Tomosynthesis-guided Vacuum-assisted Breast Biopsy of Sonographically Occult Non-calcified Breast Lesions Detected on Tomosynthesis
ORIGINAL ARTICLE
Tomosynthesis-guided Vacuum-assisted Breast Biopsy of Sonographically Occult Non-calcified Breast Lesions Detected on Tomosynthesis
WY Fung1, EPY Fung2, KM Kwok2, SK Chan3, LKM Wong2, WS Mak2, DHY Cho2
1 Department of Radiology, Princess Margaret Hospital, Hong Kong
2 Department of Diagnostic and Interventional Radiology, Kwong Wah Hospital, Hong Kong
3 Department of Pathology, Kwong Wah Hospital, Hong Kong
Correspondence: Dr WY Fung, Department of Radiology, Princess Margaret Hospital, Hong Kong. Email: fwyyuk@gmail.com
Submitted: 16 Dec 2019; Accepted: 5 May 2020.
Contributors: WYF and EPYF designed the study. WYF acquired the data. All authors analysed the data. WYF draft the manuscript. EPYF, KMK, SKC, LKMW, WSM and DHYC critically revised the manuscript for important intellectual content.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics Approval: This study was approved by the Kowloon Central / Kowloon East Research Ethics Committee (Ref: KC/KE-21-0223/ER-2).
The requirement for patient consent was waived for this retrospective study.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Acknowledgements: We would like to express our grateful thanks to Well Women Clinic, Tung Wah Group of Hospitals for the support of our project.
Abstract
Objectives
To analyse the pathological results from tomosynthesis-guided vacuum-assisted breast biopsy (VAB) of tomosynthesis-detected sonographically occult non-calcified breast lesions.
Methods
We performed a retrospective review of patients who had undergone tomosynthesis-guided VAB from December 2017 to May 2019. Imaging findings and pathological outcome were evaluated. The technical success rate and complications of tomosynthesis-guided VAB were reviewed.
Results
In our centre, all sonographically occult non-calcified lesions detected on digital breast tomosynthesis (DBT)
with grade ≥4a or above according to Breast Imaging Reporting and Data System (BI-RADS) are selected for VAB
under tomosynthesis guidance. Among the 41 cases reviewed, sampling was successful in 40 (97.6%). Among the
40 cases with pathologies, three malignancies, 14 high-risk lesions and 23 benign lesions were identified. All three
malignancies in our study presented as architectural distortion, which was the main feature of the majority of DBTdetected
sonographically occult non-calcified breast lesions (n = 38, 95%); the remaining two had focal asymmetry
(n = 2, 5%). The positive predictive value for malignancy of architectural distortion detected on DBT only was 7.9%.
All reported complications were clinically insignificant haematomas (n = 7, 17.5%).
Conclusion
Tomosynthesis-guided VAB is a safe and effective method for evaluation of sonographically occult
lesions detected on DBT. The feature associated with the majority of these lesions was architectural distortion.
Key Words: Biopsy; Breast; Mammography
中文摘要
數位斷層合成攝影定位真空輔助乳房活檢對3D乳房X光檢測到的放射隱匿性非鈣化乳腺病灶
馮惠鈺、馮寶恩、郭勁明、陳紹騏、黃嘉敏、麥詠詩、曹慶恩
目的
分析數位斷層合成攝影定位真空輔助乳腺活檢(VAB)對數位斷層合成攝影檢測到的放射隱匿性非鈣化乳腺病灶的病理結果。
方法
回顧2017年12月至2019年5月期間接受數位斷層合成攝影定位VAB的患者的影像學結果和病理結果,以及數位斷層合成攝影定位VAB的技術成功率和併發症。
結果
對3D乳房X光(DBT)檢測到,屬於乳腺影像報告和數據系統(BI-RADS)4a級或以上的所有放射隱匿性非鈣化病灶進行數位斷層合成攝影定位VAB。在檢視的41宗病例中,40宗(97.6%)成功抽樣。40例病灶中,發現惡性腫瘤3例、高危病灶14例、良性病灶23例。3例惡性腫瘤均呈結構變形,是大部分DBT 檢測到的放射隱匿性非鈣化乳腺病灶的主要特徵(n = 38,95%);其餘2例為乳房局部不對稱(n = 2,5%)。僅在DBT上檢測到的結構變形惡性腫瘤的陽
性預測值為7.9%。所有患者報告的併發症都是臨床影響不顯著的血腫(n = 7,17.5%)。
結論
數位斷層合成攝影定位VAB能安全並有效評估DBT上檢測到的放射隱匿病灶,而大部分病灶均出現結構變形。
INTRODUCTIONS
Digital breast tomosynthesis (DBT) has been found
to have several advantages over planar digital
mammography; it reduces anatomic noise by reducing
tissue overlap.[1] It improves visualisation of subtle
