Sonographic Features of Breast Fibroepithelial Masses: Distinguishing Fibroadenoma from Phyllodes Tumour
ORIGINAL ARTICLE
Sonographic Features of Breast Fibroepithelial Masses: Distinguishing Fibroadenoma from Phyllodes Tumour
GY Lee1, GW Shin1, HY Park2, HK Yoon2, TH Kim3, A Lee3, YJ Heo1, YJ Lee1, JY Han1, YM Park1
1 Department of Radiology, Busan Paik Hospital, Inje University College of Medicine, South Korea
2 Department of Pathology, Busan Paik Hospital, Inje University College of Medicine, South Korea
3 Department of Surgery, Busan Paik Hospital, Inje University College of Medicine, South Korea
Correspondence: Prof YM Park, Department of Radiology, Busan Paik Hospital, Inje University College of Medicine, South Korea. Email: nanbarkym@hanmail.net
Submitted: 23 Apr 2021; Accepted: 22 Jan 2021.
Contributors: All authors designed the study and acquired the data. GYL, GWS, YJH and YMP analysed the data. All authors drafted the
manuscript. GYL, GWS, JYH and YMP critically revised the manuscript for important intellectual content. All authors had full access to the
data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This research was funded by Dongkook Lifescience Co., Ltd., South Korea.
Data Availability: All data generated or analysed during the present study are included in this published article (and its supplementary information files).
Ethics Approval: This retrospective study was approved by the Institutional Review Board of Busan Paik Hospital, South Korea, and the requirement for informed consent was waived (Ref: BPIRB 2020-01-026-009).
Abstract
Objective
Fibroepithelial tumours of the breast, which include fibroadenoma (FA) and phyllodes tumour (PT), are
benign entities. Although they show different biological behaviours, distinguishing between them using imaging
features or core needle biopsy (CNB) is challenging. We evaluated sonographic and CNB features, that could be
useful for distinguishing between the two.
Methods
A total of 121 patients with 125 lesions diagnosed as fibroepithelial tumours on ultrasound-guided CNB
from March 2017 to April 2020 were studied. Among them, sonographic features of 68 lesions were retrospectively
analysed. Clinicopathological results of CNB and surgical excision were reviewed using electronic medical records.
Results
On ultrasound, tumour size, echogenicity, presence of an internal cleft, vascularity, and elasticity revealed
significant differences between FA and PT. Tumour size ≥ 3 cm, presence of an internal cleft, and hard elasticity were
common sonographic features in PTs. CNB revealed similar pathological results with surgical excision performed
in 61 cases of 68 cases (89%).
Conclusion
Ultrasonographic features can be useful imaging factors for distinguishing between FA and PT.
Ultrasound-guided CNB can replace surgical excision with a high diagnostic accuracy in some cases.
Key Words: Breast; Fibroadenoma; Phyllodes tumor; Ultrasonography
中文摘要
乳腺纖維上皮腫塊的超聲特徵:區分纖維腺瘤和葉狀腫瘤
GY Lee、GW Shin、HY Park、HK Yoon、TH Kim、A Lee、YJ Heo、YJ Lee、JY Han、YM Park
目的
乳腺纖維上皮腫瘤,包括纖維腺瘤(FA)和葉狀腫瘤(PT)為良性病變。儘管它們表現不同的生物學行為,但用影像學特徵或核心針活檢(CNB)來區分兩者並不容易。本研究評估可能有助於區分兩者的超聲和CNB特徵。
方法
研究2017年3月至2020年4月超聲引導CNB診斷為纖維上皮腫瘤的 121例患者共125個病灶。其中回顧性分析了68個病灶的超聲特徵,並通過電子病歷回顧CNB和手術切除標本的臨床病理學結
果。
結果
在超聲學上,腫瘤大小、迴聲、內裂的存在、血管分佈和組織彈性顯示FA 和PT間存在顯著差異。腫瘤大小≥3 cm、存在內裂和彈性較硬是PT的常見超聲特徵。CNB在68例中的61例(89%)中顯示與手術切除相似的病理結果。
結論
超聲影像學特徵有助於鑑別FA和PT。在某些情況下,超聲引導的CNB 可以替代手術切除,具有很高的診斷準確性。
INTRODUCTION
Fibroepithelial tumours of the breast, including
fibroadenoma (FA) and phyllodes tumour (PT), are
composed of a biphasic proliferation of both epithelial
and stromal components.[1] The distinction between FA
and PT is clinically important. While FAs generally
regress with age and can be safely followed without
