Clinicopathological Features, Prognostic Factors, and Treatment Outcomes in Non-metastatic Breast Cancer in Young Asian Women in Hong Kong
ORIGINAL ARTICLE CME
Clinicopathological Features, Prognostic Factors, and Treatment Outcomes in Non-metastatic Breast Cancer in Young Asian Women in Hong Kong
HS Chung1, JCH Chow1, MHC Lam2, RKC Ngan3, KH Wong1
1 Department of Clinical Oncology, Queen Elizabeth Hospital, Hong Kong
2 Department of Oncology, United Christian Hospital, Hong Kong
3 Department of Clinical Oncology, The University of Hong Kong, Gleneagles Hospital Hong Kong, Hong Kong
Correspondence: Dr HS Chung, Department of Clinical Oncology, Queen Elizabeth Hospital, Hong Kong. Email: hiushunchung@gmail.com
Submitted: 15 Jun 2021; Accepted: 16 Nov 2021.
Contributors: HSC, JCHC and MHCL designed the study. HSC acquired the data. All authors analysed the data. HSC drafted the manuscript. All authors critically revised the manuscript for important intellectual content.
Conflicts of Interest: As an editor of the journal, RKC Ngan was not involved in the peer review process. Other authors have disclosed no conflicts of interest.
Funding/Support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: The study was approved by the Research Ethics Committee of the Hospital Authority (Ref KC/KE-20-0220/ER-1). The requirement for patient consent was waived.
Abstract
Objectives
Breast cancer is the leading cause of death in young women (<40 years) and is a distinct entity. We
reviewed the clinicopathological features and survival outcomes of young females with breast cancer in Hong Kong.
Methods
We performed a retrospective study of 497 women <40 years with non-metastatic breast cancer from a single
institution in Hong Kong from 2005 to 2013, analysing clinicopathological, prognostic, survival, and treatment data.
Results
Median age at diagnosis was 36 years. The majority of patients (87.7%) had invasive ductal carcinoma.
Grade III tumours composed approximately 40%. Proportions of stage I, II and III diseases were 34.8%, 46.1%,
and 18.1%, respectively. Hormone receptor status was positive in 80.7%; human epidermal growth factor receptor 2
status was positive in 27.2%. In all, 53.7% underwent mastectomy while 46.3% received breast-conserving surgery.
In total, 85.1% had neoadjuvant and/or adjuvant chemotherapy. Adjuvant radiotherapy was delivered in 78.5% and
hormonal therapy was given in 73.4%. Over a 9.1-year median follow-up, 26% developed recurrence and 16.1%
died. The 5-year and 10-year disease-free survival were 82.1% and 74.3%, respectively. The 5-year and 10-year
overall survival were 90.5% and 83.5%, respectively. Nodal stage was the only independent prognostic factor for
disease-free survival and overall survival.
Conclusion
Breast cancer in young patients tended to have aggressive features and presented at an advanced stage.
Breast cancer in young Asian women may have distinct phenotypes with more hormone-positive disease compared
to Western patients and warrants further investigations. Improved survival may be achieved with multimodality
treatments.
Key Words: Breast neoplasms; Female; Prognosis; Treatment outcome
中文摘要
香港年輕亞洲女性非轉移性乳腺癌的臨床病理學特徵、預後因素和治療結果
鍾曉信、周重行、林河清、顏繼昌、黃錦洪
目的
乳腺癌是40歲以下年輕女性死亡的主要原因,是一個獨特實體。本文回顧香港年輕女性乳腺癌的臨床病理學特徵和存活結局。
方法
我們對2005年至2013年香港一家機構的497名40歲以下非轉移性乳腺癌女性進行回顧性研究,分析他們的臨床病理學、預後、存活和治療數據。
結果
患者平均年齡為36歲。大多數病例(87.7%)為浸潤性導管癌。組織學III級佔約40%。I、II和III期數分別佔34.8%、46.1%和18.1%。患者屬荷爾蒙受體陽性為80.7%;HER2擴增率為27.2%。整體而言,53.7%患者進行全乳切除術,46.3%則接受保乳手術。85.1%患者接受術前或術後輔助化療。78.5%病例進行輔助電療以及73.4%接受荷爾蒙治療。跟進期中位數為9.1年,有26%病例復發及16.1%患者死亡。5年和10年無病存活率分別為82.1%和74.3%。5年和10年總存活率分別為90.5%和83.5%。淋巴結轉移是唯一的獨立預後因素。
結論
年輕乳腺癌患者傾向有較高的惡性度及腫瘤期數。亞洲乳腺癌患者有獨特的表型,比起西方患者具有更多荷爾蒙受體陽性的病例,需要進一步研究。綜合治療方案能改善存活率。
INTRODUCTION
Breast cancer diagnosed at a young age requires special
attention due to its specific clinicopathological features
and unique psychosocial sequelae. It is associated
with more aggressive biology, advanced stage, and
unfavourable prognosis with increased risk of recurrence
and mortality, as compared with the disease in older
patients.[1] In fact, the differences in risk factors, gene
expression, tumour characteristics, and clinical outcomes
suggest that breast cancer arising in young women
represents a distinct entity, as reported in previous
studies.[2] [3] Because of the nature of the disease, these
patients usually undergo more aggressive multimodality
treatments that may have significant complications,
including reduced fertility and genetic concerns, which
can all pose serious impacts on patients’ lives.
Currently, there is no widely agreed consensus on the
definition of a cut-off for young age for breast cancer.
The cut-off differs among studies, but most have used
40 years of age as a threshold.[4] Guidelines have been
developed for management of this distinct disease entity.[5]
Due to its relative rarity, breast cancers in young patients are often underrepresented in clinical studies. Moreover, the majority of reports have been based on Western
cohorts and data on Asians remain limited. Breast
cancer tends to occur earlier in Chinese women than in
Caucasian women,[6] and its incidence in the under-40
Asian population may be two to three-fold greater.[7] In
Hong Kong, approximately 7.7% of all breast cancers
are diagnosed before age 40 years according to the
most recent statistics.[8] More evidence on biological
characteristics and treatment strategies is needed to help
clinicians better manage the disease in this age-group.