abnormalities, including architectural distortion and
masses with spiculated margins.[1] [2] Investigations of
DBT have demonstrated its ability to reduce recall rates,
with higher sensitivity and specificity rates compared
with planar digital mammography.[3] [4] [5] [6] [7] [8] [9] With increased
use of DBT, management of DBT-detected lesions
becomes a new challenge. Among the DBT-detected
lesions, some are sonographically occult and therefore
cannot be biopsied under ultrasound guidance. Several
studies have concluded that tomosynthesis-guided
vacuum-assisted biopsy (VAB) is a safe and feasible
procedure, which allows further evaluation of these
lesions.[10] [11] [12] Rochat et al[13] suggested a difference in
pathology outcome of tomosynthesis-guided VAB
from stereotactic guided VAB that potentially results
in a change in managing these lesions. The objective
of our study was to analyse the pathological findings of
sonographically occult non-calcified lesions biopsied
with tomosynthesis-guided VAB. The technical success
rate and complications of tomosynthesis-guided VAB
were also evaluated.
METHODS
We performed a retrospective review of 41 consecutive
cases of patients that had undergone tomosynthesis-guided
VAB from December 2017 to May 2019 from
a single institution (Well Women Clinic, Tung Wah
Group of Hospitals).
Since the implementation of DBT in our institution,
patients have been offered either planar digital
mammography or DBT (Selenia Dimensions; Hologic,
Bedford [MA], United States). Between December 2017
and May 2019, a total of 16,382 DBTs were performed
in our centre. DBT imaging data were used to generate
standard craniocaudal and mediolateral oblique views.
Tomosynthesis slices and synthetic two-dimensional
(2D) views were then generated from the raw data for
reporting. All mammograms were reported by radiology
fellows according to the Breast Imaging Reporting and
Data System (BI-RADS) 5th edition.[14] Supplementary
ultrasound was performed for evaluation of masses,
asymmetry, and architectural distortion detected on
DBT.
Suspicious lesions were discussed in multidisciplinary
meetings for management such as timeframe of follow-up
or modality of biopsy. For the suspicious mass lesions detectable on ultrasound, we proceeded to ultrasound-guided
biopsy. For suspicious calcifications, we
proceeded to stereotactic mammography-guided biopsy.
Tomosynthesis-guided VAB would be performed only
on non-calcified lesions not readily seen on ultrasound, as
it is a self-financed item in our setting. Suspicious lesions
(categorised as BI-RADS ≥4a) without sonographic
correlation were selected for tomosynthesis-guided
VAB using an erect table system (Affirm Breast Biopsy
Guidance System; Hologic, Marlborough [MA], United
States).
Data Collection
Data on patients’ demographics, DBT, ultrasound studies
with reference to the BI-RADS, and pathology results
were analysed. Patients’ medical records including
clinical notes, radiology reports, procedural records,
surgical notes, and pathology reports were reviewed.
The pathological outcome and positive predictive value
(PPV) for malignancy were analysed. The technical
success rate and complications of tomosynthesis-guided
VAB were evaluated.
Biopsy Procedure and Postprocedural Management
Tomosynthesis-guided VAB was performed using a
9-gauge Eviva biopsy needle (Hologic) with an aperture
of 20 mm. All biopsies were performed by radiology
fellows after written informed consent was obtained.
DBT scout images were acquired to determine the three-dimensional
Cartesian coordinates of target lesions. The
user was able to scroll among the DBT sections where
the target was best seen to determine the Z coordinate
(i.e., distance from target to breast support platform).
