of the parameters are shown in Table 2.
Table 2. Factors affecting localisation accuracy, measured as the distance between the centre of the mammographic target and the centre of maximum radioactivity (n = 68).
DISCUSSION
Use of radioisotopes is a safe and effective method to
localise nonpalpable breast lesions. In terms of radiation
safety, Cremonesi et al[7] calculated the effective dose
of radiation involved in 99mTc-guided localisation to be 100 to 200 times less than the radiation exposure
from the additional mammograms needed for hookwire
localisation.[6] [7] Rampaul et al[8] also demonstrated that the
hand dose to breast surgeons and radiologists is also
minimal when compared with the annual dose limit
even if 100 radioisotope-guided localisation procedures
were to be performed per year. A prior study conducted
in our centre demonstrated that radioisotope-guided
localisation is as good as hookwire localisation in terms
of specimen margin clearance and need for second
operation with a shorter procedural time.[6] There is also
the additional benefit of localisation of sentinel nodes in
the same procedure when filtered 99mTc sulphur colloid is
used. In another local study, a high surgical success rate
has been demonstrated.[9] In the present study, the surgical
success rate for malignant lesions with adequate margins
achieved by the first operation was 83.9% (47/56), which
is comparable to the previous study performed by our
centre as well as in other local and international studies,
which ranged from 75% to 100%.[6] Of nine malignant
cases with margin involvement in the present study,
seven (78%) had their mammographic targets well
localised in the first operative session. In one of the
cases requiring reoperation, the tumour margin was
focally involved in the first operation and no residual
malignancy was identified in the surgical specimen of
subsequent operation. In the remaining case, tumour
extended beyond the visualised microcalcifications upon
review of the pathology report, specimen radiograph,
and scintigraphic images.
Accurate positioning of the localisation device is a
key element for successful localisation of nonpalpable
breast lesions. Stereotactically guided placement of
localisation devices is often complicated by the effect of
breast compression. With the problem of the accordion
effect, even minimal deviation of the injection site
from the target could be augmented when the breast
compression is released.[10] In the present study, we have
identified that injection along the lateromedial aspect
could achieve significantly better localisation than along
the craniocaudal or lateromedial oblique aspects. We
postulated that it could be due to the relative difficulty in
achieving the same positioning of the breast as in previous
biopsy sessions due to a higher tendency of the breast
to roll in craniocaudal or oblique image acquisitions
that require the breast and ipsilateral arm to be extended
through the opening in the table (‘arm-through-the-hole’
technique). Rolling of the breast is commonly
be observed on craniocaudal view,[11] and may result in
deviation of the injected radioisotope in an unpredictable direction upon release of compression due to the
accordion effect. Based on our findings, in the cases
where the mammographic targets were visible on both
craniocaudal and mediolateral views, the lateromedial
approach was more accurate.
Posterior and deep targets usually require injection via an
oblique projection using the ‘arm-through-the-hole’ (or
‘drop-shoulder’) technique.[12] A prior study on stereotactic
guided breast biopsy has shown that successful retrieval
of posteriorly located microcalcifications <15 mm from
the pectoralis muscle was better achieved by a digital
add-on unit (erect table) mammographic machine than
on a prone table due to better resolution, especially when
the microcalcifications were small or poorly delineated.[13]
This suggests that radioisotope-guided localisation of
such deep lesions would better be achieved by add-on
unit on an erect table. However, this practice would be
subjected to availability of the required equipment and further research on this area should be performed.
Our study demonstrated that invasive carcinoma was
associated with worse localisation than DCIS and high-risk
lesions, such as atypical ductal hyperplasia and
papillary lesions. Loss of myoepithelial cells surrounding
tumour cells is the hallmark of invasive breast carcinoma.
Upon degradation of the basement membrane,
desmoplasia with associated recruitment of fibroblasts,
inflammatory cells and angiogenesis occur, causing the
tumour to become fibrotic and hard in texture.[14] [15] This
can lead to difficulty in injection, unintended spread of
the injected radioisotope, or spillage of the radioisotope.[16]
Previous studies have recommended peritumoural rather
than intratumoural injection if significant resistance is
encountered.[17] Others have suggested modification of
the procedure, using Luer Lock syringes for injection of
the radioisotope to prevent disconnection of syringe and
needle during injection.[16]
We identified four cases in which the biopsied
microcalcifications were faintly seen on the prone
table on the day of the localisation procedure. The scar
position, projection, and coordinates from previous
stereotactically guided biopsies; breast compression
thickness; and the depth of the lesion were referred to
for injection of radioisotope and resulted in successful
radioisotope-guided excision. In order to aid future
localisation, when residual microcalcifications are too
faint or difficult to be localised, or if near-total removal
is anticipated, we suggest that a marker should be
placed at the biopsy site at the end of core needle biopsy
procedures.
Advances have been made in interventional procedures
for breast diseases in recent years. A prone stereotactic
biopsy system with both two-dimensional and three-dimensional
tomosynthesis breast imaging has been
introduced. It utilises the same detector as its diagnostic
counterpart with a wider field of view compared with
the former two-dimensional stereotactic prone table; and
hence better detection and localisation of subtle lesions,
e.g., faint calcifications are to be expected. At the same
time, the advantage of a prone table approach with better
patient comfort compared with erect tomosynthesis
biopsy systems can be maintained.[18] Accessories for
performing hookwire insertion or radioisotope-guided
localisation procedures are available for the prone table
with tomosynthesis.