further investigations, PTs continue to grow, requiring
wide local excision to prevent local recurrence.[2] [3] [4] The
risk of local recurrence in PTs ranges from 17% in benign
PT to 27% in malignant PT, with metastasis occurring in
approximately 25% of malignant PTs.[5] Most recurrent
PTs are histologically similar to the initial tumour;
however, in up to 26% of initially benign PTs, there is a
risk of recurrence as borderline or malignant PTs.[6] [7]
Although the two disease entities show different
biological behaviours, distinguishing between them
using imaging features or core needle biopsy (CNB) is
challenging.[8] [9] Histologically, PT is usually distinguished
from FA based on the presence of hypercellular stroma
that show leaf-like projection. However, a definite
diagnosis with CNB is difficult owing to the small sample
size and because hypercellularity also can be present in
juvenile FAs and in the breast tissue of women receiving
hormone replacement therapy.[10] [11] [12]
If the disease entities can be distinguished using imaging findings and CNB results, unnecessary surgical excision
can be avoided. Therefore, we evaluated the sonographic
features that could distinguish FA from PT.
METHODS
Patient Population
This retrospective study was approved by the Institutional
Review Board of our hospital, and the requirement
for informed consent was waived. Three radiologists
retrospectively reviewed the medical and imaging records
of patients diagnosed with breast fibroepithelial tumours
on ultrasound (US)-guided CNB. From March 2017 to
April 2020, 125 lesions in 121 patients were confirmed
to be fibroepithelial tumours. Among them, cases without
surgical excision after CNB (n = 46), cases with US-guided
vacuum-assisted biopsy after CNB (n = 8), cases
confirmed as another lesion (sclerosing adenosis) [n = 1],
and cases confirmed as both FA and PT (n = 2) were
excluded, resulting in a total 68 cases in 65 patients.
Data Acquisition and Data Analysis
Breast ultrasonography was performed using a 15-4 MHz
(Aixplorer, SuperSonic Imagine, Aix-en-Provence,
France) or a 5-12 MHz (iU22; Philips Healthcare,
Bothell [WA], United States) linear array transducer. The sonographic features of the 68 lesions were analysed
independently by three dedicated breast radiologists,
with 1, 5 and 20 years of experience, according to the
ACR BI-RADS (American College of Radiology Breast
Imaging Reporting and Data System) Atlas (5th ed.).[13]
The maximum elasticity value (Emax) of shear-wave
elastography was categorised into hard (Emax ≥144 kPa),
intermediate (72 kPa < Emax < 144 kPa) and soft elasticity
(Emax ≤72 kPa).[14] US-guided CNB was performed using
a 14-gauge cutting needle with freehand technique, and
five core specimens were retrieved from each lesion.
Clinicopathological results of the CNBs and surgical
excision were reviewed using the images from the
electronic medical records. We analysed the concordance
between the pathology reports of all the CNBs and the
pathological results of surgical excision.
Statistical Analysis
Comparison of the categorical variables between FA and
PT was performed using the Chi-square and Fisher’s
exact tests. The level of significance was set at P<0.05
for all tests. The statistical analysis was conducted using
SPSS (Windows version 26.0; IBM Corp, Armonk
[NY], United States).
RESULTS
Among the 68 lesions analysed in this study, the
pathological results of surgical excision revealed
32 FAs and 36 PTs. The mean age of patients with FA
was 41.7 years (range, 20-68) and those with PT was
39.3 years (range, 18-52). Among the 36 cases of PTs, the
histological subtypes were benign in 32 cases (88.9%),
borderline PT in two cases (5.6%) and malignant PT in
two cases (5.6%).