The aim of this study was to evaluate the
clinicopathological features and survival outcomes of
young female breast cancer in the Hong Kong Chinese
population. In addition, we investigated the differences
among various molecular subtypes and assessed the
prognostic factors of survival.
METHODS
All consecutive, non-metastatic, invasive female breast cancer cases from January 2005 to December 2013 were
retrospectively identified from a single institutional
breast cancer database. Inclusion criterion was age
<40 years at the time of diagnosis. Exclusion criteria
were non-Chinese ethnicity and incomplete clinical data.
Medical records were reviewed and details of
clinicopathological features, surgery, and adjuvant
therapies were recorded. Surgery and adjuvant treatments
were performed according to standard guidelines at
the time of diagnosis (Table 1, online supplementary Appendix).
Cancer staging was carried out according to the AJCC
(American Joint Committee on Cancer) system (7th
ed). For tumour and nodal classifications, if patients
underwent neoadjuvant therapy, clinical or pathological
stage, whichever was higher, was used to reflect the
tumour burden more accurately. Hormone receptor
(HR) expression, including oestrogen receptor (ER)
and progesterone receptor (PR), was evaluated based on
the percentage of tumour cells with nuclear staining by
immunohistochemistry. The threshold for ER and PR
positivity was ≥1%. Human epidermal growth factor
receptor 2 (HER2) positivity was defined as either 3+
staining by immunohistochemistry, or the presence
of HER2 amplification by in situ hybridisation. Breast
cancer subtype projection was determined according to
HR status, HER2 status and tumour grade (Ki-67 index
was not universally available thus not included in the
projection)[9] [10]: luminal A (ER+/PR+, HER2-, grade 1-2),
luminal B (ER+/PR+, HER2+, grade 1-2 or ER+/PR+,
HER2+/-, grade 3), HER2-amplified (ER-, PR-, HER2+)
and basal-like (ER-, PR-, HER2-).
Statistical Analysis
Duration of follow-up was measured from the date of
histological diagnosis to the date of death or data cut-off,
i.e., 1 September 2020. Disease-free survival (DFS) was
defined as the duration from diagnosis to any recurrence
(local, regional, or distant relapse), contralateral breast
cancer, or death of any cause. Overall survival (OS) was
defined as the duration from diagnosis to death of any
cause.
Descriptive analyses were used to summarise patient
demographics, tumour pathological characteristics,
treatment details, and relapse pattern. The Kaplan–Meier method was used to estimate DFS and OS.
Prognostic factors were evaluated using log-rank test
and multivariable Cox regression. Significant variables
identified from the univariate analysis were included in
the Cox proportional hazard model. Statistical analyses
were conducted using SPSS (Windows version 24.0;
IBM Corp, Armonk [NY], United States). A p value
<0.05 was considered statistically significant.
RESULTS
Clinical and Pathological Features
All 6370 consecutive, non-metastatic, invasive breast
cancer cases during the study period from January 2005
to December 2013 were identified. Of them, 538 (8.4%)
were age <40 years at the time of diagnosis. A total of
41 cases were excluded: 31 had non-Chinese ethnicity
and 10 had incomplete clinical data. Finally, 497 cases
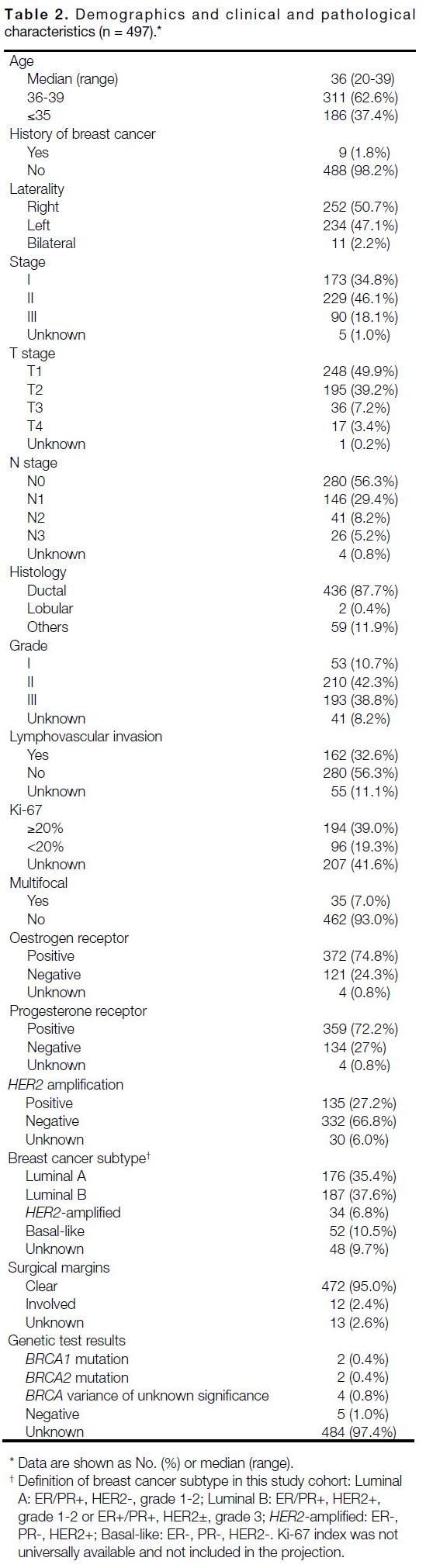
were eligible and included in the analysis (Table 2). The
median age at diagnosis was 36 years (range, 20-39),
37.4% were aged ≤35 years. Nine (1.8%) patients had
a history of breast cancer. Tumour laterality was even,
while 11 (2.2%) patients had synchronous bilateral
breast cancer.
