Magseed Localisation of Non-palpable Papillary Lesions: a Pictorial Essay
PICTORIAL ESSAY
Magseed Localisation of Non-palpable Papillary Lesions: a Pictorial Essay
MKK Law1, LWY Ma2, AYT Lai1, PL Chau2, AKY Au1, YH Ling2, WWC Wong1
1 Department of Radiology, Pamela Youde Nethersole Eastern Hospital, Hong Kong
2 Department of Surgery, Ruttonjee Hospital, Hong Kong
Correspondence: Dr MKK Law, Pamela Youde Nethersole Eastern Hospital, Hong Kong. Email: drmkklaw@gmail.com
Submitted: 30 Apr 2021; Accepted: 2 Aug 2021.
Contributors: All authors designed the study. LWY Ma, AYT Lai, PL Chau, AKY Au acquired the data. MMK Law, LWY Ma, AYT Lai, AKY Au analysed the data. MKK Law drafted the manuscript. All authors critically revised the manuscript for important intellectual content.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: The study was approved by Hong Kong East Cluster Research Ethics Committee (Ref HKECREC-2021-003). The patients were treated in accordance with the tenets of the Declaration of Helsinki. The requirement for patient consent was waived by the review board.
INTRODUCTION
Papillary lesions of the breast may be benign or malignant and are a common cause of bloody nipple discharge.
Some are clinically non-palpable and may be detected
only by radiological investigations.[1]
Papillary lesions can be radiologically detected on
mammography, ultrasound, or magnetic resonance
imaging (MRI). However, radiological features alone
cannot reliably distinguish benign from malignant
papillary lesions. In addition, biopsy using spring-loaded
devices can result in upgrade rates of 10.2% at surgery.[1]
This limitation has led to some patients and surgeons
opting for surgical excision, especially when
radiologically suspicious features, clinical features, or
presence of papilloma with atypia are evident. With more
widespread use of breast cancer screening, an increasing
number of non-palpable breast lesions are inevitably
detected so accurate localisation is increasingly
important.
The most established approach is wire-guided localisation (WGL) before surgery to guide excision of non-palpable breast lesions. However, this technique requires same
day operation, can be associated with patient discomfort,
risk of migration and may result in larger areas of tissue
dissection. These factors have led to exploration of other
techniques.[2] [3]
More popular localisation techniques include
radioguided occult lesion localisation and radioactive
iodine seed localisation, and have shown similar results
in guiding intra-operative lesion localisation compared
with WGL.[2] [3] [4] However, complex logistics and exposure
to radiation are some of the drawbacks, especially for
resections with diagnostic intent. Regarding costs, studies
have suggested that compared with WGL, radioactive
iodine seed localisation is cheaper[5] while radioguided
occult lesion localisation is a similar cost.[3]
More recently, newer techniques such as magnetic
seed localisation have been shown to be non-inferior
to WGL.[4] [6] [7] Magseed (Endomagnetics Ltd, London,
United Kingdom) is a magnetic seed containing non-radioactive
paramagnetic steel and iron oxide seed. The
device measures just under 5 mm × 1 mm and seeds can
be delivered to a target site to mark breast lesions via an 18-gauge needle. The Sentimag probe is then used to
detect the Magseed magnetic signal during the operation,
after applying an alternating magnetic field to the seed.[8]
This is presented to the reader with a numerical count
and audio tone. Advantages include easier localisation
of lesions, improved patient comfort, no need for same-day
surgery and no ionising radiation. There are however
unique limitations to Magseed that should be considered
before placement. Detection may be limited to depths
of <4 cm, interference from stainless steel surgical
instruments, MRI conditional status at 1.5 and 3T and its
signal void on MRI.[4]
The advantages of this technique have led to its adoption by many institutions. Accordingly, studies have evaluated
the potential cost of Magseed localisation compared
with established techniques. The cost of each Magseed
is higher than a wire for WGL but with similar cost of
use in the elective or outpatient setting.[9] One study set
in the Netherlands evaluated further, and concluded that
implementation of Magseed localisation was cost-saving
to its service although evaluation past this period is
more complex and unclear. Beyond this phase, the cost
of Magseed use per patient, mainly influenced by the
number of Magseeds, is a consideration.[10] This should be
balanced by the savings made from freeing up personnel
and services but is unique to every institution.