On US, tumour size, echogenicity, presence of internal
clefts, vascularity, and elasticity showed significant
differences between FA and PT (Table). FAs were
frequently small (<3 cm), hypoechoic masses with soft
or intermediate elasticity and without internal clefts
(Figure 1, Table). PTs were significantly larger than FAs
(Figure 2). Among the PTs, 11 lesions were ≥3 cm
(11/36, 30.6%), whereas only one lesion (1/32, 3.1%) was
>3 cm among FAs. The echogenicity in PTs was
frequently heterogeneous (PT = 13.9% vs. FA = 3.1%)
and showed a significantly more frequent complex
cystic and solid pattern (PT = 19.4% vs. FA = 0%). The
presence of internal clefts (PT = 44.4% vs. FA = 15.6%),
internal vascularity (PT = 75% vs. FA = 43.8%), and hard
elasticity (PT = 22.2% vs. FA = 3.1%) were significantly
more often observed in PT than in FA (Figure 3).
Table. Ultrasonographic findings in fibroadenomas and phyllodes tumours.
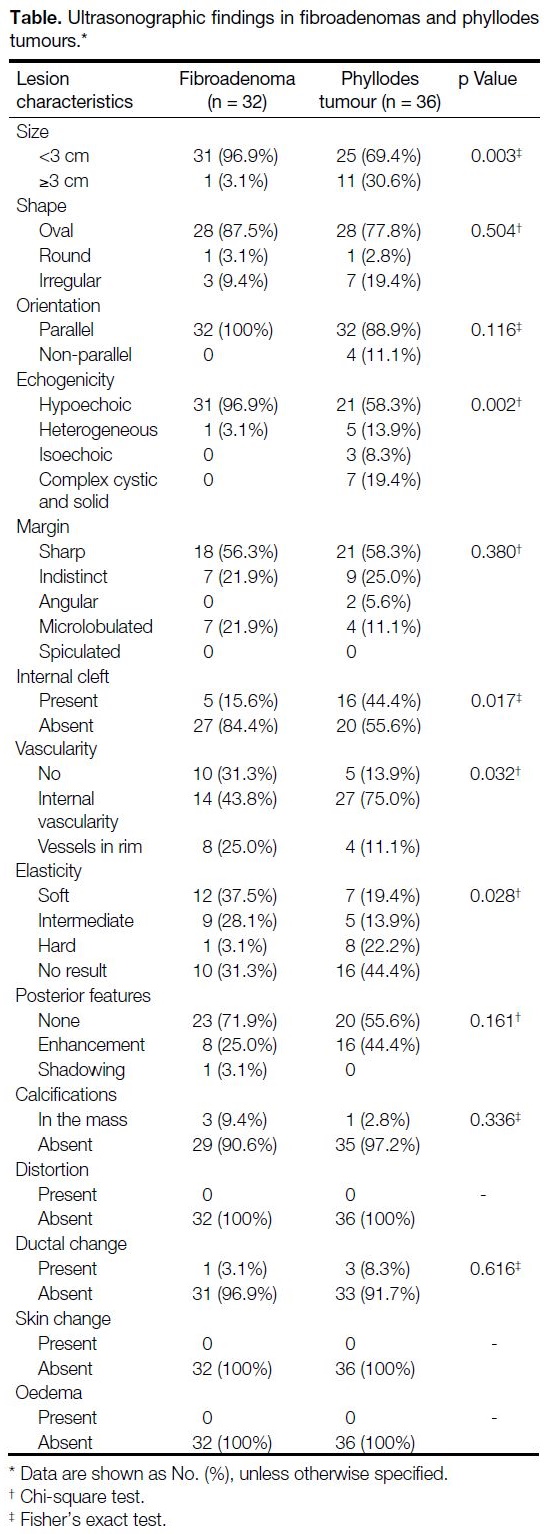
Figure 1. A 31-year-old woman with a palpable lump in the right breast. Ultrasonograms showing a 2.2 cm × 0.9 cm, oval, well-circumscribed,
parallel, hypoechoic mass (a) with minimal peripheral vascularity and no cleft formation (b) and intermediate elasticity (c, Emax = 70.4 kPa). Microscopic examination of the ultrasound-guided CNB (d, H&E stain, ×40) and surgical excision (e, H&E stain, ×40) showing periductal (arrows) and stromal (arrowhead) hyalinisation, suggestive of fibroadenoma.