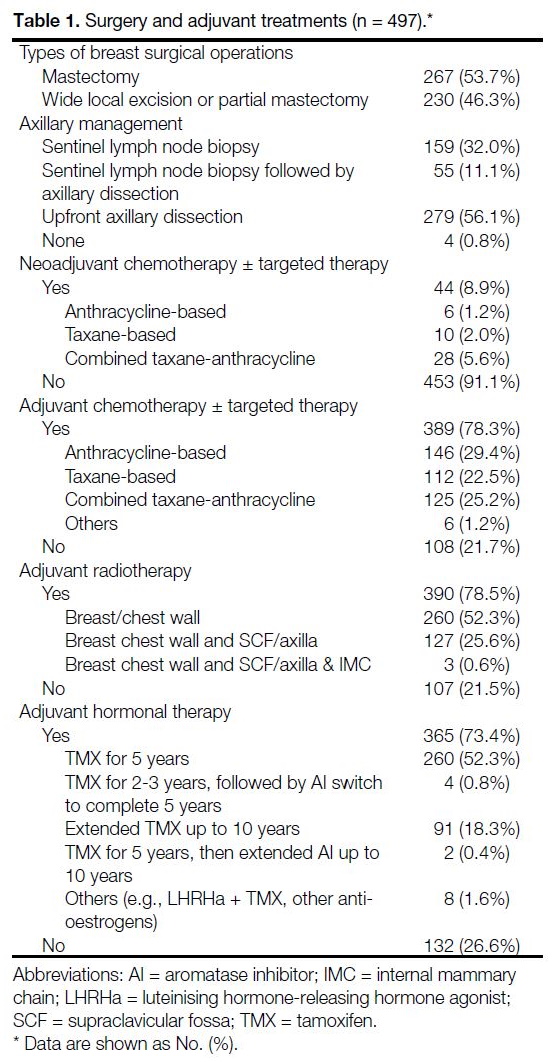
Table 1. Surgery and adjuvant treatments (n = 497)
Group stage at presentation was I (34.8%), II (46.1%)
and III (18.1%), respectively. The majority of the
tumours were invasive ductal carcinoma subtype
(87.7%), whereas invasive lobular histology constituted
0.4%. The most common histological grade was II
(42.3%), followed by III (38.8%) and I (10.7%). In all,
39% of the tumours had high Ki-67 index (≥20%) and
7% had multifocal disease. Lymphovascular invasion
and involved margins were present in 32.6% and 2.4%
of tumours, respectively.
Overall, 401 (80.7%) cases were of hormone-positive
disease (either ER or PR). In total, 135 (27.1%) of breast
cancers were HER2+. Breast cancer subtypes were
distributed as follows: 35.4% luminal A, 37.6% luminal
B, 6.8% HER2-amplified, 10.5% basal-like, and 9.7%
unclassified.
Genetic test results were available in 13 cases at the time of data analysis. Five cases had documented negative
results, while two patients had the BRCA1 mutation,
two had the BRCA2 mutation, and four had variances of
unknown significance.
Surgery and Adjuvant Treatments
Information about surgical and adjuvant oncological
treatment are detailed in Table 1. All 497 patients
underwent surgery. A total of 267 (53.7%) patients
had mastectomy, while 230 (46.3%) received breast-conserving
surgery. Axillary management included
sentinel lymph node biopsy in 159 (32%), sentinel
lymph node biopsy followed by axillary dissection in 55
(11.1%), and upfront axillary dissection in 279 (56.1%).
Table 2. Demographics and clinical and pathological characteristics (n = 497)
Among these patients, 390 (78.5%) patients underwent adjuvant radiotherapy. In all, 260 (52.3%) received
local radiotherapy to either breast or the chest wall. A
total of 127 (25.6%) patients underwent locoregional
radiotherapy that included the supraclavicular fossa or
axilla. Irradiation of the internal mammary chain was not
a routine practice in our institution.
Overall, 423 (85.1%) cases received neoadjuvant
and/or adjuvant chemotherapy, typically anthracycline
or/and taxane-based. Anti-HER2 monoclonal antibody,
trastuzumab, was administered to 54.8% HER2+
cases (74/135). We attempted to determine the rate of
chemotherapy-related amenorrhoea (CRA), which was
defined as amenorrhoea for ≥3 months during and within
12 months after the completion of adjuvant chemotherapy
in this study. Of 423 cases undergoing chemotherapy,
150 (35.5%) developed CRA, and 200 (47.3%) did
not experience CRA, while menstrual history was not
available for 73 cases (17.3%). Of those who had CRA,
113 (75.3%) regained menstruation.
Among the 401 cases with hormone-positive disease
(ER or PR+), 365 (91%) received hormonal therapy.
Most cases received tamoxifen for at least 5 years,
whereas some also received extended tamoxifen for up
to 10 years, which is the current standard of care for
selected high-risk cases. However, the discontinuation
rate of adjuvant endocrine treatment (excluding disease
progression) was approximately 10.4% (38/365). The
most common reasons were adverse effects (including
hot flashes, mood swings, and vaginal dryness) or plans for pregnancy (needed interruption of treatment due to
teratogenicity of tamoxifen).
Survival Outcomes and Relapse Pattern
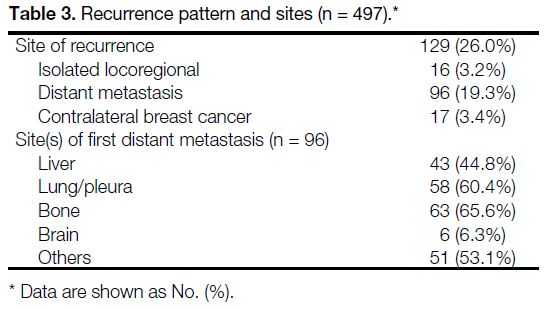
Over a median follow-up time of 9.1 years (range, 0.5-15.4), 129 (26%) cases had disease recurrences and 80
(16.1%) patients had died at the time of data collection.
The relapse patterns and sites are presented in Table 3. In total, 16 (3.2%) cases developed isolated locoregional
recurrence and 96 (19.3%) had distant metastases.