Recent studies, including one in Hong Kong and a
systematic review, have demonstrated the efficacy of
Magseed in localisation of non-palpable masses.[11] [12] [13] [14]
However, the proportion of ultrasound-guided Magseed
localisation of papillary or subcentimetre lesions was
low. To the best of our knowledge, performance and
efficacy in this group of lesions has not been established.
We report on our experience in using ultrasound-guided Magseed localisation for non-palpable subcentimetre
papillary lesions of the breast, with primary outcomes
being successful localisation and excision of the lesions.
IMAGING FINDINGS
Herein, we review the imaging findings of four patients
(mean age 59 years; range, 45-85) with clinically non-palpable
breast lesions who underwent Magseed marker
insertion. Three patients received one Magseed marker
and one patient received two markers for two adjacent
masses. All patients had their marker(s) inserted before
the day of operation (range, 3-17 d, median=14 d).
All lesions were small masses (range, 4-6 mm,
mean=4.8 mm). No patient had Magseed in situ for more than 30 days.
All patients proceeded to lumpectomy under Sentimag
guidance. Masses were successfully located using the
Sentimag device with marker removal confirmed in
the surgical specimen. Pathology confirmed intraductal
papilloma in all but one specimen that was found to be
due to cauterisation artefact. No immediate complications
were found.
There were no post excision upgrades to in situ or
invasive malignancy.
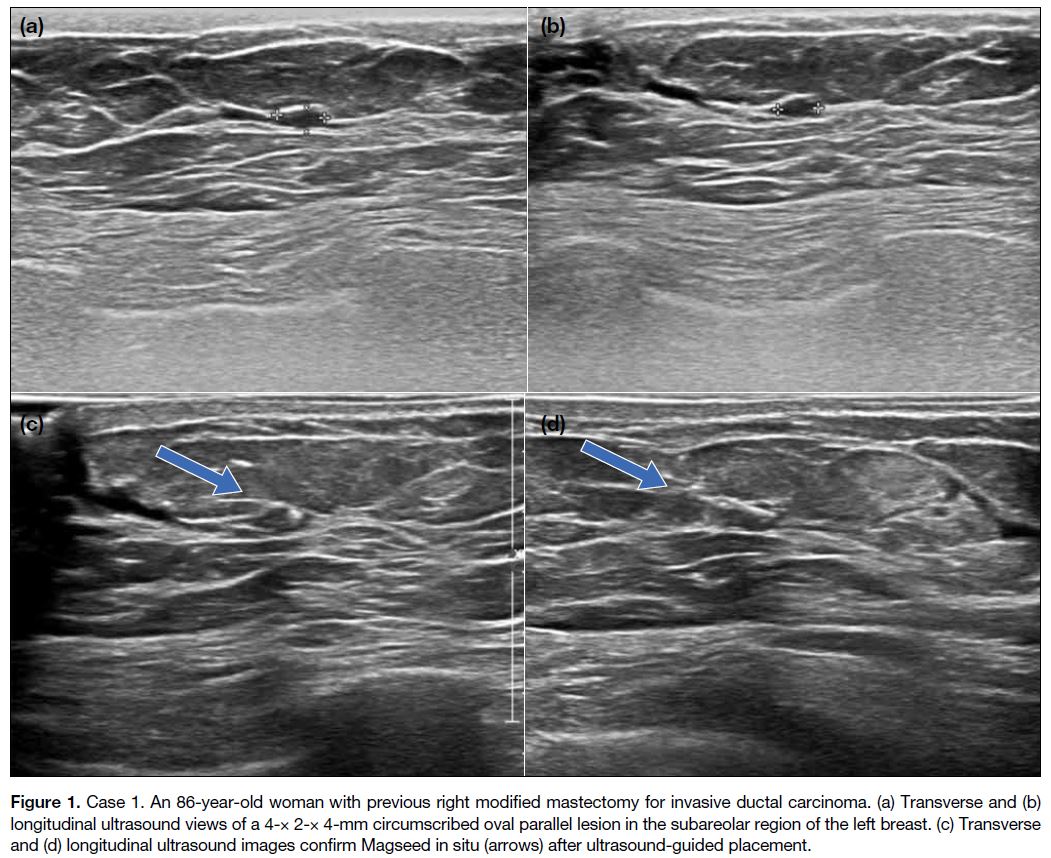
Case 1
An 86-year-old woman with a history of locally
invasive, invasive ductal carcinoma in the right breast,
for which she underwent modified mastectomy in 2017.