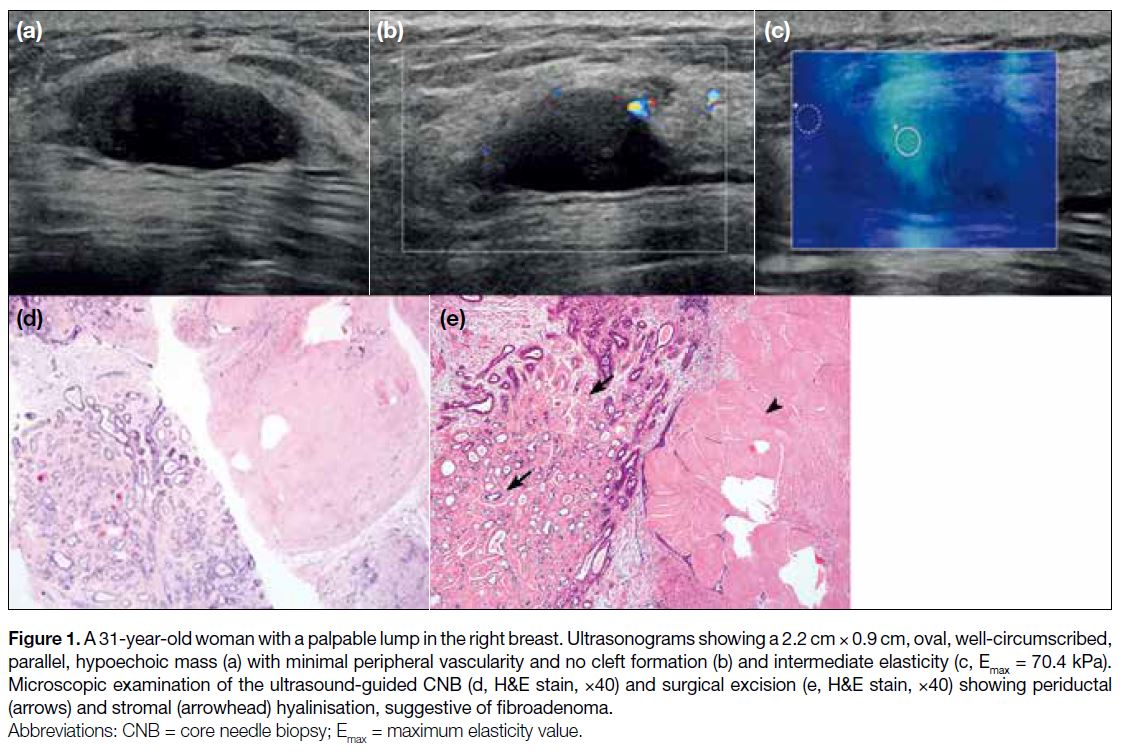
Figure 2. A 33-year-old woman with palpable lump in the right breast. Ultrasonograms showing an approximately 9-cm oval, well-circumscribed,
heterogeneous, hypoechoic mass with internal fluid-filled clefts (arrows in a, b), internal vascularity (c). Microscopic
examinations of ultrasound-guided core needle biopsy (d, H&E stain, ×40) and surgical excision (e, H&E stain, ×400) showing characteristic
leaf-like structures, suggestive of phyllodes tumour.
