Procedure Time, Efficacy, and Safety of Portal Vein Embolisation Using a Sheathless Needle-Only Technique Compared with Traditional Technique
ORIGINAL ARTICLE
Procedure Time, Efficacy, and Safety of Portal Vein Embolisation Using a Sheathless Needle-Only Technique Compared with Traditional Technique
KCH Yu1, SSM Wong1, YC Wong1, CB Tan1, JCW Siu1, HY Lau1, JCX Chan1, CSC Tsai2,
SCH Yu2,3,4
1 Department of Radiology and Nuclear Medicine, Tuen Mun Hospital, Hong Kong
2 Department of Imaging and Interventional Radiology, Prince of Wales Hospital, Hong Kong
3 Department of Imaging and Interventional Radiology, The Chinese University of Hong Kong, Hong Kong
4 Vascular and Interventional Radiology Foundation Clinical Science Centre, The Chinese University of Hong
Kong, Hong Kong
Correspondence: Prof SCH Yu, Department of Imaging and Interventional Radiology, The Chinese University of Hong Kong, Hong Kong. Email: simonyu@cuhk.edu.hk
Submitted: 16 Nov 2020; Accepted: 2 Dec 2020..
Contributors: All authors designed the study. KCHY and SSMW acquired data. KCHY, SSMW and SCHY analysed the data. KCHY drafted the manuscript. All authors critically revised the manuscript for important intellectual content.
Conflicts of Interest: The authors have no conflicts of interest to declare that are relevant to the content of this article.
Funding/Support: This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are included in this published article (and its supplementary information files).
Ethics Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the
institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
For this type of study formal consent is not required. Ethical approval was waived and approved (NTWC/REC/19133) respectively for the two
retrospective cohorts in this study.
Acknowledgement: This research was submitted to and accepted for oral presentation at the 28th Annual Scientific Meeting of the Hong Kong College of Radiologists, 14-15 November 2020.
Abstract
Introduction
Portal vein embolisation is traditionally performed through an access sheath for selective catheterisation
and embolisation of portal vein branches. This study aimed to compare the procedure time, efficacy, and safety of a
previously reported sheathless technique versus the traditional technique.
Methods
Two retrospective cohorts of portal vein embolisation from two different institutions were reviewed, one for
each technique. Baseline characteristics included patient demographics, liver and renal function tests, international
normalised ratio test, tumour type, and planned resection extent. The primary outcome was procedure time. Secondary
outcomes were technical success, procedural sedation, resection rate, complications, and 30-day mortality. For
cases with available computed tomographic volumetry, future liver remnant volume (FLRV), %FLRV, and increase
in FLRV and %FLRV were recorded. The two cohorts were then compared statistically.
Results
Fifty portal vein embolisation procedures on forty-nine patients were included in each cohort. There
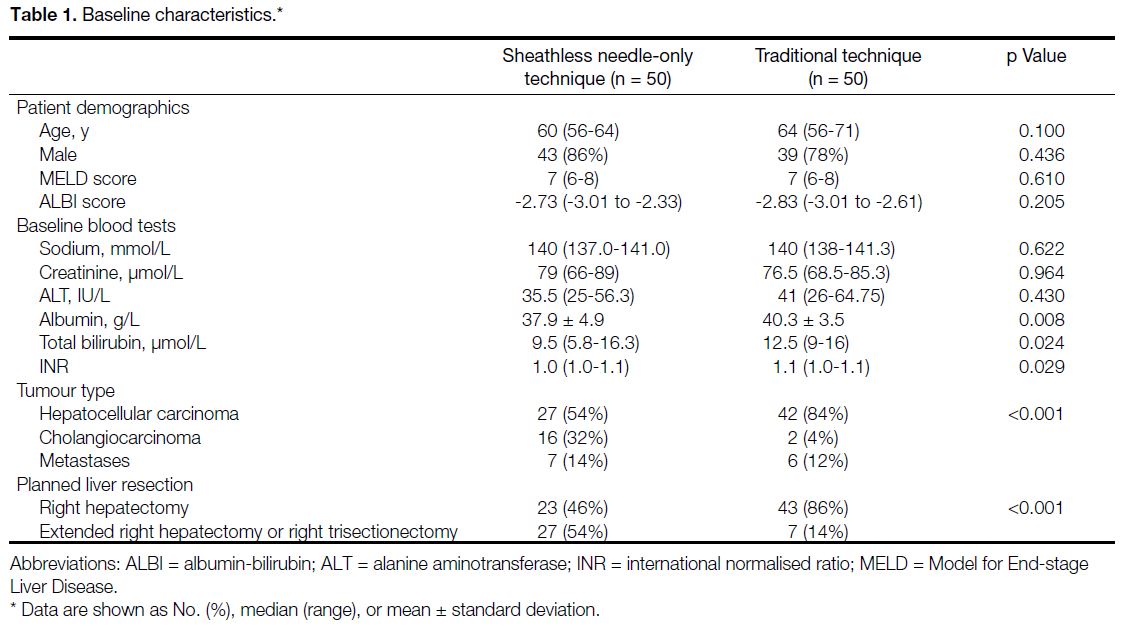
were no statistically significant differences in baseline characteristics including age, sex, Model for End-stage
Liver Disease, and albumin-bilirubin scores between the cohorts. The sheathless cohort had significantly lower
albumin, bilirubin, and international normalised ratio levels (though all within normal limits), a significantly lower
proportion of hepatocellular carcinomas and a significantly higher proportion of cholangiocarcinomas and planned
trisectionectomies. The sheathless cohort had significantly shorter procedure times and less use of procedural
sedation, with no significant differences in technical success, absolute increases in FLRV and %FLRV, resection
rate, complications, or 30-day mortality.