A cursor was placed at the target in the selected section
to determine X-Y coordinates, which were then sent to
the biopsy system. Using sterile technique, a small skin
incision was made for needle insertion after application
of local anaesthesia. The 9-gauge needle was introduced
and its position was confirmed with pre-fire stereotactic
paired images. Multiple samples could be obtained by
rotating the biopsy needle in different directions without
needle reinsertion. Post-biopsy DBT images were
taken to confirm that lesions had been correctly and
sufficiently sampled. After lavage and aspiration of the
biopsy site, a biocompatible titanium marker (TriMark)
was deployed in all the cases. A post-marking DBT was
performed to confirm marker placement at the site of
original DBT-detected lesion. After biopsy and wound
care, patients would be given a pressure wrap bandage
and ice pack to apply to the biopsy site to minimise the chance of haematoma formation. All patients were
assessed clinically to identify possible complications
during or after procedures. Complications such as
vasovagal reaction and haematoma were recorded
in the standardised procedure report and checklist.
Patients’ clinical notes were reviewed for any delayed
complication such as infection. Clinically significant
complications were defined as complications that
required additional surgical or medical intervention
as a result of the biopsy.[15] Self-limited inflammation,
ecchymosis, or minor interstitial haemorrhage were not
considered as such.[15]
Pathological Outcomes
The pathological reports from tomosynthesis-guided
VABs, and the mammographic findings, were reviewed
for radiological-pathological concordance. For patients
who underwent surgical excision or other means of
biopsies, the pathological findings were compared with
the results from VAB. The PPV for malignancy was
calculated as the number of lesions with malignancies
from tomosynthesis-guided VAB divided by the
total number of lesions with biopsy performed. Any
histological upgrade of any VAB-obtained tissue at
subsequent surgical excision, e.g., ductal carcinoma in
situ (DCIS) from VAB upgraded to invasive carcinoma
at surgical excision, was recorded.
RESULTS
Technical Success
During the study period, there were 40 patients with
41 target lesions biopsied with tomosynthesis-guided
VAB (Table 1). In one of the 40 patients (2.5%),
two biopsy attempts were made because post-biopsy
mammography after the first attempt showed that the
biopsy site did not correspond to the site of architectural
distortion. The second attempt was successful. Biopsies
were successful in 40 out of 41 cases (97.6%). The failed
one was a posteriorly located lesion that was at the edge
of the compression paddle and not accessible by the
biopsy needle.
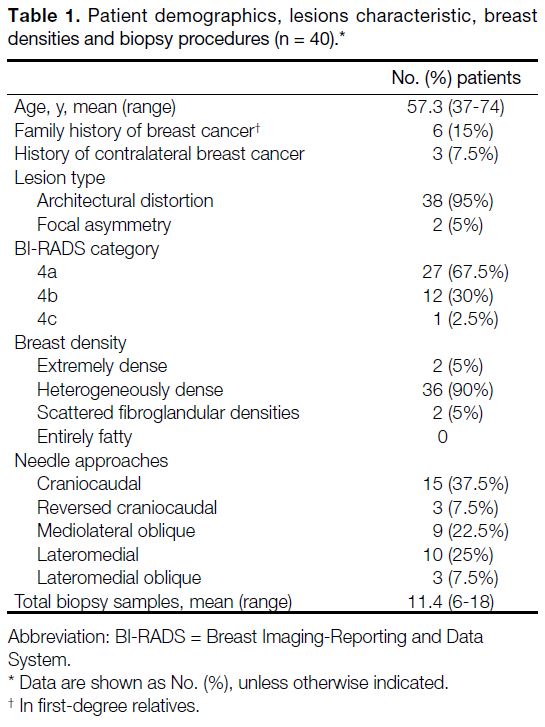
Table 1. Patient demographics, lesions characteristic, breast
densities and biopsy procedures (n = 40).
Complications
All the reported complications were minor. There were
seven cases of clinically insignificant haematoma (17.5%)
and no occurrences of vasovagal syncope. None of the
cases developed clinically significant complications that
required medical or surgical treatment.
Pathology Analysis
Pathological findings were recorded for 40 patients with 40 target lesions (Table 2). There were no histological
upgrades at surgical excision; the two DCIS were not
upstaged to invasive carcinoma.
