Spontaneous Pancreaticoduodenal Fistula is a Rare Complication of Intraductal Papillary Mucinous Neoplasm: a Case Report
CASE REPORT
Spontaneous Pancreaticoduodenal Fistula is a Rare Complication of Intraductal Papillary Mucinous Neoplasm: a Case Report
AHC Wong1, ECH Lai2, EMF Wong1
1 Department of Radiology, Pamela Youde Nethersole Eastern Hospital, Hong Kong
2 Department of Surgery, Pamela Youde Nethersole Eastern Hospital, Hong Kong
Correspondence: Dr AHC Wong, Department of Radiology, Pamela Youde Nethersole Eastern Hospital, Hong Kong. Email: amy.hc.wong@gmail.com
Submitted: 13 Aug 2020; Accepted: 7 Oct 2020.
Contributors: All authors designed the study and acquired the data. AHCW and EMFW analysed the data. AHCW drafted the manuscript. All
authors critically revised the manuscript for important intellectual content.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: The patient was treated in accordance with the tenets of the Declaration of Helsinki. The patient provided written informed
consent for all treatments and procedures.
Declaration: This case report was presented as a poster at the 8th Joint Scientific Meeting of the Royal College of Radiologists and the Hong
Kong College of Radiologists and 27th Annual Scientific Meeting of the Hong Kong College of Radiologists, held on 16-17 November 2019 in
Hong Kong.
INTRODUCTION
Pancreatic cystic lesions are increasingly identified as
cross-sectional imaging becomes more readily available.
The prevalence of pancreatic cystic lesions is up to 49.5%
in the general population.[1] In patients who undergo
abdominal imaging, 13.5% are found to have incidental
pancreatic cystic lesions.[2] Intraductal papillary mucinous
neoplasm (IPMN) is among the most common pancreatic
cystic lesions; it is characterised by the proliferation
of mucin-secreting papillary epithelial cells with
consequent dilatation of main or branch pancreatic ducts.
Owing to its potential for malignant transformation,
IPMNs are of particular interest to radiologists and
clinicians alike. Although patients with IPMN are often
asymptomatic, they can also present with abdominal
pain, jaundice, weight loss and pancreatitis; fistulation to
adjacent organs is a very rare complication of IPMN. We
report a case of spontaneous pancreaticoduodenal fistula
secondary to IPMN.
CASE REPORT
An 81-year-old man with a history of carcinoma of
the rectum treated by laparoscopic anterior resection
presented with intermittent epigastric pain for 1 week.
He denied any symptoms of jaundice, pale stool, tea-stained
urine, weight loss or fever. On examination he
was afebrile and was not jaundiced; he had epigastric
tenderness but no guarding. His blood results showed
an elevated white cell count of 13.64 × 109/L (normal
range 3.7-9.3 × 109/L) with normal amylase, liver and
renal function. He underwent a computed tomography
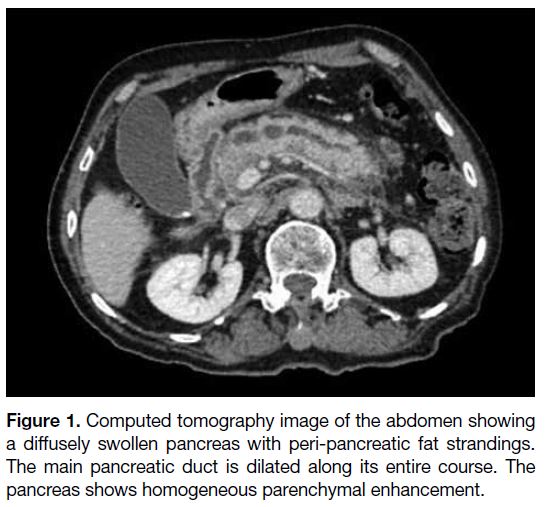
(CT) abdomen and pelvis that demonstrated a diffusely
swollen pancreas with homogeneous parenchymal
enhancement and a grossly dilated main pancreatic
duct, up to 1.3 cm at the pancreatic head (Figure 1). The
pancreatic ductal system had a bifid configuration with
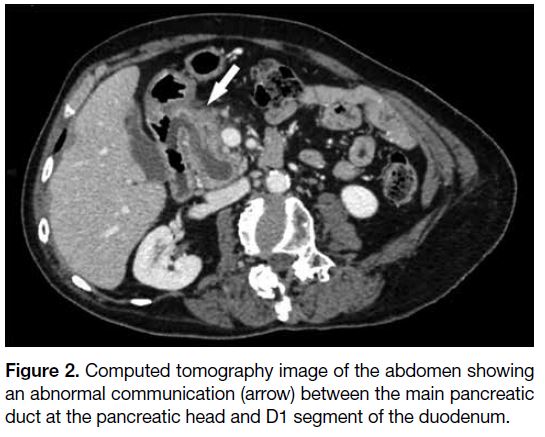
a dominant duct of Wirsung. There was an abnormal
communication between the main pancreatic duct and
adjacent D1 segment of the duodenum (Figure 2). No intramural nodule was found within the dilated main
pancreatic duct. Overall CT findings were suggestive of
pancreaticoduodenal fistula secondary to IPMN. He was
treated as a case of mild pancreatitis and discharged home.