Conclusion
The sheathless technique was associated with shorter procedure time and reduced use of procedural
sedation compared with the traditional technique, with comparable efficacy and safety.
Key Words: Carcinoma, hepatocellular; Cholangiocarcinoma; Embolization, therapeutic; Endovascular procedures; Liver neoplasms; Liver regeneration; Portal vein; Radiology, interventional
中文摘要
無鞘單針技術與傳統方法的門靜脈栓塞術:比較手術時間、療效和安全性
余俊鴻、王先民、王耀忠、陳崇文、蕭志偉、劉顯宇、陳積聖、蔡紹俊、余俊豪
引言
門靜脈栓塞術(PVE)傳統上通過通路鞘進行,用於選擇性導管插入和栓塞門靜脈分支。本研究旨在比較先前報導的無鞘技術與傳統技術的手術時間、療效和安全性。
方法
分析來自兩個不同機構的兩個PVE回顧性隊列,每個機構一種技術。基線特徵包括患者基本特點、肝功能檢測、國際標準化比值測試、腫瘤類型和計劃切除範圍。主要結果是手術時間。次要結果包括技術成功率、手術鎮靜、切除率、併發症和30天死亡率。對於具有可用計算機斷層掃描計算體積的病例,記錄術後肝殘餘體積(FLRV)、%FLRV百份比,以及FLRV和FLRV百份比的增加。這兩個隊列進行統計學比較。
結果
每個隊列包括49名患者的50次PVE手術。兩組之間的基線特徵包括年齡、性別、終末期肝病模型(MELD)或白蛋白膽紅素(ALBI)評分無統計學顯著差異。無鞘隊列患者的白蛋白、膽紅素和國際標準化比值水平顯著較低(儘管都在正常範圍內),肝細胞癌比例顯著較低,膽管癌和計劃三段切除的比例顯著較高。無鞘隊列的手術時間顯著縮短,並且較少使用程序性鎮靜,在技術成功率、FLRV和FLRV百份比的增加、切除率、併發症或30天死亡率方面無顯著差異。
結論
與傳統技術相比,無鞘技術的手術時間更短,鎮靜劑用量減少,療效和安全性相若。
INTRODUCTION
Portal vein embolisation (PVE) is an interventional
radiology procedure first reported in 1986 as an
alternative to open PV ligation.[1] It is performed prior to
hepatic resection for primary or secondary malignancy to
increase the size of future liver remnant (FLR), in patients
initially contraindicated for upfront hepatectomy due to
borderline liver function or inadequate FLR volume. This
is achieved by selective embolisation of PV branches
supplying the tumour-bearing hepatic lobe, resulting in
increased blood flow to the FLR to induce hypertrophy
with a high success rate,[2] [3] [4] along with reduction in
postoperative hepatic dysfunction, complications, and an
increase in the ability to subsequently perform hepatic
resections with curative intent.[5] [6] PVE traditionally
involves transhepatic puncture of a segmental or sectoral
PV branch, insertion of an access sheath, portography
for anatomical delineation and planning, and selective
embolisation of PV branches.[7] Various embolic agents
including liquid, spherical, particulate agents, and coils
have been utilised.[8]
Performing PVE with a simplified sheathless needle-only
technique, in which direct portography and glue
embolisation are performed via the puncture needle, has been reported to have a high technical success rate
and satisfactory FLR hypertrophy.[9] This study aimed
to compare the procedure time, efficacy, and safety of
PVE with the sheathless technique versus the traditional
technique.
METHODS
Two retrospective patient cohorts were retrieved from
two tertiary institutions, one comprised of patients that
had undergone the sheathless technique and the other
comprised of patients that had undergone the traditional
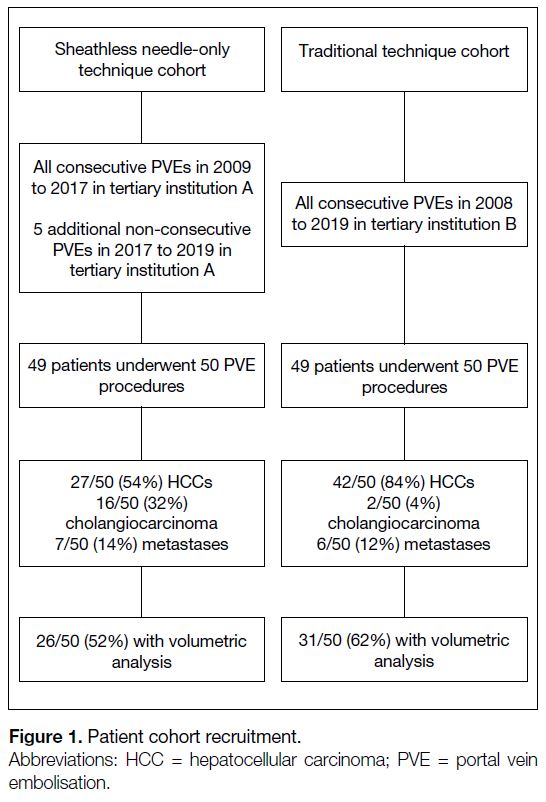
technique (Figure 1). The decision to perform PVE
had been made by a multidisciplinary consensus with
hepatobiliary surgeons and interventional radiologists
in accordance with local guidelines.[10] [11] [12] Ethical
approval was waived for the sheathless technique
cohort and approved for the traditional technique cohort
(NTWC/REC/19133) by their respective institutional
review boards. Patient consent was waived due to the
retrospective nature of the study.
