Inactivated and mRNA COVID-19 Vaccines Affect 18F-fluorodeoxyglucose Positron Emission Tomography/Computed Tomography in Oncology Patients
ORIGINAL ARTICLE
Inactivated and mRNA COVID-19 Vaccines Affect
18F-fluorodeoxyglucose Positron Emission Tomography/Computed
Tomography in Oncology Patients
IWC Wong, CWI Li, DKK Ng, KS Chu, JBT Kung, TK Au-Yong
Nuclear Medicine Unit, Queen Elizabeth Hospital, Hong Kong
Correspondence: Dr IWC Wong. Nuclear Medicine Unit, Queen Elizabeth Hospital, Hong Kong. Email: wwc949@ha.org.hk
Submitted: 8 Dec 2021; Accepted: 3 May 2022.
Contributors: All authors designed the study, acquired the data, analysed the data, drafted the manuscript, and critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: As an editor of the journal, TKA was not involved in the peer review process. Other authors have disclosed no conflicts of interest.
Funding/Support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: The study was approved by the Hong Kong Hospital Authority Kowloon Central Cluster / Kowloon East Cluster Research Ethics Committee (Ref (KC/KE)-21-0245/ER-2), and the requirement for informed consent was waived. The patients were treated in accordance with the tenets of the Declaration of Helsinki. The patients provided written informed consent for all treatments and procedures.
Declaration: Preliminary results of the present study were presented in part at the 9th Joint Scientific Meeting of the Royal College of Radiologists
(RCR) & the Hong Kong College of Radiologists (HKCR) and 29th Annual Scientific Meeting of HKCR on 13-14 November 2021.
Abstract
Introduction
We aimed to analyse the effect of coronavirus disease 2019 (COVID-19) vaccination on
18F-fluorodeoxyglucose positron emission tomography/computed tomography (FDG PET/CT) imaging findings in cancer patients.
Methods
A total of 165 oncology patients who underwent FDG PET/CT between 1 May 2021 and 30 September
2021 after their first or second COVID-19 vaccination with were included in this retrospective study. The occurrence
and pattern of FDG uptake at the injection site (usually deltoid), ipsilateral axillary and other regional lymph nodes,
were measured.
Results
Overall, the incidence of FDG-avid ipsilateral regional nodal uptake was 26.7% (44/165), with a median
maximal standardised uptake value of 3.2 (range, 1.7-13.8). Vaccine-associated hypermetabolic lymphadenopathy
(VAHL) was found in 11.4% (5/44) of the subjects beyond 6 weeks after vaccination. VAHL was more common
in patients receiving BioNTech-Fosun mRNA vaccine (compared with patients receiving the Sinovac CoronaVac
inactivated vaccine), and in women (p < 0.05).
Conclusion
VAHL is common and can be observed beyond 6 weeks after vaccination. It was seen more frequently
in women and in patients receiving the mRNA-based vaccine. Proper vaccination history documentation, locating
the vaccination site contralateral to the primary cancer, and appropriate scheduling of FDG PET/CT are advisable
for correct image interpretation.
Key Words: COVID-19 vaccines; Lymphadenopathy; Fluorodeoxyglucose F18; Positron emission tomography
computed tomography
中文摘要
滅活和mRNA新冠疫苗接種對腫瘤患者18F-氟脫氧葡萄糖PET/CT掃描的影響
黃偉宗、李煒燁、吳冠橋、朱競新、龔本霆、歐陽定勤
引言
旨在分析新冠疫苗接種對癌症患者18F-氟脫氧葡萄糖(FDG)PET/CT成像結果的影響。
方法
本回顧研究納入165名第一次或第二次新冠疫苗接種後,於2021年5月1日至2021年9月30日期間接受FDG PET/CT檢查的腫瘤患者。測量注射部位(通常是三角肌)、同側腋窩和其他區域淋巴結以及脾臟產生FDG攝取極其模式。
結果
整體而言,同側區域淋巴結FDG-強烈攝取的發生率為26.7%(47/165),最大標準化攝取值中位數為3.2(介乎1.7-13.8)。接種疫苗6 週後,11.4%名受試者(5/44)出現與疫苗相關高代謝性淋巴結改變(VAHL)。與接受科興滅活疫苗的患者相比,VAHL在接受 BioNTech-復星mRNA疫苗的患者和女性中更為常見(p < 0.05)。
結論
VAHL常見,可在疫苗接種後6週後觀察到,在女性和接種mRNA疫苗的患者中更常見。正確的圖像解釋須參考疫苗接種記錄、考慮原發癌對側部位接種疫苗、以及適當安排FDG PET/CT檢查日期。
INTRODUCTION
The coronavirus disease 2019 (COVID-19) pandemic
has resulted in a tremendous burden on public health
and the economy; universal vaccination is an important
measure against the pathogen, SARS-CoV-2 (severe
acute respiratory syndrome coronavirus 2), to lessen
serious infections and hospitalisation. There are currently
two types of COVID-19 vaccine available in Hong
Kong: Sinovac CoronaVac (inactivated vaccine) and
Comirnaty (BNT162b2 mRNA vaccine, manufactured
by BioNTech in collaboration with Fosun Pharma); both
have been validated by World Health Organization for
emergency use, and both require two doses to induce
adequate protection against SARS-CoV-2.[1]
Reactive axillary, supraclavicular, and lower cervical
lymphadenopathy following COVID-19 vaccination
has been reported in several case reports and cohort
studies on different imaging modalities.[2] [3] [4] [5] [6] Being an
essential imaging tool for various types of cancers,
18F-fluorodeoxyglucose positron emission tomography/computed tomography (FDG PET/CT) is no exception.