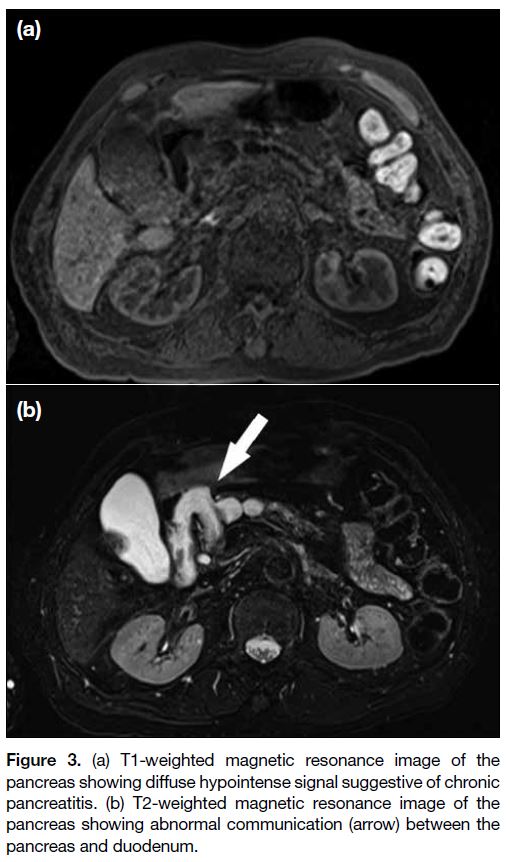
Subsequent magnetic resonance images of the pancreas
showed similar findings to the CT with an abnormal
communication between the pancreas and duodenum
(Figure 3). There was a small number of dependent
hypointense signals within the main pancreatic duct,
suggestive of mucin. The pancreatic parenchyma showed
diffuse T1 hypointense signals suggestive of chronic
pancreatitis; no enhancing components were seen in the
arterial or portovenous phases. The patient underwent
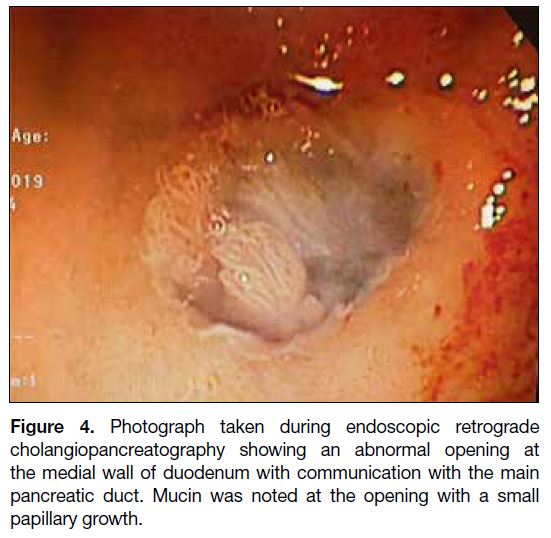
endoscopic retrograde cholangiopancreatography as an outpatient that demonstrated an abnormal opening over
the medial wall of D1 with mucus plugging and small
internal papillary growth. This confirmed the diagnosis
of pancreaticoduodenal fistula secondary to IPMN
(Figure 4). Biopsy results revealed moderate dysplasia.
His carbohydrate antigen 19-9 level was 44.8 U/mL
(normal range <35 U/mL). Surgery was offered to the
patient at the time but was declined given his advanced
age.
Figure 1. Computed tomography image of the abdomen showing
a diffusely swollen pancreas with peri-pancreatic fat strandings.
The main pancreatic duct is dilated along its entire course. The
pancreas shows homogeneous parenchymal enhancement.
Figure 2. Computed tomography image of the abdomen showing
an abnormal communication (arrow) between the main pancreatic
duct at the pancreatic head and D1 segment of the duodenum.