Seventeen (3.4%) patients developed contralateral breast
cancer. The most common sites of distant metastases were bone (n = 63), followed by lung or pleura (n = 58),
and liver (n = 43).
Table 3. Recurrence pattern and sites (n = 497)
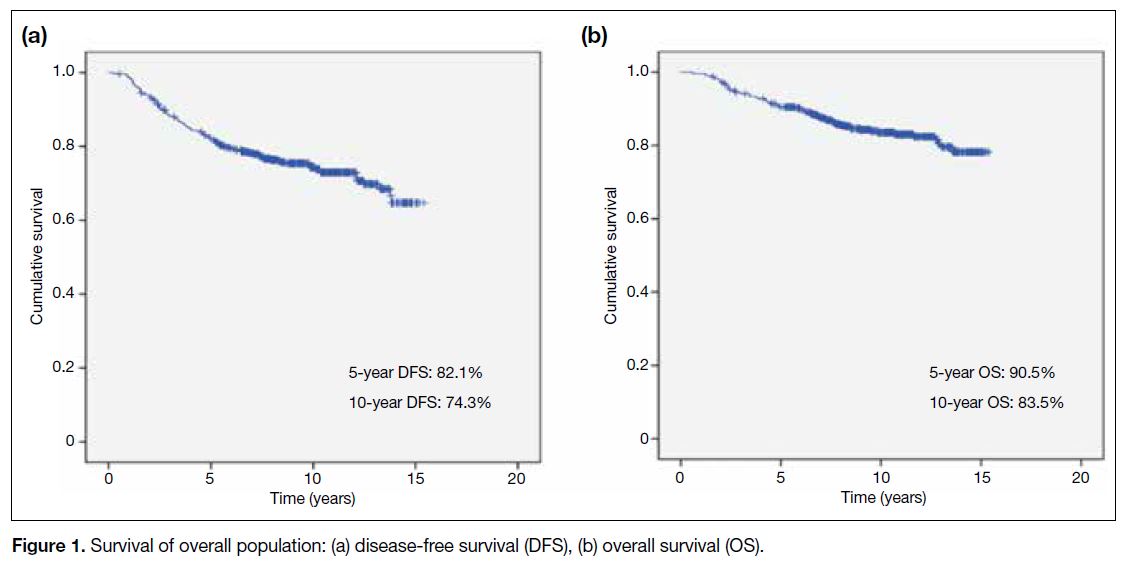
The survival curves of the overall population are shown in Figure 1. DFS at 5 years and 10 years was 82.1%
and 74.3%, respectively. OS at 5 years and 10 years
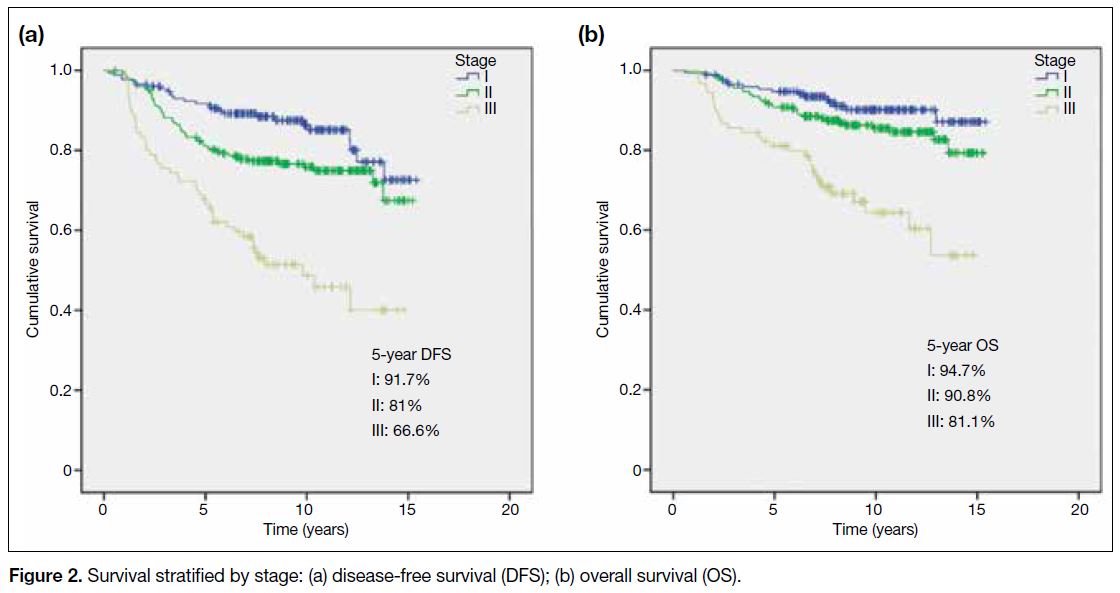
was 90.5% and 83.5%, respectively. Figure 2 depicts
the stage-specific survivals. The survival outcomes
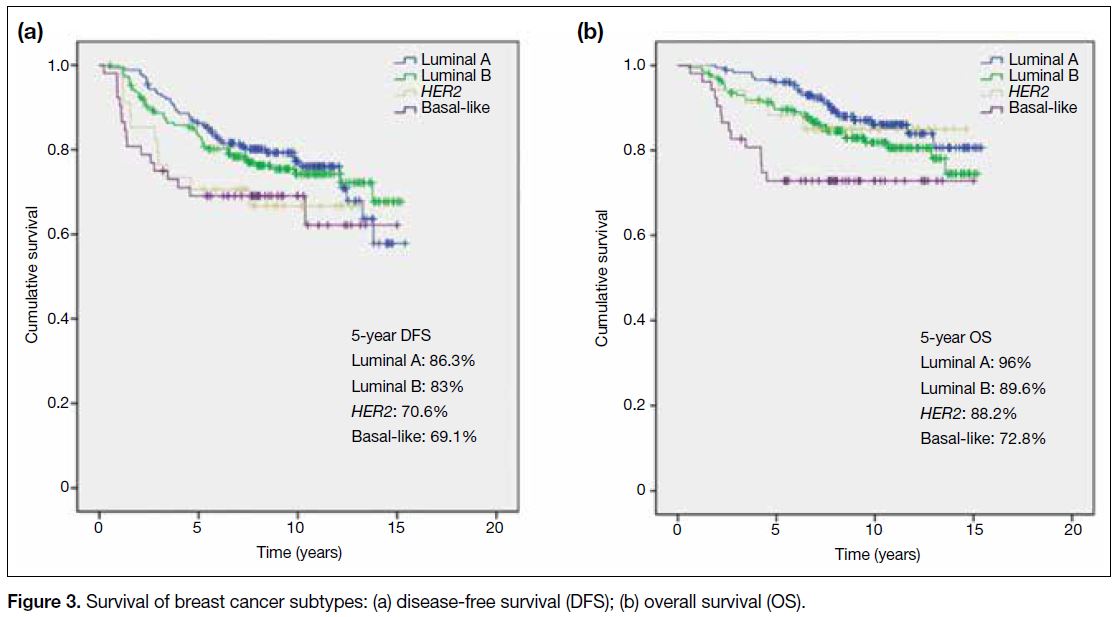
for different breast cancer subtypes are illustrated in
Figure 3; luminal A and B phenotypes had the best
prognoses, followed by HER2+ disease, while basal-like
tumours had the worst survival.