Subsequent ultrasound 7 months later showed a small
circumscribed oval hypoechoic lesion in the subareolar
region of the left breast, measuring 4 × 2 × 4 mm
(Figure 1). Fine needle aspiration pathology showed
papillary neoplasm with epithelial proliferation. She
proceeded to lumpectomy 3 days after Magseed insertion,
which was uneventful. Cauterisation artefacts were seen
but pathology revealed no evidence of malignancy.
Figure 1. Case 1. An 86-year-old woman with previous right modified mastectomy for invasive ductal carcinoma. (a) Transverse and (b)
longitudinal ultrasound views of a 4-× 2-× 4-mm circumscribed oval parallel lesion in the subareolar region of the left breast. (c) Transverse and (d) longitudinal ultrasound images confirm Magseed in situ (arrows) after ultrasound-guided placement.
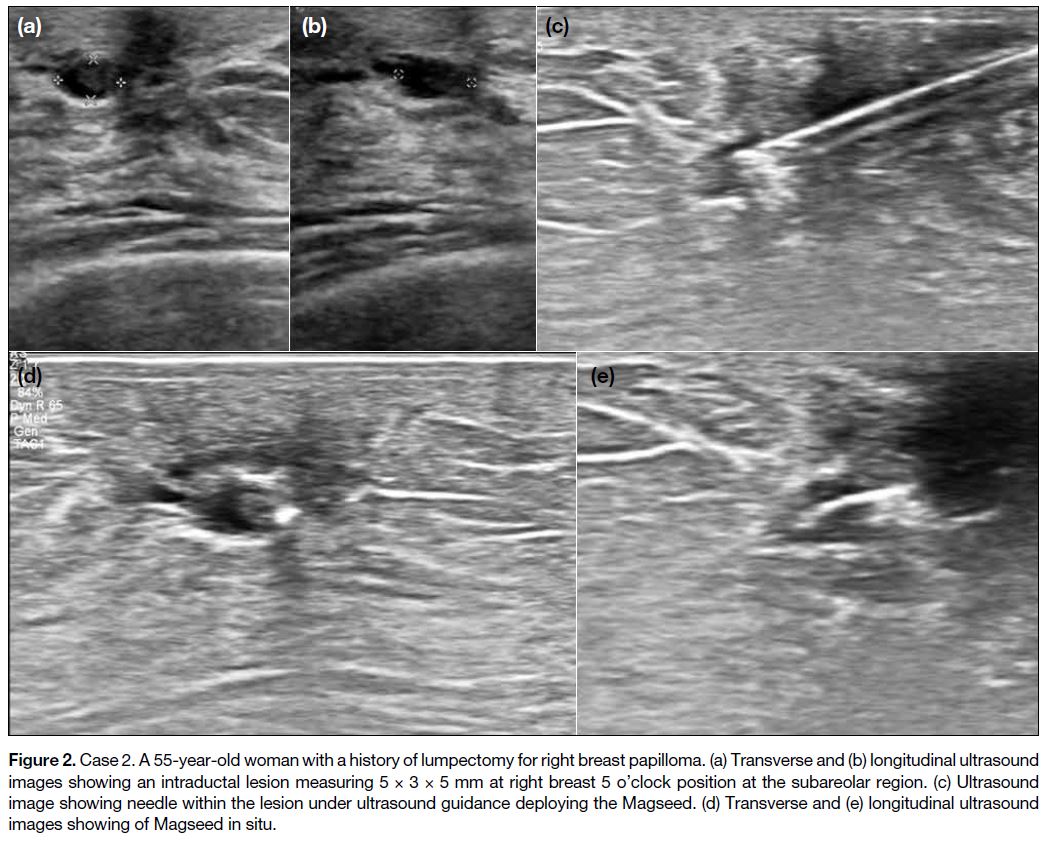
Case 2
A 55-year-old woman with a history of right breast lump excision confirmed as breast papilloma. Routine follow-up
ultrasound examination revealed mild duct dilatation
at the right breast 5 o’clock position subareolar region
with intraductal oval circumscribed hypoechoic nodule,
measuring 5 × 3 × 5 mm (Figure 2). Ultrasound-guided
fine needle aspiration pathology revealed intraductal
papilloma. Magseed was inserted without complications
and the patient proceeded to lumpectomy 17 days later.