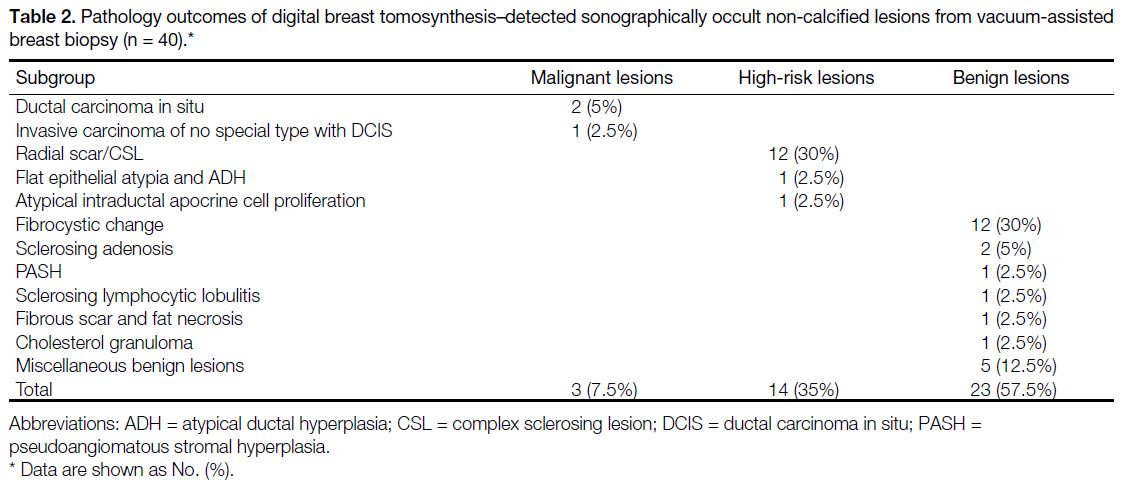
Table 2. Pathology outcomes of digital breast tomosynthesis–detected sonographically occult non-calcified lesions from vacuum-assisted breast biopsy (n = 40).
Among the architectural distortions (n = 38), three (7.9%) were malignant, 14 (36.8%) were high-risk, and
21 (55.3%) were benign. Both focal asymmetries (n = 2)
were benign. The PPV for malignancy of DBT-detected
sonographically occult non-calcified architectural
distortion was 7.9%. The PPV for malignancy of DBT-detected
sonographically occult focal asymmetries from
VAB was 0%.
One patient had undergone left mastectomy with
sentinel lymph node biopsy (Figure 1). The surgical
specimen showed a 25-mm invasive carcinoma of no
special type with DCIS, i.e., stage II disease (pT2, N0,
M0) without evidence of nodal and distant metastasis
(Figure 2).
Figure 1. Invasive carcinoma of no special type: a 69-year-old woman with a history of right breast cancer and right mastectomy presented
for screening mammography. (a) Left mediolateral oblique view generated from digital breast tomosynthesis (DBT) data with (b) a synthetic
C view reveal architectural distortion (arrows) in the upper outer quadrant of the left breast, which is less conspicuous on the craniocaudal
view (c). Tomosynthesis-guided vacuum-assisted biopsy was performed from a mediolateral (ML) approach. (d) DBT ML scout image
confirms the location of architectural distortion (arrow). (e) Pre-fire paired stereotactic images confirmed the needle placement. (f) Post-deployment
DBT confirmation marker (arrow) corresponds to the site of architectural distortion, suggesting a successful biopsy. (g)
Microscopic examination shows an invasive carcinoma and ductal carcinoma in situ of low nuclear grade (original magnification: 100×). (h)
The invasive carcinoma cells are arranged in cords dissecting through the fibrous stroma (original magnification: 200×).
Figure 2. Radial scar without atypia: a 42-year-old woman presented for screening mammography. (a) A right craniocaudal (CC) view
generated from digital breast tomosynthesis (DBT) data reveals an architectural distortion (arrow) in the central mid depth region of the right
breast, which was not visible on right mediolateral oblique view (b). Tomosynthesis-guided vacuum assisted biopsy was performed from
a CC approach. (c) Post-fire three-dimensional CC DBT image confirmed marker placement (arrow) corresponding to site of architectural
distortion, indicating successful biopsy of the target lesion. Surgical excision was not performed after discussion in multidisciplinary
meeting. (d) H<E-stained section showing angulated mammary ductules lined by a benign epithelium embedded in a fibroelastic stroma,
characteristic of a radial scar/complex sclerosing lesion (original magnification: 100×). Associated florid usual epithelial hyperplasia is shown
in (e) [original magnification: 100×]. (f) Follow-up DBT at 1 year demonstrated stability of the architectural distortion (arrow).