Figure 1. Survival of overall population: (a) disease-free survival (DFS), (b) overall survival (OS)
Figure 2. Survival stratified by stage: (a) disease-free survival (DFS); (b) overall survival (OS)
Figure 3. Survival of breast cancer subtypes: (a) disease-free survival (DFS); (b) overall survival (OS)
Prognostic Factors for Disease-free Survival
and Overall Survival
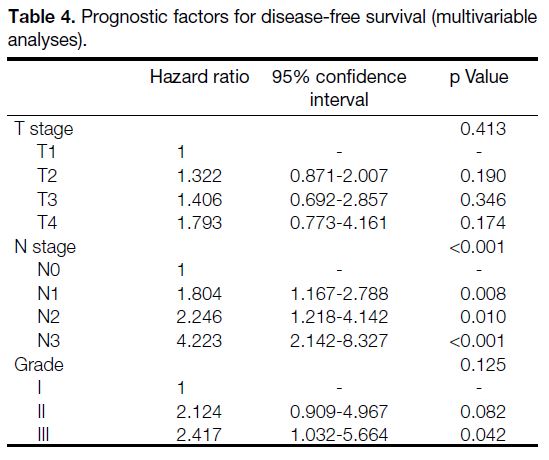
The clinicopathological prognostic factors for DFS and OS are highlighted in Tables 4 and 5. Ki-67 index was
not included in the model because of the high proportion
(41.6%) of missing data. In univariate analyses, high
T-stage, N-stage, and tumour grade were associated
with a higher risk of disease recurrence. In multivariable
analyses, advanced nodal status remained as the only
independent negative prognostic factor for DFS (p < 0.001).
Table 4. Prognostic factors for disease-free survival (multivariable analyses)
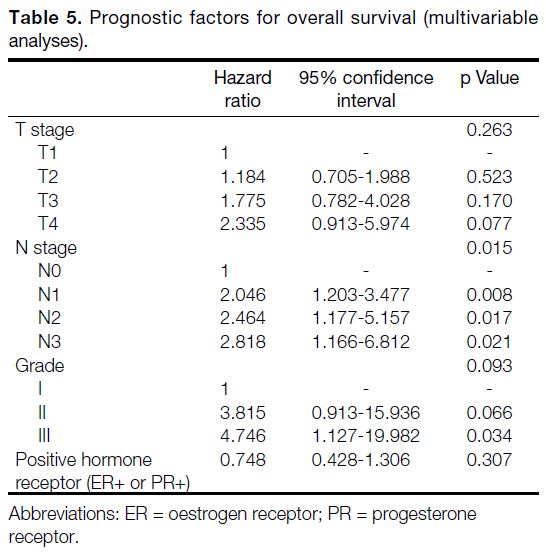
Table 5. Prognostic factors for overall survival (multivariable analyses)
For OS, in univariate analyses, high T-stage, N-stage, and grade were significant negative prognostic factors,
while positive hormonal status was associated with better
OS. Multivariate analyses revealed only advanced nodal
status to be an independent factor for OS (p = 0.015).
DISCUSSION
To our knowledge, the present study is the first in the literature to report the clinicopathological profile and
treatment outcomes of young breast cancer cases in the
Hong Kong Chinese population. Although this study was
based on a single centre, our hospital had a large patient
population and detailed records from an institutional
breast cancer registry. With almost 500 patients and a 9-year follow-up period in this study, we were able to
analyse and report the long-term clinical outcomes in an
in-depth manner.
The biological features of breast cancer under age 40
were consistent with other published works in the West,
with overrepresentation of aggressive characteristics,
such as nodal positivity and high grade.[11] [12] In our cohort, nearly half of the cases had node-positive disease, with
64.2% having stage II or III disease. Approximately
40% had grade III disease, in contrast to only around
10% with grade I. Additionally, HER2 overexpression
was detected in almost a third of cases (27.2%), similar
to the high proportion reported in the literature.[13] It is
well-known that HER2 positivity correlates with more
aggressive disease behaviour and a poorer prognosis.[14]
Our cohort consisted of a larger percentage of hormonepositive
tumours (80.7%), whereas the reported figure
in the West was approximately 70%.[15] This supports the
previous finding that a higher proportion of hormone-positive
disease is found in young breast cancer patients
in China and East Asia.[16] [17] It adds to the evidence that
the Asian pattern and pathology of premenopausal breast
cancer may be different from that of the West, requiring
more focused study.