Localisation at surgery was uneventful.
Figure 2. Case 2. A 55-year-old woman with a history of lumpectomy for right breast papilloma. (a) Transverse and (b) longitudinal ultrasound
images showing an intraductal lesion measuring 5 × 3 × 5 mm at right breast 5 o’clock position at the subareolar region. (c) Ultrasound
image showing needle within the lesion under ultrasound guidance deploying the Magseed. (d) Transverse and (e) longitudinal ultrasound
images showing of Magseed in situ.
Case 3
A 45-year-old woman presented with a 3-year history
of bilateral mastalgia and breast imaging from another
centre that identified a small right breast 12 o’clock
position subareolar complex cystic lesion measuring
5 × 5 × 6 mm (Figure 3). Biopsy of the lesion suggested
intraductal papilloma and surgical excision was planned.
In the interim, follow-up breast imaging at another centre
identified another lesion in the same breast, 12 o’clock
position, 3 cm from the nipple, measuring 3 × 3 × 5 mm
(Figure 3). Biopsy pathology also showed intraductal
papilloma. The patient underwent preoperative
localisation of these two lesions with Magseed (Figures 4 and 5). Magseeds were inserted into both lesions,
with inter-Magseed distance measured to facilitate
surgical localisation. Lumpectomy was performed for
both lesions 14 days after Magseed localisation. Two
spikes corresponding to the two Magseed markers
were detected on Sentimag intra-operatively. The final
pathology report for the surgical specimen confirmed
intraductal papilloma with usual ductal hyperplasia.
Figure 3. Case 3. A 45-year-old woman with two right breast 12 o’clock position fine needle aspiration-proven intraductal papillomata,
one at the subareolar region and the other at 3 cm from the nipple. (a) Transverse and (b) longitudinal ultrasound of right breast 12
o’clock position subareolar lesion. (c) Transverse and (d) longitudinal ultrasound of right breast 12 o’clock position 3 cm from nipple lesion,
measuring 3 × 3 × 5 mm.
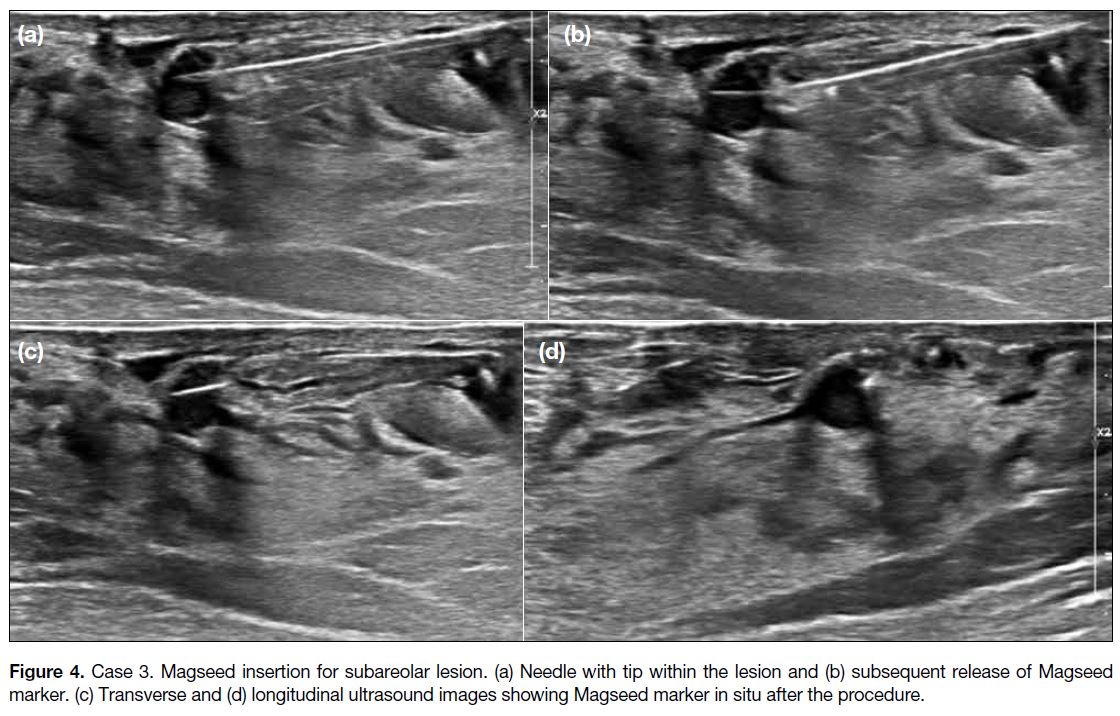
Figure 4. Case 3. Magseed insertion for subareolar lesion. (a) Needle with tip within the lesion and (b) subsequent release of Magseed marker. (c) Transverse and (d) longitudinal ultrasound images showing Magseed marker in situ after the procedure.