For the case with flat epithelial atypia with atypical
ductal hyperplasia, supplementary ultrasound
revealed two suspicious masses (BI-RADS grade 4a)
in the ipsilateral breast that did not correspond to
the architectural distortion. At 3 months after VAB,
ultrasound-guided biopsy of both of the masses revealed
DCIS. Lumpectomy of breast masses was performed.
The pathology of surgical specimen showed DCIS with
margin involvement. Second operation with mastectomy
showed complete removal of residual tumour. The
pathology of surgical specimen from second operation
also showed DCIS. In this case, the patient had concurrent
malignancy from masses detected incidentally from
the supplementary ultrasound. It was not counted as a
malignant upgrade because the masses did not correspond
to the architectural distortion. Therefore, the malignant
upgrade from high-risk lesion in our study is 0%.
After multidisciplinary discussion, it was decided that
surgical excision was not required for other high-risk
lesions if they were removed during VAB. All the
benign lesions were considered as concordant. Follow-up
mammography was suggested for all benign and
high-risk lesions to ensure stability and benignity.
The follow-up period after biopsies ranged from 4 to
21 months (mean, 10.7 months).
DISCUSSION
Architectural distortion is the most common DBT-only finding,[16] and the most commonly missed as interval
cancer in planar digital mammography.[17] It is well
established that there has been an increase in the detection
of architectural distortion with the advent of DBT,[18] [19] [20]
thus increasing the cancer detection rate. Similar to the
rest of the Asian population, most of the cases in our
study had high breast density. In a recent meta-analysis,
Phi et al[21] showed that DBT significantly increases the
cancer detection rate in dense breasts.
The purpose of the present study was to analyse the performance of tomosynthesis-guided VAB and
pathological outcome for DBT-detected sonographically
occult non-calcified lesions. The present study highlights
that, after calcifications, the second most common
finding in DBT-detected sonographically occult lesions
was architectural distortion. A total of 44.7% instances
of architectural distortion were found to be malignancy
and high-risk lesions. The PPV for malignancy of
DBT-detected sonographically occult non-calcified
architectural distortion from tomosynthesis-guided VAB
was 7.9%. Several studies have shown that architectural
distortion is much less likely to represent malignancy if
detected only on DBT,[22] is sonographically occult,[23] [24]
or is detected in a screening population.23 Recent
studies have shown similar PPV for DBT-only detected
sonographically occult architectural distortion ranges
from 7.7% to 26%,[22] [24] [25] [26] [27] which are not low enough to
forgo biopsy. Therefore, histological correlations are
warranted for these lesions. In the present study, we
achieved a high technical success rate with tomosynthesis-guided
VAB without any reported clinically significant
complications.
Radial scars/complex sclerosing lesions (n = 12,
31.6%) are one of the most common non-malignant
findings in our study. Bahl et al[19] showed that radial
scars are more commonly found with DBT. Among
architectural distortions detected on DBT in our study,
about one-third of cases were radial scars. In a recent
meta-analysis, Farshid and Buckley[28] found that the
upgrade rate of radial scars without atypia from VAB
by 8- to 11-gauge needles (1%) was much lower than
from core-needle biopsy by 14-gauge needle (5%).
Due to the low malignant upgrade rate of radial scars/complex sclerosing lesions removed with VAB, there
has been a shift in management of these lesions towards
close surveillance instead of surgical excision.[29] [30] This
is why the radial scars/complex sclerosing lesions in this
study were not subjected to further surgical excision
(Figure 2).
There are several advantages of tomosynthesis-guided
biopsy, which can overcome some of the technical
difficulties of 2D-guided biopsy. There is a higher
chance of inaccurate targeting in 2D-guided biopsy of
low-contrast lesions, as the operator may fail to identify
the same lesion on the paired images.[12] DBT improves
lesion conspicuity and provides depth information
without the need for triangulation and paired images.[11]
It allows accurate lesion targeting and calculation of
the distance between target and skin. It facilitates better biopsy planning with a safer and easier approach to
avoid complications such as skin injury. The procedural
time can be reduced by faster lesion detection and hence
patients’ comfort can be improved. In our study, we
seldom encountered difficulty in lesion targeting even
though all of our targets were low-contrast lesions.