Because our centre is also used for training, ROLL
procedures are performed by radiologists with varying
amounts of experience (breast radiologists in-training to
specialists with over 20 years of experience). Although
the success rate is well maintained, the present study
demonstrated no statistically significant difference in
the success rate in good targeting between trainees and
specialists. This could be attributed to the dedicated
training and supervision, as well as the experience of the
surgeons. We suggest that the ROLL technique can be
acquired in a relatively short period of time and should
be widely adopted.
There are a few limitations to the present study. Because it was a single-centre retrospective study, measurements of
the original lesion size and location could not be retrieved
for the cases referred from outside facilities because the
images were not available. The relative perceptibility of
the target on craniocaudal and mediolateral projections
on the prone table could not be assessed. Some factors
such as the size and weight of the operative specimen, and the intraoperative counts were not mentioned in the
operative record to control the comparison.
CONCLUSION
Stereotactic ROLL is an effective method for localising
nonpalpable breast lesions for surgical excision with
a high surgical success rate. There is a significant
association between invasive carcinoma with worse
localisation. Injection of radioisotope in lateromedial
directions is associated with better localisation accuracy
and we would suggest injection of the radioisotope in
this direction if technically feasible.
REFERENCES
1. Hong Kong Cancer Registry, Hospital Authority, Hong Kong
SAR Government. Overview of Hong Kong cancer statistics of
2016. Available from: https://www3.ha.org.hk/cancereg/pdf/overview/Overview%20of%20HK%20Cancer.... Accessed 10 Feb 2019.
2. Perry NM, EUSOMA Working Party. Quality assurance in the
diagnosis of breast disease. EUSOMA Working Party. Eur J Cancer.
2001;37:159-72. Crossref
3. Lovrics PJ, Cornacchi SD, Vora R, Goldsmith CH, Kahnamoui K.
Systematic review of radioguided surgery for non-palpable breast
cancer. Eur J Surg Oncol. 2011;37:388-97. Crossref
4. Jha D, Deo SV, Malhotra MS. Radioguided occult lesion
localization and sentinel node and occult lesion localization in
breast cancer: The future beckons. Asian J Oncol. 2015;1:73-6. Crossref
5. Luini A, Zurrida S, Galimberti V, Paganelli G. Radioguided surgery
of occult breast lesions. Eur J Cancer. 1998;34:204-5.
6. Chu TY, Lui CY, Hung WK, Kei SK, Choi CL, Lam HS. Localisation of occult breast lesion: a comparative analysis
of hookwire and radioguided procedures. Hong Kong Med J.
2010;16:367-72.
7. Cremonesi M, Ferrari M, Sacco E, Rossi A, De Cicco C, Leonardi L,
et al. Radiation protection in radioguided surgery of breast cancer.
Nucl Med Commun. 1999;20:919-24. Crossref
8. Rampaul RS, Dudley NJ, Thompson JZ, Burrell H, Evans AJ,
Wilson AR, et al. Radioisotope for occult lesion localisation
(ROLL) of the breast does not require extra radiation protection
procedures. Breast. 2003;12:150-2. Crossref
9. Au AK, Wan AY, Leung BS, Lo SS, Wong WW, Khoo JL. Efficacy
of radioguided occult lesion localisation: how well are we doing?
Hong Kong J Radiol. 2016;19:269-78. Crossref
10. Esserman LE, Cura MA, DaCosta D. Recognizing pitfalls in early
and late migration of clip markers after imaging-guided directional
vacuum-assisted biopsy. Radiographics. 2004;24:147-56. Crossref
11. Popli MB, Teotia R, Narang M, Krishna H. Breast positioning
during mammography: mistakes to be avoided. Breast Cancer
(Auckl). 2014;8:119-24. Crossref
12. Chesebro AL, Chikarmane SA, Ritner JA, Birdwell RL, Giess CS.
Troubleshooting to overcome technical challenges in image-guided
breast biopsy. Radiographics. 2017;37:705-18. Crossref
13. Lee CY, Wan WS, Lui CY. Stereotactic-guided biopsy of
mammographic microcalcifications: when shall we use digital
add-on unit instead of prone table machine? Hong Kong J Radiol.
2014;17:152-61. Crossref
14. Khamis ZI, Sahab ZJ, Sang QX. Active roles of tumor stroma in
breast cancer metastasis. Int J Breast Cancer. 2012;2012:574025. Crossref
15. Ellis IO, Le AH, Pinder SE, Rakha EA. Tumors of the breast. In: Fletcher CD. Diagnostic Histopathology of Tumors. 4th ed. New York: Churchill Livingstone; 2013. p 1057-145.
16. Landman J, Kulawansa S, McCarthy M, Troedson R, Phillips M,
Tinning J, et al. Radioguided localisation of impalpable breast
lesions using 99m-Technetium macroaggregated albumin: Lessons
learnt during introduction of a new technique to guide preoperative
localisation. J Med Radiat Sci. 2015;62:6-14. Crossref
17. De Cicco C, Pizzamiglio M, Trifirò G, Luini A, Ferrari M, Prisco G,
et al. Radioguided occult lesion localisation (ROLL) and surgical
biopsy in breast cancer. Technical aspects. Q J Nucl Med.
2002;46:145-51.
18. Shin K, Teichgraeber D, Martaindale S, Whitman GJ. Tomosynthesis-guided core biopsy of the breast: why and how to
use it. J Clin Imaging Sci. 2018;8:28. Crossref