Figure 1. Patient cohort recruitment.
Data Collection
Data collection and reporting was done with reference to
the Strengthening the Reporting of Observational Studies
in Epidemiology (STROBE) checklist.[13] Patient data were retrieved via the electronic patient record system.
These included age, sex, baseline blood test results
(sodium, creatinine, alanine transferase, albumin, total
bilirubin, international normalised ratio [INR]), tumour
type (hepatocellular carcinoma, cholangiocarcinoma,
or metastasis), technical success, use of procedural
sedation, planned hepatectomy extent (right hepatectomy
or trisectionectomy), eventual hepatectomy, and
complications. Model for End-stage Liver Disease
(MELD) and albumin-bilirubin (ALBI) scores were used
as markers of chronic hepatic derangement, and were
calculated with their original formulas.[14] [15]
Traditional Technique Cohort
Forty-nine consecutive cases of PVE in 2008 to 2019
were analysed. Traditional PVEs were performed by
interventional radiology specialists with 5 to >20 years
of experience, in one of two dedicated interventional
angiography suites (Allura Clarity, Philips Healthcare,
Best, the Netherlands; Artis Q with PURE®, Siemens
Healthcare, Erlangen, Germany).
Under local anaesthesia (1% lidocaine) and using
sonographic guidance, a segmental PV branch was
located and punctured with a 20-22 G needle (Chiba;
Cook Incorporated, Bloomington [IN], US; Inrad,
Inc., Kentwood [MI], US). Procedural sedation with
fentanyl or midazolam was administered if necessary.
The puncture was ipsilateral to the tumour-bearing lobe.
Typically, right lobe anterior sectoral branches, which
have been shown to be safer and would not violate the
FLR, were chosen.[16] Contralateral (left-sided) puncture
was performed in cases of markedly distorted anatomy
or difficult access of the right PV system. Position
was confirmed by free aspiration of venous blood and
direct portography (Omnipaque 300; GE Healthcare,
Shanghai, China). An access sheath was then inserted
via an introducer set (Skater 6 Fr × 18 cm; Argon
Medical Devices Inc., Athens [TX], US) or a 4 to 5 Fr
vascular sheath (Cook Incorporated, Bloomington [IN],
US; Radifocus Introducer II, Terumo Corporation,
Tokyo, Japan). Using a 0.035-inch guidewire (0.035-inch
Angled Terumo guidewire; Radifocus, Terumo
Corporation, Tokyo) and angiographic catheters (4-5 Fr
C1; SHK, Rim, MPA etc., Cordis Corporation, Miami
Lakes [FL], US), portography was performed to
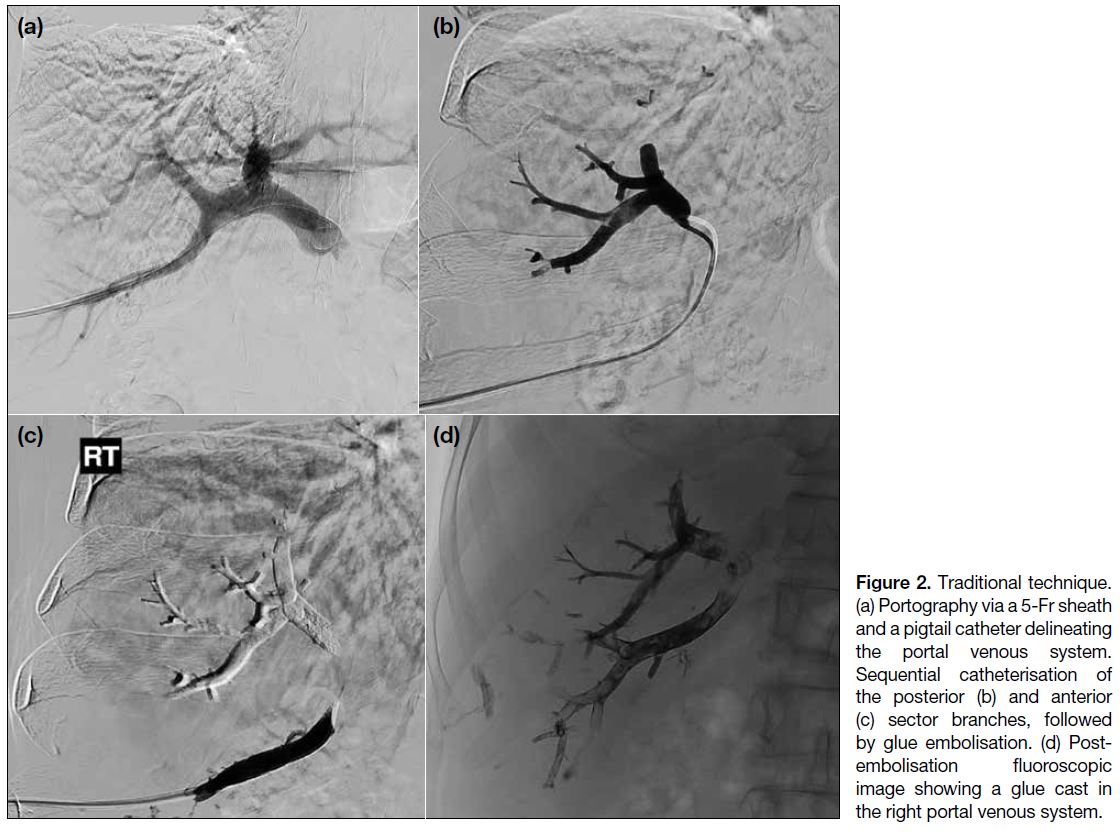
delineate the anatomy (Figure 2). Branches of the right
PV were then selectively catheterised and embolised
with microcatheters (2.4 Fr Merit Maestro; Merit
Medical Systems Inc., South Jordan [UT], US and 2.8 Fr
Renegade Hi-Flo; Boston Scientific, Marlborough
[MA], US) and a microguidewire (0.014-inch Traxcess;
MicroVention Terumo, Tustin [CA], US). The embolic
agent of choice was n-butyl-2-cyanoacrylate (Histoacryl;
B Braun Surgical S.A., Rubi, Spain) diluted to 10% to
25% in iodised oil (Lipiodol® Ultra Fluid; Guerbet LLC,
Princeton [NJ], US). In a few selected cases, 100- to
300-μm polyvinyl alcohol particles (Contour; Boston
Scientific) were employed. The angiographic endpoint
was satisfactory occlusion of the selected right PV
branches by glue cast or polyvinyl alcohol particles.