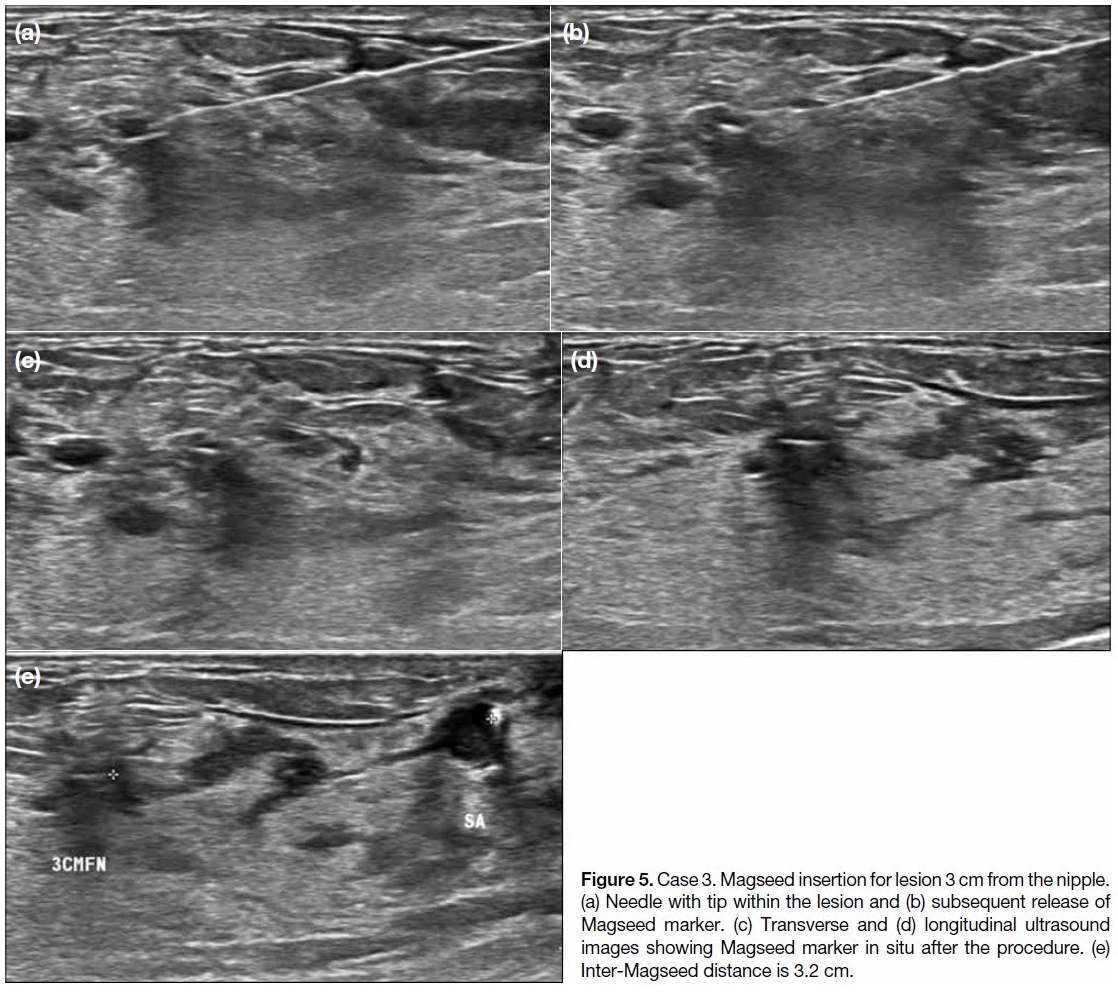
Figure 5. Case 3. Magseed insertion for lesion 3 cm from the nipple.