Figure 3. (a) T1-weighted magnetic resonance image of the
pancreas showing diffuse hypointense signal suggestive of chronic
pancreatitis. (b) T2-weighted magnetic resonance image of the
pancreas showing abnormal communication (arrow) between the
pancreas and duodenum.
Figure 4. Photograph taken during endoscopic retrograde
cholangiopancreatography showing an abnormal opening at
the medial wall of duodenum with communication with the main
pancreatic duct. Mucin was noted at the opening with a small
papillary growth.
On routine follow-up 15 months after his initial
presentation, the patient was found to have grossly
deranged liver function with an elevated bilirubin
64 μmol/L (normal range 3-21 μmol/L), alkaline
phosphatase 473 IU/L (normal range 47-168 IU/L)
and alanine aminotransferase 254 IU/L (normal range
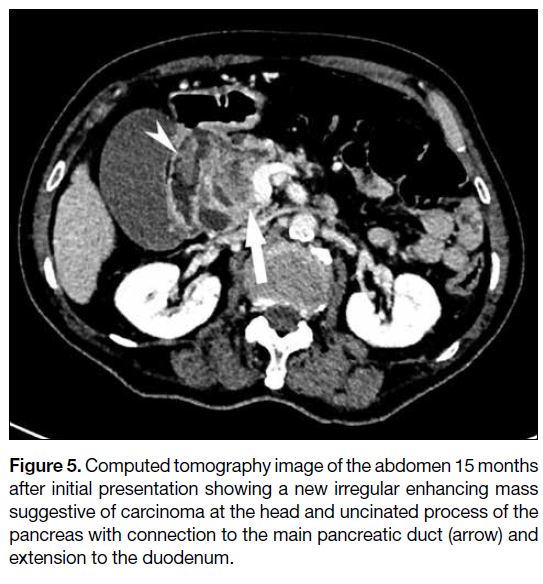
<49 IU/L). CT abdomen demonstrated a new heterogeneous and irregular lesion with enhancing
solid components at the pancreatic head and uncinated
process (Figure 5) suggestive of carcinoma. The
lesion was connected to the main pancreatic duct with
suspicious extension to the duodenum via the fistula.
The common bile duct was now grossly dilated to
1.9 cm with associated bilateral intrahepatic duct
dilatation. Most recent carbohydrate antigen 19-9 was markedly elevated at 90.0 U/mL. The patient
underwent internal external percutaneous transhepatic
biliary drainage with subsequent biliary stent insertion
and his liver function gradually improved. The lesion
was deemed inoperative, and the patient subsequently
received palliative radiotherapy.
Figure 5. Computed tomography image of the abdomen 15 months
after initial presentation showing a new irregular enhancing mass
suggestive of carcinoma at the head and uncinated process of the
pancreas with connection to the main pancreatic duct (arrow) and
extension to the duodenum.
DISCUSSION
IPMN is one of the most common pancreatic cystic
lesions, classified according to its location: main duct
IPMN, branch duct IPMN, or mixed type IPMN. They
can also be classified according to varying degrees of
dysplasia, ranging from benign adenoma to invasive
carcinomas. Main duct IPMN is defined as diffuse or
segmental dilatation of the main pancreatic duct >5 mm
in the absence of other causes of obstruction. Main
duct IPMN is known to have a higher risk of malignant
transformation of up to 60% whereas the risk of
malignancy of branch duct IPMN is 11%.[3] [4] Magnetic
resonance cholangiopancreatography is currently
considered the investigation of choice to evaluate
pancreatic cystic lesions as it allows better visualisation
of ductal communication, nodules and septae.[5]
Most IPMNs do not progress to carcinoma, but
radiological risk stratification remains an important part
of clinical assessment. Patients with lesions with high-risk
features can be offered early surgical intervention
or appropriate follow-up examination. The international
consensus Fukuoka guidelines describe several features
that suggest a high risk for malignancy, including a main
pancreatic duct diameter ≥10 mm, enhancing mural
nodule >5 mm and biliary obstruction. Patients with
these features who are surgically fit should undergo
surgical resection. Patients with worrisome features
including cyst size ≥3 cm, enhancing mural nodule
<5 mm, or main duct diameter 5 to 9 mm should
undergo endoscopic ultrasound. Surgery should be
considered in the presence of any of the following on
endoscopic ultrasound: mural nodules ≥5 mm, main
duct features suspicious of involvement, or cytology
suspicious of or positive for malignancy. If no such
feature is present, patients should be followed up with
either CT, magnetic resonance imaging or endoscopic
ultrasound, depending on the size of the lesion.[5]
Although many patients with IPMN remain
asymptomatic, some present with symptoms such as
abdominal pain or weight loss, or complications such as
pancreatitis. Fistulation to the adjacent organs is a very