Figure 2. Traditional technique. (a) Portography via a 5-Fr sheath and a pigtail catheter delineating the portal venous system. Sequential catheterisation of the posterior (b) and anterior (c) sector branches, followed by glue embolisation. (d) Post-embolisation fluoroscopic image showing a glue cast in the right portal venous system.
Sheathless Technique Cohort
The same 45 consecutive cases of PVE from 2009
to 2017 from our previous study[9] plus five additional
cases from 2017 to 2019 were analysed. Full technical
details and considerations are as previously described.[9]
In brief, a right PV branch, typically segment V/VI, was
punctured with a Cook 15 cm 18 G Diamond needle under
sonographic guidance, followed by direct portography
with iodinated contrast for anatomical delineation,
and finally injection of 16% N-butyl cyanoacrylate
glue diluted in Lipiodol® Ultra Fluid all from the same needle under careful manual control of injection rate
and fluoroscopy to avoid nontarget embolisation to
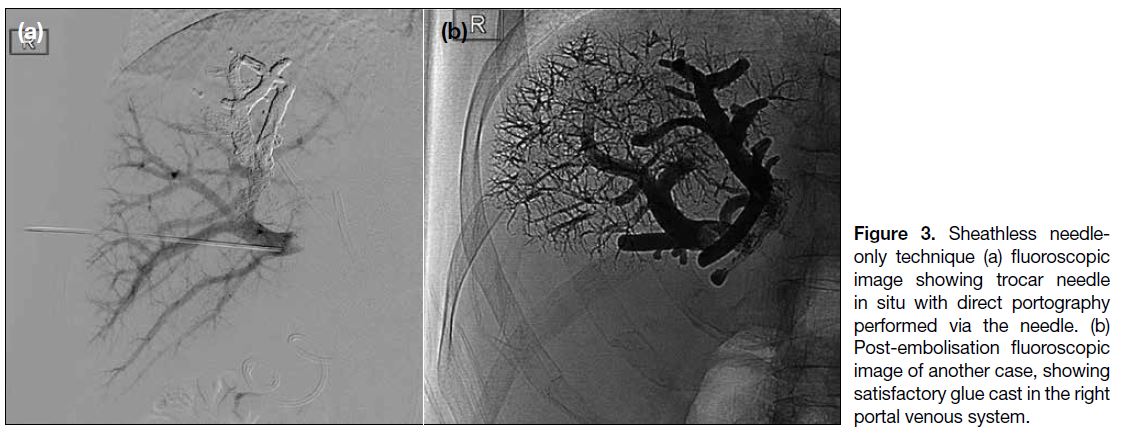
the FLR (Figure 3). Additional puncture(s) was / were
needed in cases with short right PVs (<2 cm), main PV
trifurcating into right / left / segment IV branches, or
the segment IV branch arising from the right PV. These
were performed by a single interventional radiology
fellow with >20 years of experience. Intravenous (IV)
procedural sedation (single-dose 50 μg IV fentanyl) and
cone beam computed tomography (CT) were both used
at the operator’s discretion.
Figure 3. Sheathless needle-only technique (a) fluoroscopic image showing trocar needle in situ with direct portography performed via the needle. (b) Post-embolisation fluoroscopic image of another case, showing satisfactory glue cast in the right portal venous system.
Volumetric Analysis
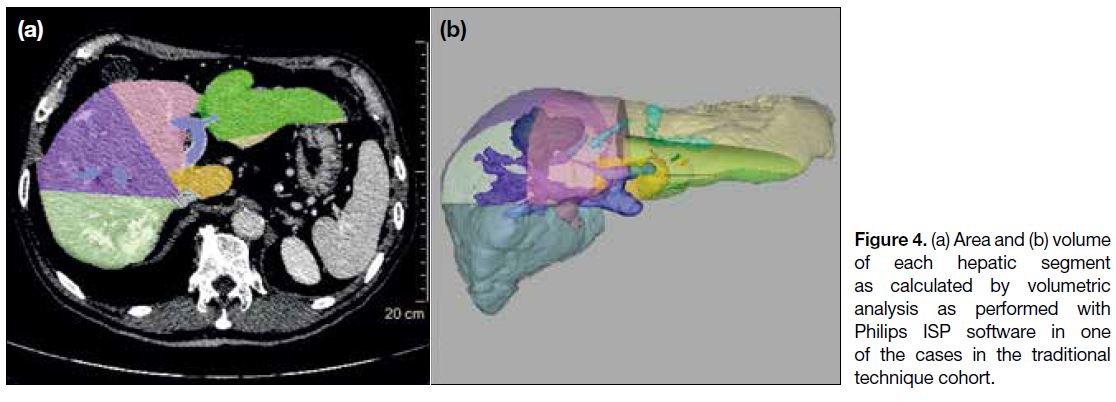
In cases where DICOM data of both pre- and post-PVE
CTs were available (Figure 4), volumetric analysis
was performed using commercial imaging software
(IntelliSpace Portal v5.0.2.30010; Philips Healthcare
or OsiriX v7.0.3, Pixmeo, Bernex, Switzerland) on
5 mm sections by two radiology fellows with 5 and 9 years of experience in liver volumetry, respectively.