In this cohort, the basal-like/triple-negative breast cancer rate was not particularly high. While the occurrence of
many of the young breast cancer cases may be related
to BRCA hereditary breast cancer, and the association of
triple-negative breast cancer with the BRCA1 mutation in
particular, one may expect that the proportion of basal-like
breast cancer would be much higher in young women compared with older patients. However, the prevalence
of BRCA1/2 mutations might also play a role. In a local
study, which recruited 2549 ‘high-risk’ breast or ovarian
cancer patients based on age, personal, and family
history, BRCA mutations were found in 244 patients
(9.6%), of which 110 (45.1%) had the BRCA1 mutation
and 134 (54.9%) had the BRCA2 mutation.[18] Unlike
Caucasian populations, there is a relative predominance
of the BRCA2 mutation over the BRCA1 in the Chinese
population.[19] In that case, since BRCA2 disease tends to
express ER/PR in contrast to BRCA1, it would seem to
contribute to the HR-positive phenotypes. Nevertheless,
data on BRCA1/2 mutations specific to the young breast
cancer population in our locality are lacking and further
investigation is needed.
In this study, we performed univariate and multivariable
analyses to gain further insights into the tumour
characteristics of young breast cancer patients. Various
clinicopathological factors, including large tumour
size, positive nodal status, and high grade, have been
found to be adverse prognostic factors of survival.[20] [21] [22]
We demonstrated that nodal metastasis is a strong
independent negative prognostic factor for both DFS
and OS in this patient group. It highlighted the clinical
significance of nodal staging on prognosis and might
aid the prediction of relapse risk and guide treatment
strategies.
Regarding treatment outcomes, previous studies of
young breast cancer patients reported poor 5-year OS of
70% to 80% in older series.[23] [24] However, the OS figure
in our cohort was somewhat better, and comparable
to the more recently published studies with 5-year OS
up to 90%.[20] [25] The possible reasons could include our
universal access to tertiary healthcare, relatively high
socio-economic status and recent advances in treatments
for locoregional and recurrent or metastatic disease.
Looking into the disease course, 68.2% (88/129) of the
relapses occurred within 5 years of surgery, including
61.6% of HR-positive (61/99) and 90% of HR-negative
(27/30) diseases. This was compatible with the known
observation that HR-positive disease can develop
relapses in later years, in contrast to HR-negative
disease, which mostly recurs within the first 5 years.[26]
Five years also represents the time point when most of
the HR-positive patients have discontinued their anti-oestrogen
therapy. Studies have reported that recurrence
rate of breast cancer continues to rise over 5 to 20 years
following treatment,[27] and prolonged endocrine therapy has been proven beneficial for patients with a high
recurrence risk.[28] Thus, there was increasing use of
extended hormonal therapy observed in the latter part of
the period in our cohort.
The observation that breast cancer subtypes carry
different prognoses was obvious in this cohort of young
women. Luminal disease had the best prognosis while
triple negative disease had the worst DFS and overall
OS. In this study, HER2 disease had equally poor DFS
similar to that of triple negative disease, although its OS
was superior. The high relapse rate of HER2 disease in
our cohort, which began in the year 2005, could be due
to the fact that only 54.8% of HER2+ cases (74/135) had
received adjuvant trastuzumab, since it had just became
the standard of care and was not universally reimbursed
during that period. In fact, the importance of breast cancer
subtypes is also reflected in the latest (eighth) edition
of the AJCC Cancer Staging Manual.[29] With evolving
knowledge of breast cancer biology, the incorporation of
biomarkers, including hormonal receptor status, HER2
receptor status, and histological grade into anatomic
staging indicates that biologic subtypes have become
increasingly important in defining prognosis, estimating
survival, and influencing the selection of therapies.
Treatment strategies for breast cancer have evolved
rapidly over the past decade. In the adjuvant setting, for
example, additional ovarian function suppression with
luteinising hormone-releasing hormone agonists was not
used outside of clinical trials. We started to discuss it with
premenopausal patients with HR-positive breast cancer
only in recent years, after the SOFT trial demonstrating
its superior survival than that with tamoxifen alone.[30]
In addition, the neoadjuvant approach has been more
frequently used in recent years, especially for triple
negative and HER2+ disease. Not only it can downstage
the disease to improve resectability, but also it allows
early assessment of response to systemic treatment and
tailored adjuvant options based on pathological response
as in the CREATE-X and KATHERINE trials.[31] [32] With
current effective treatments, we can expect survival for
young women to continue to improve in the future.
Several points need to be emphasised during the care
of patients in this age-group. Much of the treatment
offered, both surgical and adjuvant, may have significant
impacts on patients’ lives. Organ preservation is
important in young women. There is no evidence that
mastectomy improves OS in this group of patients.[33] A
secular trend was observed in our study with increasing use of conservative breast treatment over mastectomy
(49.4% patients [134/271] received breast-conserving
surgery from 2009 to 2013, compared to 42.5% [96/226]
from 2005 to 2008). The overall local recurrence
rate remained low at 4% (20/497) in our study; 5.2%
(12/230) after breast-conserving surgery and 3% (8/267)
after mastectomy. A breast-conserving approach with
oncoplastic surgical techniques should be discussed with
patients in order to optimise cosmesis and body image. Looking at chemotherapy or endocrine therapy, fertility
preservation options and family planning concerns
need to be addressed before initiation of treatment.