rare complication of IPMN with only a few reports in the literature. In a retrospective study of 423 patients
with IPMN, 26 fistulas to adjacent organs were found
in eight patients (1.9%).[6] The majority of fistulas were
found in the duodenum, followed by the stomach,
common bile duct and the colon. The type of IPMN
most commonly involved with fistulation was main duct
IPMN, followed by mixed type IPMN and branch duct
IPMN. Although the imaging findings of all patients with
IPMNs and fistulations were suggestive of malignancy,
histology from 27% of fistulas following surgery did
not demonstrate atypia. It was previously thought that
fistulations could only be found in malignant IPMNs,
but studies have now shown that they can also be found
in benign lesions.[6] [7] [8] Hypotheses on the pathogenesis of
fistula formation include excessive mucin production
resulting in an increase in mechanical pressure on the
pancreatic ducts or direct invasion by the primary
tumour.[9] [10]
CONCLUSION
Fistulation to adjacent organs is a rare complication
of IPMN and can occur in both malignant and benign
lesions. The duodenum is the most commonly involved
organ. Radiologists and clinicians should be aware
of this potential complication since pancreatic cystic
lesions including IPMN are increasingly detected due to
wide availability of cross-sectional imaging. We present
a rare case of spontaneous pancreaticoduodenal fistula
secondary to IPMN that later progressed to carcinoma.
REFERENCES
1. Kromrey ML, Bülow R, Hübner J, Paperlein C, Lerch MM, Ittermann T, et al. Prospective study on the incidence, prevalence
and 5-year pancreatic-related mortality of pancreatic cysts in a
population-based study. Gut. 2018;67:138-45. Crossref
2. Lee KS, Sekhar A, Rofsky NM, Pedrosa I. Prevalence of incidental
pancreatic cysts in the adult population on MR imaging. Am J
Gastroenterol. 2010;105:2079-84. Crossref
3. Salvia R, Fernández-del Castillo C, Bassi C, Thayer SP, Falconi M,
Mantovani W, et al. Main-duct intraductal papillary mucinous
neoplasms of the pancreas: clinical predictors of malignancy and
long-term survival following resection. Ann Surg. 2004;239:678-85. Crossref
4. Rodriguez JR, Salvia R, Crippa S, Warshaw AL, Bassi C,
Falconi M, et al. Branch-duct intraductal papillary mucinous
neoplasms: observations in 145 patients who underwent resection.
Gastroenterology. 2007;133:72-9. Crossref
5. Tanaka M, Fernández-del Castillo C, Kamisawa T, Jang JY,
Levy P, Ohtsuka T, et al. Revisions of international consensus
Fukuoka guidelines for the management of IPMN of the pancreas.
Pancreatology. 2017;17:738-53. Crossref
6. Ravaud S, Laurent V, Jausset F, Cannard L, Mandry D, Oliver A,
et al. CT and MR imaging features of fistulas from intraductal
papillary mucinous neoplasms of the pancreas to adjacent organs:
a retrospective study of 423 patients. Eur J Radiol. 2015;84:2080-8. Crossref
7. Koizumi M, Sata N, Yoshizawa K, Tsukahara M, Kurihara K,
Yasuda Y, et al. Post-ERCP pancreatogastric fistula associated with
an intraductal papillary-mucinous neoplasm of the pancreas — a
case report and literature review. World J Surg Oncol. 2005;3:70. Crossref
8. Jausset F, Delvaux M, Dumitriu D, Bressenot A, Bruot O,
Mathias J, et al. Benign intraductal papillary-mucinous neoplasm
of the pancreas associated with spontaneous pancreaticogastric and
pancreaticoduodenal fistulas. Digestion. 2010;82:42-6. Crossref
9. Okada K, Furuuchi T, Tamada T, Sasaki T, Suwa T, Shatari T,
et al. Pancreatobiliary fistula associated with an intraductal
papillary-mucinous pancreatic neoplasm manifesting as obstructive
jaundice: report of a case. Surg Today. 2008;38:371-6. Crossref
10. Harino T, Tomimaru Y, Noguchi K, Nagase H, Ogino T, Hirota M,
et al. A case of intraductal papillary-mucinous neoplasm of the
pancreas penetrating into the stomach and spleen successfully
treated by total pancreatectomy. Surg Case Rep. 2018;4:117. Crossref