There are a few limitations of this study. First, it was a
retrospective study, which has its inherent limitations. In
our study, the precise record of procedural time could not
be achieved in most of the cases. Second, the sample size
was relatively small (n = 40). The pathological analysis
of focal asymmetry was also limited due to very small
sample size (n = 2). Third, the study was performed in a
single institution from a breast screening programme. All
diagnostic and biopsy procedures in our institution were
interpreted and performed by trained breast radiologists
which may not be generalisable to other practices. Fourth,
there is a lack of complete follow-up data and imaging
(i.e., >2 y of stability) for the benign or high-risk lesions
due to short follow-up period of this retrospective study.
This would potentially underestimate the malignancy
rate. Lastly, tomosynthesis-guided VAB were performed
in selected cases (i.e., sonographically occult non-calcified
lesions) and as self-financed basis, which
would introduce selection bias. Further studies (such as
a prospective study with larger sample size and complete
follow-up data) are suggested for confirmation of our
findings. As the use of DBT becomes more popular, it is
important for breast radiologists to familiarise themselves
with tomosynthesis-guided biopsy techniques.
CONCLUSION
Tomosynthesis-guided VAB is a safe, minimally
invasive, and cost-effective method for evaluation of
sonographically occult lesions detected on DBT. The
majority of DBT-detected sonographically occult non-calcified
breast lesions were architectural distortion with
a PPV of 7.9%.
REFERENCES
1. Andersson I, Ikeda DM, Zackrisson S, Ruschin M, Svahn T,
Timberg P, et al. Breast tomosynthesis and digital mammography:
a comparison of breast cancer visibility and BIRADS classification
in a population of cancers with subtle mammographic findings. Eur
Radiol. 2008;18:2817-25. Crossref
2. Skaane P, Gullien R, Bjørndal H, Eben EB, Ekseth U, Haakenaasen U,
et al. Digital breast tomosynthesis (DBT): initial experience in a
clinical setting. Acta Radiol. 2012;53:524-9. Crossref
3. Friedewald SM, Rafferty EA, Rose SL, Durand MA, Plecha DM, Greenberg JS, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA. 2014;311:2499-507. Crossref
4. McDonald ES, Oustimov A, Weinstein SP, Synnestvedt
MB, Schnall M, Conant EF. Effectiveness of digital breast
tomosynthesis compared with digital mammography: outcomes
analysis from 3 years of breast cancer screening. JAMA Oncol.
2016;2:737-43. Crossref
5. Ciatto S, Houssami N, Bernardi D, Caumo F, Pellegrini M, Brunelli S, et al. Integration of 3D digital mammography with
tomosynthesis for population breast-cancer screening (STORM):
a prospective comparison study. Lancet Oncol. 2013;14:583-9. Crossref
6. Rose SL, Tidwell AL, Bujnoch LJ, Kushwaha AC, Nordmann AS, Sexton R Jr. Implementation of breast tomosynthesis in a routine screening practice: an observational study. AJR Am J Roentgenol.
2013;200:1401-8. Crossref
7. Skaane P, Bandos AI, Gullien R, Eben EB, Ekseth U, Haakenaasen U,
et al. Comparison of digital mammography alone and digital
mammography plus tomosynthesis in a population-based screening
program. Radiology. 2013;267:47-56. Crossref
8. McCarthy AM, Kontos D, Synnestvedt M, Tan KS, Heitjan DF,
Schnall M, et al. Screening outcomes following implementation
of digital breast tomosynthesis in a general-population screening
program. J Natl Cancer Inst. 2014;106:dju316. Crossref
9. Houssami N, Skaane P. Overview of the evidence on digital breast tomosynthesis in breast cancer detection. Breast. 2013;22:101-8. Crossref
10. Viala J, Gignier P, Perret B, Hovasse C, Hovasse D, Chancelier-Galan M, et al. Stereotactic vacuum-assisted biopsies on a digital
breast 3D-tomosynthesis system. Breast J. 2013;19:4-9. Crossref
11. Schrading S, Distelmaier M, Dirrichs T, Detering S, Brolund L,
Strobel K, et al. Digital breast tomosynthesis-guided vacuum-assisted
breast biopsy: initial experiences and comparison
with prone stereotactic vacuum-assisted biopsy. Radiology.