The vast majority of cases (85.1%) were administered
chemotherapy in the study. With young age itself being
a risk factor of relapse,[34] most of the patients would be
recommended to receive chemotherapy. In our study, the
rate of CRA (no menses ≥3 months within 12 months of
chemotherapy) was found to be at least 35.5%. According
to a local study which used the same definition of CRA,
91.1% young breast cancer patients (age ≤45) developed
CRA.[35] The difference can be explained by the fact that
our study recruited younger patients (age <40) who were
less susceptible to CRA as suggested in the literature.[36] Other possible reasons include underreporting of CRA
and missing data in our study, since menstrual history
was not routinely recorded during follow-up. While
some patients might regain menstruation later on (75.3%
in our study vs. 66.7% in the local study), the potential
consequences of chemotherapy-related infertility
and premature menopause should not be overlooked
and more data on these aspects are needed. Overall,
personalised treatment plans are required for young
women. Chemotherapy might be omitted in selected
cases of low-risk HR+ early breast cancer after careful
discussion of risks and benefits, and further studies
on risk stratification by genomic signatures in young
women might also help guide treatment decisions.[5] As
for adjuvant endocrine therapy, one major issue is the
high discontinuation rate observed in young patients,
as up to 10.4% in our study, due to reasons including
adverse effects and their effect on mood, work, and
sexual function, and the desire to become pregnant.[37]
Nonadherence to hormonal therapy has been found to
be associated with increased mortality.[38] Education and
interventions to improve compliance may be critical to
improve breast cancer survival. Management of breast
cancer in young patients is indeed challenging, and
physicians should take into account these psychosocial
aspects as well.
A few inherent limitations should be considered in our study. First, it was a retrospective hospital-based
study. There was potential selection bias, and our study
cohort might not fully represent the whole population.
However, as one of the largest public oncology centres
in Hong Kong, the data should reasonably reflect the
real-world outcomes in our locality. Second, we did
not have detailed records of family history or BRCA
mutation status, which would be helpful in studying
this young population. Breast cancer at an early age is
more likely to be associated with underlying genetic
abnormalities, especially germline BRCA mutations,
which have potential impacts on prognosis and treatment
options. Genetic screening is not widely carried out in
Hong Kong, but it has been gaining more attention in
recent years. Third, the Ki-67 index, a marker of cell
proliferation, was missing in almost half of the cases in
our cohort. According to the St. Gallen Consensus 2011,
it is chiefly important for the distinction between ‘luminal
A’ and ‘luminal B (HER2-)’ subtypes.[9] However, it was
not routinely determined due to lack of standardisation
of laboratory techniques and cut-off points. Therefore,
histological grade was used as an alternative assessment
of proliferation in this study. Routine analysis of Ki-67
index would be helpful to better classify and further
understand the disease in the future.
CONCLUSION
This study described the clinicopathological features, prognostic factors, and treatment outcomes of breast
cancer in young Chinese women (age <40 years) in
Hong Kong. Young women tended to present with
aggressive diseases with high grade and lymph node
involvement. We found more hormone-positive disease
seen in Asian than in Western populations. Breast
cancer in young Asian women may represent a distinct
subgroup that deserves more research. Addressing not
only potential differences in host and tumour biology,
but also psychosocial and behavioural issues will likely
improve disease outcomes in this unique population.
REFERENCES
1. El Saghir NS, Seoud M, Khalil MK, Charafeddine M, Salem ZK,
Geara FB, et al. Effects of young age at presentation on survival
in breast cancer. BMC Cancer. 2006;6:194. Crossref
2. Azim HA Jr, Michiels S, Bedard PL, Singhal SK, Criscitiello C,
Ignatiadis M, et al. Elucidating prognosis and biology of breast
cancer arising in young women using gene expression profiling.
Clin Cancer Res. 2012;18:1341-51. Crossref
3. Anders CK, Hsu DS, Broadwater G, Acharya CR, Foekens JA,
Zhang Y, et al. Young age at diagnosis correlates with worse
prognosis and defines a subset of breast cancers with shared patterns
of gene expression. J Clin Oncol. 2008;26:3324-30. Crossref
4. Cardoso F, Loibl S, Pagani O, Graziottin A, Panizza P, Martincich L, et al. The European Society of Breast Cancer Specialists recommendations for the management of young women with breast cancer. Eur J Cancer. 2012;48:3355-77. Crossref
5. Paluch-Shimon S, Cardoso F, Partridge AH, Abulkhair O, Azim HA Jr, Bianchi-Micheli G, et al. ESO-ESMO 4th International Consensus Guidelines for Breast Cancer in Young Women (BCY4). Ann Oncol. 2020;31:674-96. Crossref
6. Fan L, Strasser-Weippl K, Li JJ, St Louis J, Finkelstein DM, Yu KD, et al. Breast cancer in China. Lancet Oncol. 2014;15:e279-89. Crossref
7. Han W, Kim SW, Park IA, Kang D, Kim SW, Youn YK, et al.
Young age: an independent risk factor for disease-free survival in
women with operable breast cancer. BMC Cancer. 2004;4:82. Crossref
8. Hong Kong Breast Cancer Foundation. Hong Kong Breast Cancer
Registry Report No. 12. (Issue 2020). Available from: https://www.hkbcf.org/en/our_research/main/519/upload/category/519/self/.... Accessed 12 Sep 2021.
9. Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thürlimann B,
Senn HJ, et al. Strategies for subtypes — dealing with the diversity
of breast cancer: highlights of the St. Gallen International Expert
Consensus on the Primary Therapy of Early Breast Cancer 2011.