The latest pre-PVE CT and post-PVE CT, typically
4 weeks before and 4 to 6 weeks after PVE, were used
for volumetric analysis. Hepatic segments were defined
according to the Couinaud classification. Since segment
I is usually partially or completely resected during
hepatectomy, FLR refers to segments II-IV for right
hepatectomy candidates, and to segments II/III for right
trisectionectomy candidates. The percentage of FLR
volume (%FLRV) is defined as [FLRV × 100%/(total
liver volume - tumour volume)]. Tumour volume was
included except for infiltrative or ill-defined tumours.
Figure 4. (a) Area and (b) volume of each hepatic segment as calculated by volumetric analysis as performed with Philips ISP software in one of the cases in the traditional technique cohort.
Outcome Measures
Primary outcome was procedure time, defined as time
elapsed from the first to the final angiographic runs,
plus time taken for sonographic evaluation, universally
assumed to be 10 minutes. Secondary outcomes included
technical success, use of procedural sedation, degree of FLR hypertrophy, resection rate, and complications.
Technical success was defined as satisfactory occlusion
of right PV branches by glue cast.
Use of procedural sedation was defined as any use of any
IV sedation agents, regardless of dose.
The degree of FLR hypertrophy was assessed by the
absolute increase in FLRV, absolute increase in %FLRV,
and percentage increase of %FLRV after PVE. The
resection rate was the proportion of patients eventually
undergoing hepatectomy. Staged hepatectomy was
performed in patients with satisfactory FLR hypertrophy
or improvement in liver function tests, and delayed or
cancelled in patients with inadequate FLR hypertrophy,
inadequate liver function, tumour progression, or new contraindications to surgery. Complications
were classified according to Society of Interventional
Radiology guidelines.[17]
Data and Statistical Analysis
Sample size was calculated with the formula for
difference in two independent sample means for our
primary outcome (procedure time). The ratio of controls
to cases was 1:1. Two-sided significance level (α) and
statistical power (1-β) were taken as 0.05 and 80%,
respectively. From the previously published series,[9] the
mean procedure time and standard deviation (SD) for
the sheathless technique was 19.4 ± 15.3 minutes. The
most recent 10 cases of PVE with traditional technique
performed in 2018 to 2019 were reviewed as a pilot
series, with mean procedure time of 84.3 ± 38.1 minutes. The expected difference in means was 64.9 minutes.
However, we consider a 50% decrease in procedure time
with the sheathless technique (42 min) to be clinically
meaningful. The required sample size for each cohort
was therefore 13.
The normality of data was tested by a Shapiro–Wilk test,
with p > 0.05 indicating normal distribution. Categorical
variables were listed as number and percentage to total
and compared using Fisher’s exact test (two-tailed).
Continuous parametric variables were expressed as
mean ± SD and compared using an independent-samples
t test. Continuous nonparametric variables were
expressed as median and interquartile range (Q1-Q3)
and compared using the Mann-Whitney U test. A p
value of <0.05 (two-tailed) was defined as statistically
significant. Data collection was performed using
commercial software (Office Excel version 16.29.1,
Microsoft, Redmond [WA], US). Statistical analyses and
graph plots were also performed on commercial software
(SPSS Windows version 24.0; IBM Corp, Armonk
[NY], US).
RESULTS
Forty-nine patients undergoing 50 PVE procedures
were included in each cohort. Baseline characteristics
are summarised in Table 1. There was no statistical difference in age, sex, MELD, or ALBI scores. The
sheathless technique cohort showed significantly lower
serum albumin, bilirubin, and INR levels (all p < 0.05),
although all levels were within normal limits. There
was a significantly lower proportion of hepatocellular
carcinoma, higher proportion of cholangiocarcinoma
and higher proportion of planned trisectionectomy (all
p < 0.05). For the sheathless group, 38 patients (76%)
required only a single puncture while the others (n = 12,
24%) required two punctures. None of the patients
required three or more punctures.
Table 1. Baseline characteristics.