2015;274:654-62. Crossref
12. Waldherr C, Berclaz G, Altermatt HJ, Cerny P, Keller P, Dietz U,
et al. Tomosynthesis-guided vacuum-assisted breast biopsy: a
feasibility study. Eur Radiol. 2016;26:1582-9. Crossref
13. Rochat CJ, Baird GL, Lourenco AP. Digital mammography
stereotactic biopsy versus digital breast tomosynthesis-guided
biopsy: differences in biopsy targets, pathologic results, and
discordance rates. Radiology. 2020;294:518-27. Crossref
14. D’Orsi CJ, Sickles EA, Mendelson EB, et al. ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System. Reston, VA, American College of Radiology; 2013.
15. Parker SH, Burbank F, Jackman RJ, Aucreman CJ, Cardenosa G, Cink TM, et al. Percutaneous large-core breast biopsy: a multi-institutional
study. Radiology. 1994;193:359-64. Crossref
16. Ray KM, Turner E, Sickles EA, Joe BN. Suspicious findings
at digital breast tomosynthesis occult to conventional digital
mammography: imaging features and pathology findings. Breast
J. 2015;21:538-42. Crossref
17. Burrell HC, Sibbering DM, Wilson AR, Pinder SE, Evans AJ, Yeoman LJ, et al. Screening interval breast cancers: mammographic features and prognosis factors. Radiology. 1996;199:811-7. Crossref
18. Partyka L, Lourenco AP, Mainiero MB. Detection of
mammographically occult architectural distortion on digital breast
tomosynthesis screening: initial clinical experience. AJR Am J
Roentgenol. 2014;203:216-22. Crossref
19. Bahl M, Lamb LR, Lehman CD. Pathologic outcomes of
architectural distortion on digital 2D versus tomosynthesis
mammography. AJR Am J Roentgenol. 2017;209:1162-7. Crossref
20. Dibble EH, Lourenco AP, Baird GL, Ward RC, Maynard AS,
Mainiero MB. Comparison of digital mammography and digital
breast tomosynthesis in the detection of architectural distortion.
Eur Radiol. 2018;28:3-10. Crossref
21. Phi XA, Tagliafico A, Houssami N, Greuter MJ, de Bock GH.
Digital breast tomosynthesis for breast cancer screening and
diagnosis in women with dense breasts — a systematic review and
meta-analysis. BMC Cancer. 2018;18:380. Crossref
22. Alshafeiy TI, Nguyen JV, Rochman CM, Nicholson BT, Patire JT,
Harvey JA. Outcome of architectural distortion detected only
at breast tomosynthesis versus 2D mammography. Radiology.
2018;288:38-46. Crossref
23. Bahl M, Baker JA, Kinsey EN, Ghate SV. Architectural distortion
on mammography: correlation with pathologic outcomes and
predictors of malignancy. AJR Am J Roentgenol. 2015;205:1339-45. Crossref
24. Pujara AC, Hui J, Wang LC. Architectural distortion in the era
of digital breast tomosynthesis: outcomes and implications for
management. Clin Imaging. 2019;54:133-7. Crossref
25. Ariaratnam NS, Little ST, Whitley MA, Ferguson K. Digital breast
tomosynthesis vacuum assisted biopsy for tomosynthesis-detected
sonographically occult lesions. Clin Imaging. 2018;47:4-8. Crossref
26. Walcott-Sapp S, Garreau J, Johnson N, Thomas KA. Pathology
results of architectural distortion on detected with digital breast
tomosynthesis without definite sonographic correlate. Am J Surg.
2019:217;857-61. Crossref
27. Patel BK, Covington M, Pizzitola VJ, Lorans R, Giurescu M,
Eversman W, et al. Initial experience of tomosynthesis-guided
vacuum-assisted biopsies of tomosynthesis-detected (2D
mammography and ultrasound occult) architectural distortions.
AJR Am J Roentgenol. 2018;210:1395-400. Crossref
28. Farshid G, Buckley E. Meta-analysis of upgrade rates in 3163 radial
scars excised after needle core biopsy diagnosis. Breast Cancer Res
Treat. 2019;174:165-77. Crossref
29. Kim EM, Hankins A, Cassity J, McDonald D, White B,
Rowberry R, et al. Isolated radial scar diagnosis by core-needle
biopsy: is surgical excision necessary? Springerplus. 2016;5:398. Crossref
30. Leong RY, Kohli MK, Zeizafoun N, Liang A, Tartter PI. Radial
scar at percutaneous breast biopsy that does not require surgery. J
Am Coll Surg. 2016;223:712-6. Crossref