(a) Needle with tip within the lesion and (b) subsequent release of
Magseed marker. (c) Transverse and (d) longitudinal ultrasound
images showing Magseed marker in situ after the procedure. (e)
Inter-Magseed distance is 3.2 cm.
Case 4
A 42-year-old woman with an incidental ultrasound
finding of left breast 12 o’clock position nodule, 1 cm
from the nipple, measuring 6 × 3 × 5 mm, associated
with a prominent duct (Figure 6). Ultrasound-guided
biopsy showed a papillary lesion, likely to be intraductal
papilloma. Magseed insertion was performed before the
procedure and was uneventful (Figure 7). Seventeen days later, lumpectomy was performed and localisation was
uneventful. Pathological examination of the specimen
confirmed intraductal papilloma.
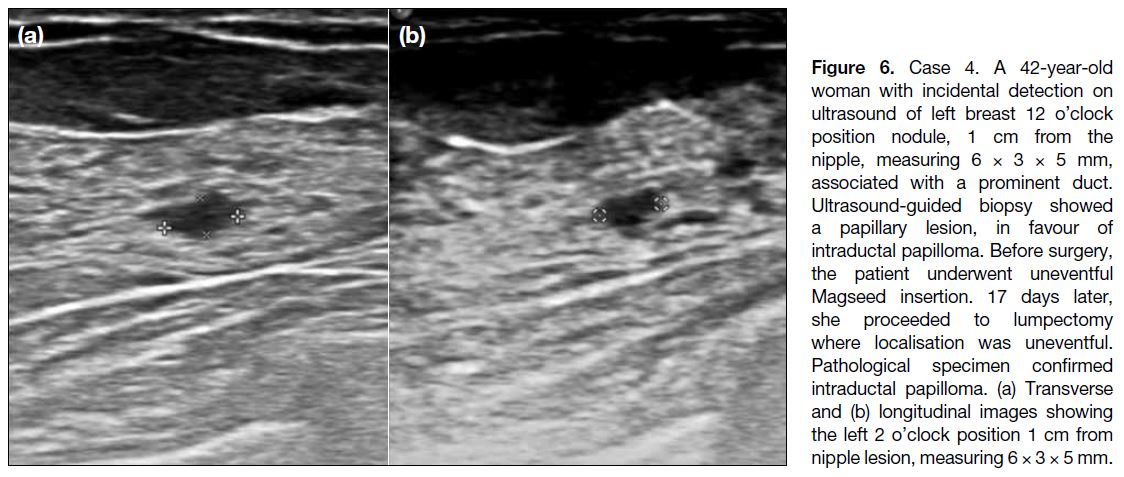
Figure 6. Case 4. A 42-year-old woman with incidental detection on ultrasound of left breast 12 o’clock position nodule, 1 cm from the nipple, measuring 6 × 3 × 5 mm, associated with a prominent duct. Ultrasound-guided biopsy showed a papillary lesion, in favour of intraductal papilloma. Before surgery, the patient underwent uneventful Magseed insertion. 17 days later, she proceeded to lumpectomy where localisation was uneventful. Pathological specimen confirmed intraductal papilloma. (a) Transverse and (b) longitudinal images showing the left 2 o’clock position 1 cm from nipple lesion, measuring 6 × 3 × 5 mm.