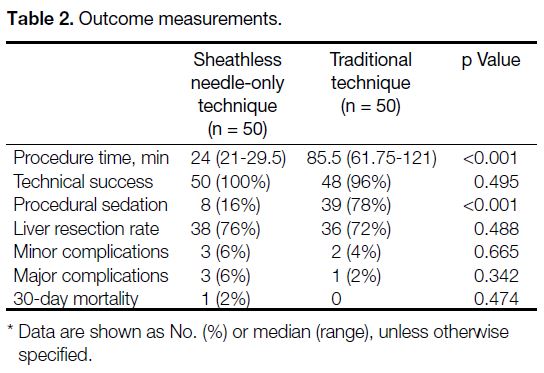
Outcome measures are shown in Table 2. The sheathless
technique cohort showed significantly shorter procedure
time (24 min vs 85.5 min; p < 0.001) and a lower
proportion of patients required procedural sedation (16%
vs 78%; p < 0.001). There was no significant difference in
technical success rate (p > 0.05). There was a comparable
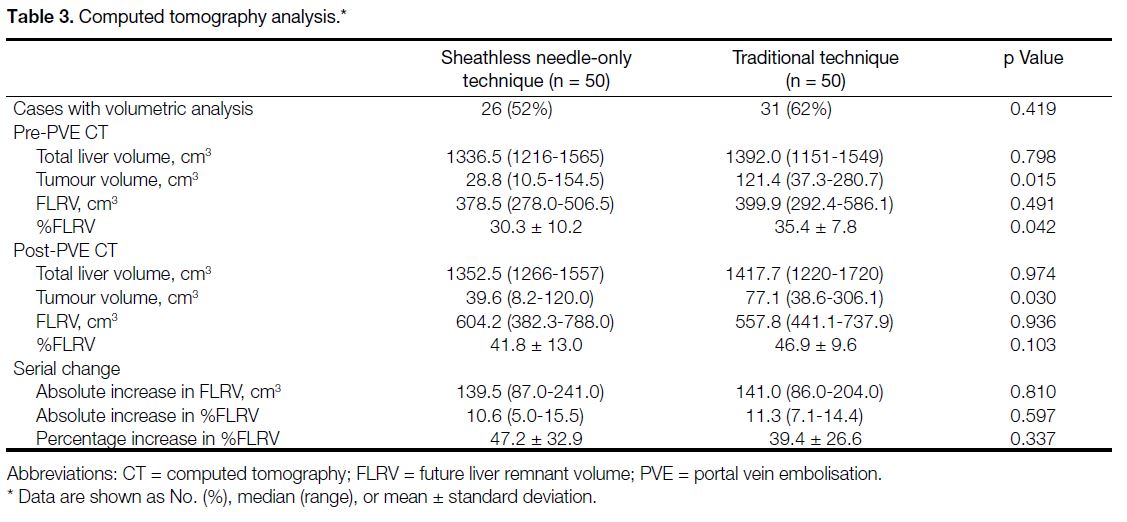
number of CT volumetry data sets available in the two
cohorts, with no significant difference in absolute FLRV
increase, absolute %FLRV increase, or percentage
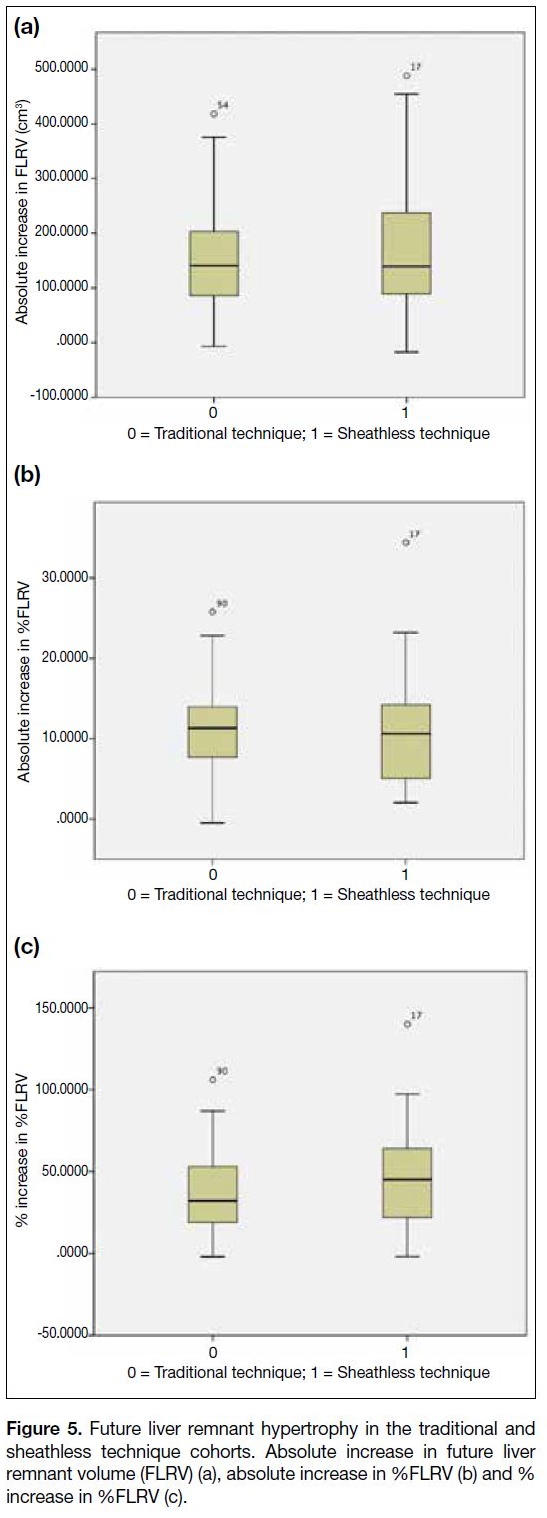
increase in %FLRV (p > 0.05) [Table 3 and Figure 5].
There was no significant difference in resection rate,
minor complications, major complications, or 30-day
mortality (p > 0.05).
Table 2. Outcome measurements.
Table 3. Computed tomography analysis.
Figure 5. Future liver remnant hypertrophy in the traditional and
sheathless technique cohorts. Absolute increase in future liver
remnant volume (FLRV) (a), absolute increase in %FLRV (b) and %
increase in %FLRV (c).