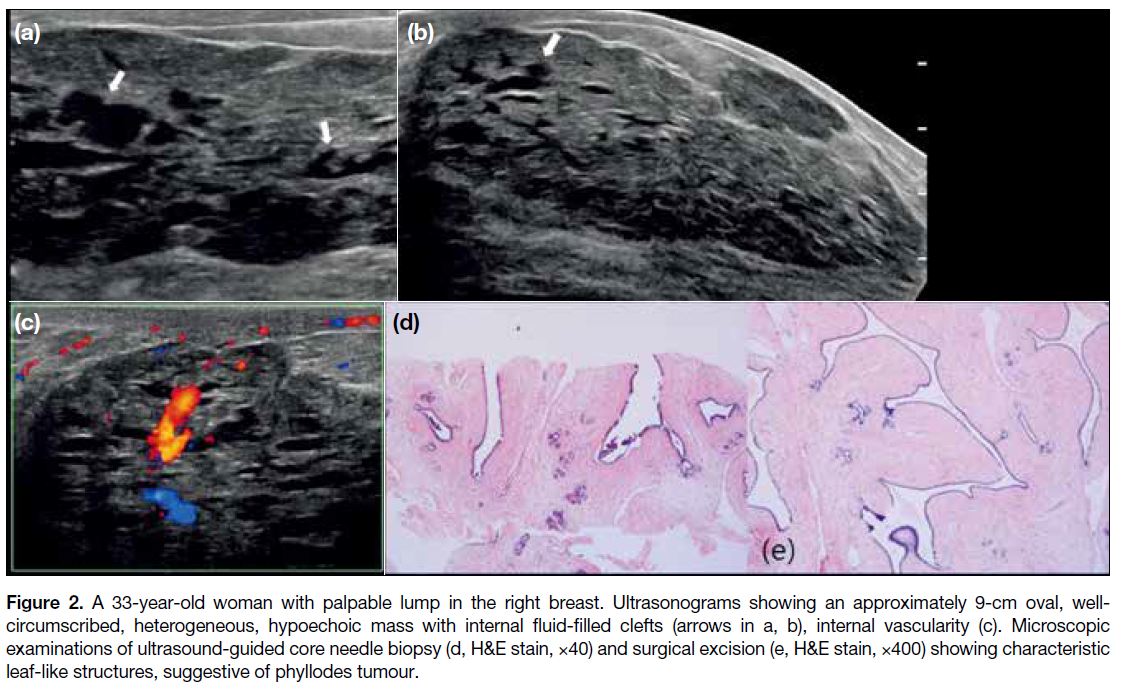
Figure 3. A 35-year-old woman with palpable lump in the left breast. Ultrasonograms showing a 2.5-cm oval, well-circumscribed, isoechoic
mass with an internal cystic cleft (arrow in a), internal vascularity (b) and hard elasticity (c, Emax = 85.2 kPa). Microscopic examinations of ultrasound-guided core needle biopsy (d, H&E stain, ×100) and surgical excision (e, H&E stain, ×100) showing mildly increased cellularity and cellular atypia in stroma, suggestive of a benign phyllodes tumour.
Other sonographic features including shape, orientation,
margin, posterior features, associated calcifications,
distortion, ductal change, skin change, and oedema
showed no significant differences between FA and PT.
The pathological results of CNB were similar to that
of surgical excision in 61 cases (61/68, 89.7%). Seven
cases with discordant results were all reported as FA on
CNB and benign PT at surgical excision.
DISCUSSION
In this study, we evaluated sonographic features that
could be helpful to distinguish between FA and PT.
Distinguishing between these two entities using imaging
features or CNB has been challenging to radiologists
and pathologists.[8] [9] Moreover, as US-guided CNB
specimens only reveal a part of the total lesion and as the
two disease entities can have overlapping histological
features, pathologists are often unable to make a
definitive distinction between FA and PT without an
excised specimen.[15] [16] [17]
We found that a large tumour with heterogeneous/complex cystic and solid echogenicity and the presence of internal clefts on greyscale ultrasonography are
characteristic findings of PT, in accordance with
previous studies.[3] [8] [9] [10] Duman et al[8] reported that FAs
were smaller than PTs and were homogeneously
hypoechoic masses, while PTs showed heterogeneous/complex cystic and solid echogenicity. The presence
of internal cystic lesions was significantly more
commonly detected in PTs than in FAs. The presence
of internal cystic lesions and clefts is regarded as a
useful US feature to diagnose PTs.[3] [9] [10] [18] In a study
by Wiratkapun et al,[3] the presence of cystic spaces
and clefts within the solid mass was significantly
associated with PTs on both univariate (p < 0.001) and
multivariate analyses (cystic space; p = 0.032, clefts;
p = 0.006). This finding is related to microscopic
features of PTs, with a prominent stromal proliferation
into the epithelial-lined spaces, forming a slit-like space
or a leaf-like pattern (Figure 3d and e).[19]
Posterior acoustic enhancement was also found to be an
important sonographic feature of PTs in some studies.[9] [20]
In our study, posterior acoustic enhancement was more
frequently observed in PTs, but not to a significant
degree (PT = 44.4% vs. FA = 25%; p = 0.161).