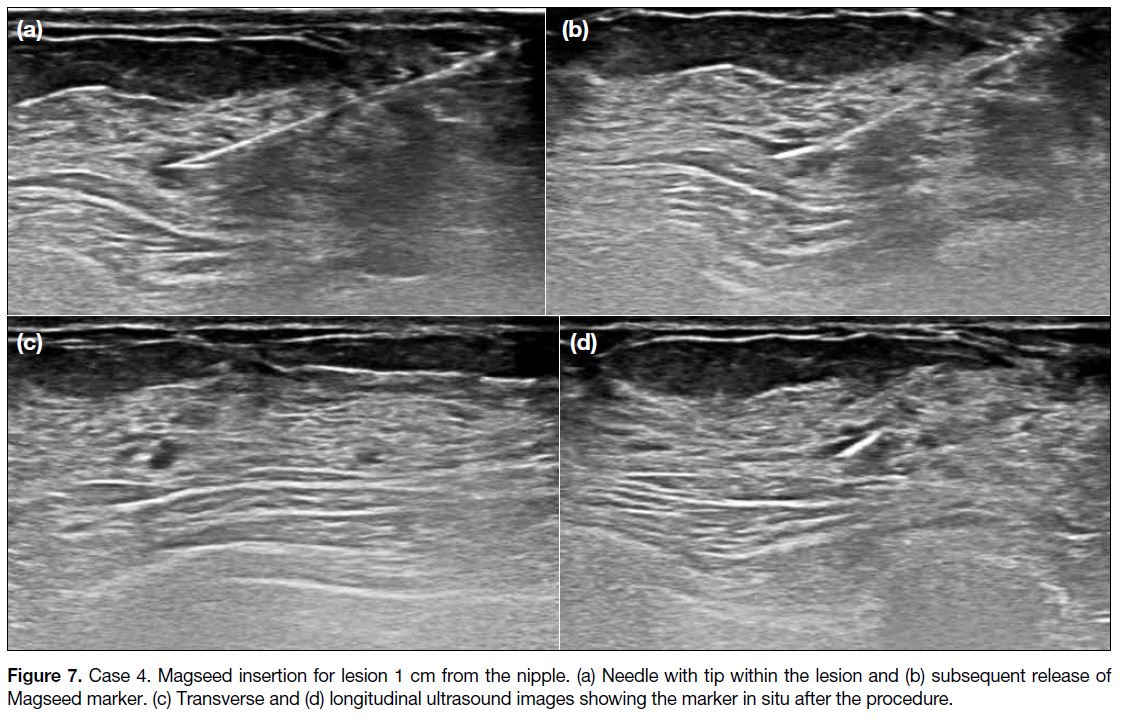
Figure 7. Case 4. Magseed insertion for lesion 1 cm from the nipple. (a) Needle with tip within the lesion and (b) subsequent release of
Magseed marker. (c) Transverse and (d) longitudinal ultrasound images showing the marker in situ after the procedure.
DISCUSSION
Our experience suggests that success rates for ultrasound Magseed localisation and re-excision are comparable
to existing studies of lesions with heterogeneous
characteristics, including for lesion sizes >1 cm.[13] [14]
Insertion of Magseed and localisation of lesions with
the Sentimag detector was relatively simple in these
cases, even when two masses were close together (3 cm
apart) and in the same breast. Most Magseed insertions
were performed >1 week before surgery and were easily
located without clinically consequent displacement, as
evidenced by resection pathology results.
None of the excised papillary lesions were upgraded
to cancer after surgical resection. However, there are
significant papillary lesions diagnosed via fine needle
aspiration or biopsy that may require resection of
the index lesion with diagnostic intent to confirm the
absence of cancer. Therefore, accurate localisation is
important.
Using this technique enables flexibility in scheduling without the need for same day surgery or use of
radioisotopes. Our surgical colleagues also reported
more accurate assessment of the depth of the lesion
compared with other techniques such as skin marking or
WGL, potentially enabling far less tissue to be excised.
The potential to lower services and personnel needs may compensate for some of the drawbacks of Magseed.
In our subgroup of patients, MRI examination of
the breast between placement and surgery was less
likely, for example. Although the impact of potential
instrumentation interference is possible, studies have
shown that re-excision rates following Magseed are non-inferior
to WGL.[4] The manufacturer and other studies have revealed that Magseed can be detected beyond
4 cm, even up to 12 cm through palpation in a supine
position.[4]
Future studies that focus on this population would be
helpful to confirm the efficacy of Magseed localisation
of small papillary breast masses. Further secondary
outcome analysis or objective measures such as tissue
excision weight can be explored. Cost-benefit analysis
should be individualised to governing health bodies or
individual institutions.