In the sheathless technique cohort, there were three major complications (6%). One patient with
cholangiocarcinoma developed an infected right
subphrenic collection after PVE, which resolved 4 days
after percutaneous drainage, but was further complicated
with cholangitic abscesses that were successfully
managed conservatively with IV antibiotics. Resection
was cancelled due to disease progression. In another
patient with hepatocellular carcinoma, there was
glue reflux into the common hepatic artery leading to
infarction of the right posterior segment, which was
resected during right hepatectomy. In one patient with
cholangiocarcinoma, a glue cast migrated into the
hepatic vein and right atrium, which was successfully
captured and removed by inferior vena cava filter
insertion, retrieval, and aspiration thrombectomy. This
patient subsequently underwent right trisectionectomy uneventfully. There were three minor complications,
all of which involved asymptomatic nonocclusive
emboli in FLR detected on post-embolisation CT. Two
of these patients underwent resection uneventfully, and
the third did not undergo resection as a result of disease
progression.
In the traditional technique cohort, there was one major
complication (2%). A patient with cholangiocarcinoma
developed a liver abscess and cholangitis a week after
PVE, requiring intensive care unit admission and
repeated percutaneous drainages. The patient later
underwent hepatectomy uneventfully. There were two
minor complications. In a patient with hepatocellular
carcinoma, there was nontarget embolisation to a
segmental pulmonary artery detected on cone beam
CT upon completion of PVE and confirmed on
CT pulmonary angiography. The patient remained
asymptomatic, not requiring additional treatment,
and was discharged on day 4, which only qualified
as a minor complication according to Society of
Interventional Radiology guidelines. Another patient
with hepatocellular carcinoma was readmitted on day
2 for right upper quadrant pain, which was successfully
managed conservatively.
There was only one case of 30-day mortality, specifically
in the sheathless technique cohort, without statistically
significant difference between the two cohorts. This
patient had hepatocellular carcinoma on a background of
multiple medical comorbidities including motor neuron disease, with an initially uneventful post-PVE recovery,
but later presented on day 27 with a fall and head injury,
with sudden asystole while still in the emergency
department. No significant abnormality was found on CT
of the brain or plain radiograph of the chest, including
intracranial haemorrhage, airspace opacification, or
inadvertent glue migration. Liver function tests were also
normal. The patient succumbed on the same day after
joint consensus of comfort care was made. The cause
of mortality was presumed to be more likely related to
the underlying motor neuron disease rather than as a
consequence of PVE.
DISCUSSION
The sheathless technique was found to be a feasible
alternative to the traditional technique in a previous
study.[9] This study further confirmed its advantages in
terms of shorter procedure time and a reduced need
for sedation while maintaining similar efficacy and
safety profiles. The traditional technique of PVE has
a few obvious disadvantages. It requires placement
of an access sheath, which is painful, and frequently
requires sedation. Selective segmental catheterisation for
portography and embolisation can also be tedious with
significantly longer procedure times, up to 214 minutes
in our series. This is likely also associated with higher
radiation dose to the patient. Last but not least, tract
embolisation also has to be performed. Complications of
the traditional technique reported in the literature include
bleeding, vascular or bile duct injury, pneumothorax,
inadvertent embolisation of the FLR, and migration of
embolic agents, among others.[16] [18] There were instances
of nontarget embolisation in both of our series, without
statistically significant differences. Specifically, this
involved the hepatic vein and hepatic artery in the
sheathless group, and probable hepatic vein followed
by segmental pulmonary artery in the traditional group.
This was likely due to either vascular shunts, which
are common in hepatic malignancies, or inadvertent
double puncture of the involved hepatic artery or
hepatic vein, which is in theory equally likely in both
techniques. The cases of minor asymptomatic FLR
emboli in the sheathless group may be attributable to
the more technically demanding nature of the sheathless
technique, requiring a painstakingly careful steady
injection and backflow of glue to the right PV in order
to reach all the targeted right PV branches. Importantly,
none of these complications was significant enough to
preclude subsequent hepatectomy.
As shown in our results, the sheathless technique negates a few major drawbacks of the traditional technique
in selected patients, while maintaining comparable
outcomes and safety profile.
There are a few important technical issues. First, the pre-PVE CT should be carefully analysed for PV anatomy.
This is especially important for the sheathless technique
because the image quality of direct portography via
the 18 G needle is not as good as via the access sheath
or catheter in the traditional technique. Second, test
runs using iodinated contrast should be performed
before embolisation to estimate the optimal flow rate
and volume, to avoid nontarget embolisation as well
as premature polymerisation of the glue cast before it
reaches all target PV branches. Third, in cases where PV
anatomy is grossly distorted by tumour or with complex
variant anatomy, the traditional technique probably
remains the safer and more conservative approach.
The major limitation of this study is the separate
patient cohorts for the two different techniques. There
were statistically significant differences in the baseline
serum albumin, bilirubin, and INR levels. Given that
these parameters were within normal range, such a
small difference might not be clinically significant. The
sheathless technique cohort also contained significantly
higher proportion of cholangiocarcinoma and planned
trisectionectomy, as well as smaller tumour volume
and %FLRV. These were likely due to different
degree of utilisation of PVE in cholangiocarcinoma in
different institutions. Although this may imply different
prevalence of chronic hepatitis in the two cohorts, the
degrees of liver derangement were comparable, as there
was no significant difference in their MELD and ALBI
scores. As such, the difference in tumour type in the two
cohorts should not influence the outcome. The MELD[14] [19]
and ALBI[15] scores were adopted in this study instead of
Child–Pugh score, as the latter incorporates subjective
factors such as presence of ascites and encephalopathy.