Ann Oncol. 2011;22:1736-47. Crossref
10. Brouckaert O, Laenen A, Vanderhaegen J, Wildiers H, Leunen K,
Amant F, et al. Applying the 2011 St Gallen panel of prognostic
markers on a large single hospital cohort of consecutively treated
primary operable breast cancers. Ann Oncol. 2012;23:2578-84. Crossref
11. Liukkonen S, Leidenius M, Saarto T, Sjöström-Mattson J. Breast
cancer in very young women. Eur J Surg Oncol. 2011;37:1030-7. Crossref
12. Kheirelseid EH, Boggs JM, Curran C, Glynn RW, Dooley C,
Sweeney KJ, et al. Younger age as a prognostic indicator in breast
cancer: a cohort study. BMC Cancer. 2011;11:383. Crossref
13. Keegan TH, DeRouen MC, Press DJ, Kurian AW, Clarke CA.
Occurrence of breast cancer subtypes in adolescent and young
adult women. Breast Cancer Res. 2012;14:R55. Crossref
14. Gusterson BA, Gelber RD, Goldhirsch A, Price KN, Säve-Söderborgh J, Anbazhagan R, et al. Prognostic importance of c-erbB-2 expression in breast cancer. International (Ludwig) Breast Cancer Study Group. J Clin Oncol. 1992;10:1049-56. Crossref
15. Collins LC, Marotti JD, Gelber S, Cole K, Ruddy K, Kereakoglow
S, et al. Pathologic features and molecular phenotype by patient
age in a large cohort of young women with breast cancer. Breast
Cancer Res Treat. 2012;131:1061-6. Crossref
16. Lin CH, Yap YS, Lee KH, Im SA, Naito Y, Yeo W, et al. Contrasting epidemiology and clinicopathology of female breast cancer in Asians vs the US population. J Natl Cancer Inst. 2019;111:1298-306. Crossref
17. Xue C, Wang X, Peng R, Shi Y, Qin T, Liu D, et al. Distribution,
clinicopathologic features and survival of breast cancer subtypes
in Southern China. Cancer Sci. 2012;103:1679-87. Crossref
18. Hong Kong Hereditary Breast Cancer Family Registry. Hereditary cancers. Our statistics. Available from: https://www.asiabreastregistry.com/en/hereditary-cancers/our-statistics. Accessed 12 Sep 2021.
19. Kwong A, Wong LP, Wong HN, Law FB, Ng EK, Tang YH, et al. Clinical and pathological characteristics of Chinese patients with BRCA related breast cancer. Hugo J. 2009;3:63-76. Crossref
20. Plichta JK, Rai U, Tang R, Coopey SB, Buckley JM, Gadd MA, et al. Factors associated with recurrence rates and long-term survival
in women diagnosed with breast cancer ages 40 and younger. Ann
Surg Oncol. 2016;23:3212-20. Crossref
21. Choi DH, Kim S, Rimm DL, Carter D, Haffty BG. Immunohistochemical biomarkers in patients with early-onset breast carcinoma by tissue microarray. Cancer J. 2005;11:404-11. Crossref
22. Abdel-Razeq H, Almasri H, Abdel Rahman F, Abdulelah H,
Abu Nasser M, Salam M, et al. Clinicopathological characteristics
and treatment outcomes of breast cancer among adolescents
and young adults in a developing country. Cancer Manag Res.
2019;11:9891-7. Crossref
23. Chung M, Chang HR, Bland KI, Wanebo HJ. Younger women with breast carcinoma have a poorer prognosis than older women. Cancer. 1996;77:97-103. Crossref
24. Karihtala P, Winqvist R, Bloigu R, Jukkola-Vuorinen A. Long-term
observational follow-up study of breast cancer diagnosed in women
≤40 years old. Breast. 2010;19:456-61. Crossref
25. Sabiani L, Houvenaeghel G, Heinemann M, Reyal F, Classe JM, Cohen M, et al. Breast cancer in young women: pathologic features and molecular phenotype. Breast. 2016;29:109-16. Crossref
26. Pagani O, Price KN, Gelber RD, Castiglione-Gertsch M,
Holmberg SB, Lindtner J, et al. Patterns of recurrence of early breast
cancer according to estrogen receptor status: a therapeutic target
for a quarter of a century. Breast Cancer Res Treat. 2009;117:319-
24. Crossref
27. Pan H, Gray R, Braybrooke J, Davies C, Taylor C, McGale P, et al. 20-Year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med. 2017;377:1836-46. Crossref
28. Davies C, Pan H, Godwin J, Gray R, Arriagada R, Raina V, et al.
Long-term effects of continuing adjuvant tamoxifen to 10 years
versus stopping at 5 years after diagnosis of oestrogen receptorpositive breast cancer: ATLAS, a randomised trial. Lancet.
2013;381:805-16. Crossref
29. Gabriel NH, James LC, Carl JD, Stephen BE, Elizabeth AM,
Hope SR, et al. Breast. In: Mahul BA, editor. AJCC Cancer Staging
Manual. 8th ed. New York (NY): Springer; 2017. p 589-628.
30. Francis PA, Regan MM, Fleming GF, Láng I, Ciruelos E, Bellet M, et al. Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J Med. 2015;372:436-46. Crossref
31. Toi M, Masuda N, Ohashi Y. Adjuvant capecitabine for breast cancer. N Engl J Med. 2017;377:791-2. Crossref
32. von Minckwitz G, Huang CS, Mano MS, Loibl S, Mamounas EP, Untch M, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380:617-28. Crossref
33. Vila J, Gandini S, Gentilini O. Overall survival according to type
of surgery in young (≤40 years) early breast cancer patients: a
systematic meta-analysis comparing breast-conserving surgery
versus mastectomy. Breast. 2015;24:175-81. Crossref
34. Winchester DP, Osteen RT, Menck HR. The National Cancer Data Base report on breast carcinoma characteristics and outcome in relation to age. Cancer. 1996;78:1838-43. Crossref
35. Liem GS, Mo FK, Pang E, Suen JJ, Tang NL, Lee KM, et al.
Chemotherapy-related amenorrhea and menopause in young
Chinese breast cancer patients: analysis on incidence, risk factors
and serum hormone profiles. PLoS One. 2015;10:e0140842. Crossref
36. Jacobson MH, Mertens AC, Spencer JB, Manatunga AK, Howards PP. Menses resumption after cancer treatment-induced amenorrhea occurs early or not at all. Fertil Steril. 2016;105:765-72.e4. Crossref
37. Llarena NC, Estevez SL, Tucker SL, Jeruss JS. Impact of fertility concerns on tamoxifen initiation and persistence. J Natl Cancer Inst. 2015;107:djv202. Crossref
38. Hershman DL, Shao T, Kushi LH, Buono D, Tsai WY, Fehrenbacher L, et al. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased
mortality in women with breast cancer. Breast Cancer Res Treat.
2011;126:529-37. Crossref
| Attachment | Size |
|---|---|
| v25n2_Clinicopathological_1.pdf | 351.61 KB |