Colour Doppler US and elastography have been
investigated as ancillary tools to improve the diagnostic
accuracy of conventional B-mode US for breast lesions.
Kim et al[21] analysed the colour Doppler ultrasonography
and shear-wave elastography features of fibroepithelial
lesions and reported that PTs tend to have higher
stiffness and vascularity than FAs. The median Emax
was significantly higher in PTs than in FAs (76.7 vs.
21.0 kPa, p < 0.01), and a high vascularity (≥2 vessels)
on colour Doppler US was more frequent in PTs than in
FAs (p < 0.01).[21] Similar to that reported in a previous
study, internal vascularity (PT = 75% vs. FA = 43.8%;
p = 0.032) and hard elasticity (PT = 22.2% vs. FA = 3.1%;
p = 0.028) were more frequently seen in PTs than FAs in
this study. Histologically, on shear-wave elastography,
the more abundant cellular stroma in PTs is associated
with their hard elasticity.[15] [19]
A definitive distinction between FA and PT is
challenging, particularly with CNB specimens.[15] [16] [17]
Based on the degree of stromal hypercellularity,
stromal overgrowth, nuclear atypia, mitoses counts,
and the amount of stroma relative to the epithelium and
infiltrative borders of the tumour, PTs are distinguished
from FAs and categorised into benign, borderline, and
malignant lesions.[3] [12] [15] However, the representation of
these features in CNB has not been proven to be reliably
adequate for pathologists to make a definitive diagnosis
of PT using CNB.[12] Wiratkapun et al[3] found a high
concordance between pathologists’ suggested diagnosis
of FA or PT from CNB specimens and the surgical
pathology after excision (p < 0.001). Komenaka et al[22]
also found similar results, showing an 83% positive
predictive value of diagnosis for fibroepithelial tumours
using CNB. Our study showed a high diagnostic accuracy
of the pathological results of CNB for distinguishing
between FA and PT, which is similar to the findings with
previous studies.[3] [22]
There are several limitations to our study. First, this
study had a retrospective single-centre design. Second,
the small sample size could have prevented an accurate
statistical analysis, with a degree of selection bias as only
excised lesions were included. Third, the analysis in this
study was based the comparison of only ultrasonographic
findings with surgically confirmed cases of PT and FA.
CONCLUSION
In conclusion, ultrasonographic features can be helpful
imaging factors for differentiating FAs and PTs. Imaging features such as a large tumour size, heterogeneous or
complex cystic and solid echogenicity, presence of
internal clefts, internal vascularity, and hard elasticity
were significant findings in PT. US-guided CNB may
replace surgical excision with high diagnostic accuracy
in some cases.
REFERENCES
1. Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, van de Vijver MJ, editors.
WHO Classification of Tumours of the Breast. 4th ed. Geneva:
World Health Organization; 2012.
2. Karim RZ, O’Toole SA, Scolyer RA, Cooper CL, Chan B,
Selinger C, et al. Recent insights into the molecular pathogenesis
of mammary phyllodes tumours. J Clin Pathol. 2013;66:496-505. Crossref
3. Wiratkapun C, Piyapan P, Lertsithichai P, Larbcharoensub N.
Fibroadenoma versus phyllodes tumor: distinguishing factors in
patients diagnosed with fibroepithelial lesions after a core needle
biopsy. Diagn Interv Radiol. 2014;20:27-33. Crossref
4. Montagna G, Ng CK, Vlajnic T, Paradiso V, Dellas S, Reina H,
et al. Fibroepithelial breast lesion: when sequencing can help
to make a clinical decision. a case report. Clin Breast Cancer.