Another limitation is the limited generalisability of the
results of the sheathless technique series, which was
performed by one experienced operator in a tertiary
academic institution. We do not have reference outcome
measures of the traditional technique in this institution
by this operator to compare with our control group,
which was performed by multiple operators with varying
levels of experience in another tertiary institution, and
this may be a confounding factor. Undeniably, operator
experience has significant bearings on both procedure
time and utilisation of procedural sedation, while institutional differences likely also account for baseline
differences such as tumour type, planned hepatectomy
extent, procedural sedation protocol, etc. to name just a
few. Due to this study’s retrospective nature, we were
unable to standardise or control for these factors. CT
volumetry data was also incomplete, as some patients
received their CT in outside facilities.
In conclusion, the sheathless technique for PVE
results in a shorter procedure time and reduced need
for sedation compared with the traditional technique,
with a comparable degree of FLR hypertrophy and
complication rate.
REFERENCES
1. Kinoshita H, Sakai K, Hirohashi K, Igawa S, Yamasaki O, Kubo S. Preoperative portal vein embolization for hepatocellular carcinoma.
World J Surg. 1986;10:803-8. Crossref
2. May BJ, Madoff DC. Portal vein embolization: rationale, technique,
and current application. Semin Intervent Radiol. 2012;29:81-9. Crossref
3. Broering DC, Hillert C, Krupski G, Fischer L, Mueller L,
Achilles EG, et al. Portal vein embolization vs. portal vein
ligation for induction of hypertrophy of the future liver remnant. J
Gastrointest Surg. 2002;6:905-13. Crossref
4. Pandanaboyana S, Bell R, Hidalgo E, Toogood G, Prasad KR,
Bartlett A, et al. A systematic review and meta-analysis of portal
vein ligation versus portal vein embolization for elective liver
resection. Surgery. 2015;157:690-8. Crossref
5. Shoup M, Gonen M, D’Angelica M, Jarnagin WR, DeMatteo RP,
Schwartz LH, et al. Volumetric analysis predicts hepatic dysfunction
in patients undergoing major liver resection. J Gastrointest Surg.
2003;7:325-30. Crossref
6. Ribero D, Abdalla EK, Madoff DC, Donadon M, Loyer EM, Vauthey JN. Portal vein embolization before major hepatectomy
and its effects on regeneration, resectability and outcome. Br J Surg.
2007;94:1386-94. Crossref
7. Avritscher R, de Baere T, Murthy R, Deschamps F, Madoff DC.
Percutaneous transhepatic portal vein embolization: rationale,
technique, and outcomes. Semin Intervent Radiol. 2008;25:132-45. Crossref
8. Madoff DC, Hicks ME, Abdalla EK, Morris JS, Vauthey JN. Portal
vein embolization with polyvinyl alcohol particles and coils in
preparation for major liver resection for hepatobiliary malignancy:
safety and effectiveness — study in 26 patients. Radiology.
2003;227:251-60. Crossref
9. Wong SS, Yuen BT, Lee RK, Tsai CS, Cheung YS, Lee KF, et al.
Percutaneous portal vein embolization using a simplified sheathless
18-gauge trocar needle approach: review of efficacy and safety. J
Vasc Interv Radiol. 2019;30:440-4. Crossref
10. Poon RT, Cheung TT, Kwok PC, Lee AS, Li TW, Loke KL, et al.
Hong Kong consensus recommendations on the management of
hepatocellular carcinoma. Liver Cancer. 2015;4:51-69. Crossref
11. Cheung TT, Kwok PC, Chan S, Cheung CC, Lee AS, Lee V,
et al. Hong Kong consensus statements for the management of
unresectable hepatocellular carcinoma. Liver Cancer. 2018;7:40-54. Crossref
12. Yau T, Tang VY, Yao TJ, Fan ST, Lo CM, Poon RT. Development
of Hong Kong Liver Cancer staging system with treatment
stratification for patients with hepatocellular carcinoma.
Gastroenterology. 2014;146:1691-700.e3. Crossref
13. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement:
guidelines for reporting observational studies. Lancet.
2007;370:1453-7. Crossref
14. Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM,
Kosberg CL, et al. A model to predict survival in patients with
end-stage liver disease. Hepatology. 2001;33:464-70. Crossref
15. Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M,
Reeves HL, et al. Assessment of liver function in patients with
hepatocellular carcinoma: a new evidence-based approach — the
ALBI grade. J Clin Oncol. 2015;33:550-8. Crossref
16. Kodama Y, Shimizu T, Endo H, Miyamoto N, Miyasaka K. Complications of percutaneous transhepatic portal vein
embolization. J Vasc Interv Radiol. 2002;13:1233-7. Crossref
17. Sacks D, McClenny TE, Cardella JF, Lewis CA. Society of
Interventional Radiology clinical practice guidelines. J Vasc Interv
Radiol. 2003;14:S199-202. Crossref
18. Yeom YK, Shin JH. Complications of portal vein embolization:
evaluation on cross-sectional imaging. Korean J Radiol.
2015;16:1079-85. Crossref
19. Freeman RB Jr, Wiesner RH, Harper A, McDiarmid SV, Lake J,
Edwards E, et al. The new liver allocation system: moving toward
evidence-based transplantation policy. Liver Transpl. 2002;8:851-8. Crossref