CONCLUSION
Ultrasound-guided Magseed localisation is safe, less
complex and allows more flexibility of scheduling. In
our case series of non-palpable subcentimetre papillary
breast masses, there were no upgraded cases nor need for
re-excision. Potential issues with Magseed such as MRI
artifacts are less of an issue in this patient subgroup. Our
experience suggests that ultrasound-guided Magseed
localisation of these lesions shows similar efficacy to
that of other studies that have explored non-palpable
lesions in general.
REFERENCES
1. Seely JM, Verma R, Kielar A, Smyth KR, Hack K, Taljaard M,
et al. Benign papillomas of the breast diagnosed on large-gauge
vacuum biopsy compared with 14 gauge core needle biopsy — do
they require surgical excision? Breast J. 2017;23:146-53. Crossref
2. Brown KJ, Bashir MR, Baker JA, Tyler DS, Paulson EK.
Imaging-guided preoperative hookwire localization of nonpalpable
extramammary lesions. AJR Am J Roentgenol. 2011;197:W525-7. Crossref
3. Postma EL, Verkooijen HM, van Esser S, Hobbelink MG, van der
Schelling GP, Koelemij R, et al. Efficacy of ‘radioguided occult
lesion localisation’ (ROLL) versus ‘wire-guided localisation’
(WGL) in breast conserving surgery for non-palpable breast cancer:
a randomised controlled multicentre trial. Breast Cancer Res Treat.
2012;136:469-78. Crossref
4. Gera R, Tayeh S, Al-Reefy S, Mokbel K. Evolving role of
Magseed in wireless localization of breast lesions: systematic
review and pooled analysis of 1,559 procedures. Anticancer Res.
2020;40:1809-15. Crossref
5. Zhang Y, Seely J, Cordeiro E, Hefler J, Thavorn K, Mahajan M,
et al. Radioactive seed localization versus wire-guided localization
for nonpalpable breast cancer: A cost and operating room efficiency
analysis. Ann Surg Oncol. 2017;24:3567-73. Crossref
6. Zacharioudakis K, Down S, Bholah Z, Lee S, Khan T, Maxwell AJ,
et al. Is the future magnetic? Magseed localisation for non palpable
breast cancer. A multi-centre non randomised control study. Eur J
Surg Oncol. 2019;45:2016-21. Crossref
7. Chan BK, Wiseberg-Firtell JA, Jois RH, Jensen K, Audisio RA.
Localization techniques for guided surgical excision of non-palpable
breast lesions. Cochrane Database Syst Rev. 2015;(12):CD009206. Crossref
8. Harvey JR, Lim Y, Murphy J, Howe M, Morris J, Goyal A, et al. Safety and feasibility of breast lesion localization using magnetic
seeds (Magseed): a multi-centre, open-label cohort study. Breast
Cancer Res Treat. 2018;169:531-6. Crossref
9. National Institute for Health and Care Excellence. Magseed for
locating impalpable breast cancer lesions. Available from: https://www.nice.org.uk/advice/mib236/resources/magseed-for-locating-imp.... Accessed 6 Jul 2021.
10. Lindenberg M, van Beek A, Retèl V, van Duijnhoven F, van
Harten W. Early budget impact analysis on magnetic seed
localization for non-palpable breast cancer surgery. PLoS One.
2020;15:e0232690. Crossref
11. Thekkinkattil D, Kaushik M, Hoosein MM, Al-Attar M, Pilgrim S,
Gvaramadze A, et al. A prospective, single-arm, multicentre clinical
evaluation of a new localisation technique using non-radioactive
Magseeds for surgery of clinically occult breast lesions. Clin Radiol.
2019;74:974.e7-11. Crossref
12. Powell M, Gate T, Kalake O, Ranjith C, Pennick MO. Magnetic seed localization (Magseed) for excision of impalpable breast lesions — the North Wales experience. Breast J. 2021;27:259-36. Crossref
13. Garzotto F, Comoretto RI, Michieletto S, Franzoso G, Lo Mele M,
Gregori D, et al. Preoperative non-palpable breast lesion
localization, innovative techniques and clinical outcomes in
surgical practice: a systematic review and meta-analysis. Breast.
2021;58:93-105. Crossref
14. Fung WY, Wong T, Chau CM, Yu EL, Chan TS, Chan RL, et al.
Safety and efficacy of magnetic seed localisation of non-palpable
breast lesions: pilot study in a Chinese population. Hong Kong
Med J. 2020;26:500-9. Crossref