2019;19:e1-6. Crossref
5. Zhang Y, Kleer CG. Phyllodes tumor of the breast: histopathologic
features, differential diagnosis, and molecular/genetic updates. Arch
Pathol Lab Med. 2016;140:665-71. Crossref
6. Xiao M, Zhu Q, Jiang Y, Li J, Wang H, Zhang J, et al. Local
recurrent phyllodes tumors of the breast: clinical and sonographic
features. J Ultrasound Med. 2015;34:1631-8. Crossref
7. Barrio AV, Clark BD, Goldberg JI, Hoque LW, Bernik SF,
Flynn LW, et al. Clinicopathologic features and long-term
outcomes of 293 phyllodes tumors of the breast. Ann Surg Oncol.
2007;14:2961-70. Crossref
8. Duman L, Gezer NS, Balcı P, Altay C, Başara I, Durak MG, et al.
Differentiation between phyllodes tumors and fibroadenomas based
on mammographic sonographic and MRI features. Breast Care
(Basel). 2016;11:123-7. Crossref
9. Yilmaz E, Sal S, Lebe B. Differentiation of phyllodes tumors versus
fibroadenomas. Acta Radiol. 2002;43:34-9. Crossref
10. Bode MK, Rissanen T, Apaja-Sarkkinen M. Ultrasonography and
core needle biopsy in the differential diagnosis of fibroadenoma
and tumor phyllodes. Acta Radiol. 2007;48:708-13. Crossref
11. Shousha S. Issues in the interpretation of breast core biopsies. Int
J Surg Pathol. 2003;11:167-76. Crossref
12. Dillon MF, Quinn CM, McDermott EW, O’Doherty A, O’Higgins N, Hill AD. Needle core biopsy in the diagnosis of
phyllodes neoplasm. Surgery. 2006;140:779-84. Crossref
13. D’Orsi CJ, editor. 2013 ACR BI-RADS Atlas: Breast Imaging
Reporting and Data System. 5th ed. American College of
Radiology; 2013.
14. Lee SH, Chang JM, Cho N, Koo HR, Yi A, Kim SJ, et al. Practice
guideline for the performance of breast ultrasound elastography.
Ultrasonography. 2014;33:3-10. Crossref
15. Jacobs TW, Chen YY, Guinee DG Jr, Holden JA, Cha I,
Bauermeister DE, et al. Fibroepithelial lesions with cellular stroma
on breast core needle biopsy: Are there predictors of outcome on
surgical excision? Am J Clin Pathol. 2005;124:342-54. Crossref
16. Resetkova E, Khazai L, Albarracin CT, Arribas E. Clinical and
radiologic data and core needle biopsy findings should dictate
management of cellular fibroepithelial tumors of the breast. Breast
J. 2010;16:573-80. Crossref
17. Choi J, Koo JS. Comparative study of histological features between core needle biopsy and surgical excision in phyllodes tumor. Pathol
Int. 2012;62:120-6. Crossref
18. Chao TC, Lo YF, Chen SC, Chen MF. Sonographic features
of phyllodes tumors of the breast. Ultrasound Obstet Gynecol.
2002;20:64-71. Crossref
19. Tse GM, Niu Y, Shi HJ. Phyllodes tumor of the breast: an update.
Breast Cancer. 2010;17:29-34. Crossref
20. Buchberger W, Strasser K, Heim K, Müller E, Schröcksnadel H.
Phyllodes tumor: findings on mammography, sonography, and aspiration cytology in 10 cases. AJR Am J Roentgenol.
1991;157:715-9. Crossref
21. Kim GR, Choi JS, Han BK, Ko EY, Ko ES, Hahn SY. Combination
of shear-wave elastography and color Doppler: Feasible method
to avoid unnecessary breast excision of fibroepithelial lesions
diagnosed by core needle biopsy. PLoS One. 2017;12:e0175380. Crossref
22. Komenaka IK, El-Tamer M, Pile-Spellman E, Hibshoosh H. Core
needle biopsy as a diagnostic tool to differentiate phyllodes tumor
from fibroadenoma. Arch Surg. 2003;138:987-90. Crossref