Increased FDG uptake at vaccine inoculation site and
spleen after COVID-19 vaccination has also been
described. Nevertheless, compared with mRNA vaccine, fewer reports about after vaccination imaging findings
for other types of COVID-19 vaccines have been
found.[7] In addition, vaccine-associated hypermetabolic
lymphadenopathy (VAHL) on FDG PET/CT in the
Chinese population has rarely been reported in the
English-language literature.
In this study, we aimed to analyse the incidence, pattern, and potential clinical effects of VAHL and other imaging
findings on FDG PET/CT in patients with cancer who
previously received inactivated or mRNA COVID-19
vaccine.
METHODS
Study Population
This was a retrospective single-centre study. All
consecutive cases with suspected or known malignancy,
who had received at least one dose of COVID-19
vaccine (either Sinovac inactivated or BioNTech-Fosun
mRNA vaccine), and subsequently undergone whole-body
FDG PET/CT at our centre between 1 May 2021
and 30 September 2021 were enrolled in the current
study. Exclusion criteria included: (1) non-whole body
PET/CT studies; (2) incomplete vaccination records;
(3) non-oncology patients; (4) known malignancy that involved or was likely to involve the draining
lymph nodes of the vaccine injection site (axillary,
supraclavicular or lower cervical lymph nodes
ipsilateral to the vaccine injection site in the deltoid
muscle; for instance, ipsilateral breast cancer); (5)
conditions and interventions that may affect lymphatic
drainage pattern ipsilateral to the vaccine injection site
(e.g., lymphoedema, local infection, axillary lymph
node dissection, radiotherapy); and (6) patients with
vaccinations in both deltoid muscles, and vaccination
sites outside the upper extremities. For lymphoma,
additional exclusion criteria included: no baseline
PET/CT, no treatment response of previously identified
lymphoma(s), and new lymphomatous lesion(s) detected
in the rest of body. COVID-19 vaccination history
(such as vaccine type, number of doses, vaccination
date[s], injection site) and other relevant demographic
and clinical data (including age, sex, and indication for
PET/CT exam) were obtained from the electronic
medical record as well as direct inquiry of each patient
upon arrival for PET/CT.
Positron Emission Tomography/Computed
Tomography
Imaging studies were performed on a PET/CT system
(Discovery 710, GE Healthcare, Milwaukee [WI], US)
according to our centre’s scanning protocol. Patients
fasted for at least 6 hours prior to FDG injection. Blood
glucose levels were required to be <11 mmol/L. 370 MBq
of FDG was injected intravenously (550 MBq for patients
with body weight >80 kg). It was a standard procedure
in our centre to inject tracer into the limb contralateral
to the primary tumour, or via the lower extremities (e.g.,
in cases of bilateral breast tumours) whenever possible.
A total of 19 patients had FDG administration and
vaccination into the same limb. No patient experienced
tracer extravasation or local infection.
Image acquisition started approximately 60 minutes
following FDG injection. Spiral CT was first obtained
for attenuation correction and anatomical localisation
using the following technical parameters: tube voltage
120 kVp, modulated tube current 80 to 300 mA, gantry
rotation speed 0.5 s per rotation, pitch 0.984. Emission
PET scan was then acquired from the skull vertex to
mid-thigh (or to toes, depending on clinical indications)
with 2 minutes per bed position in three-dimensional
mode. PET image datasets were reconstructed using a
time-of-flight ordered subset expectation maximisation
algorithm with point spread function modelling
(4 iterations, 18 subsets, 5.5-mm cut-off filter).
Image Analysis
PET/CT images were reviewed using AW Volume
Viewer (version 12.3, Ext 8, GE Healthcare, Milwaukee
[WI], US) by a reader with 4 years of experience in nuclear
medicine and molecular imaging. Results of previous
imaging studies, type, and site of primary tumours, and
location of regional nodal or distant metastases were
taken into account for image interpretation.
The maximal standardised uptake value (SUVmax,
normalised for body weight) was measured by placing
spherical volumes of interest at the vaccine injection site
(usually the deltoid muscle), at ipsilateral draining lymph
nodes (e.g., axillary, supraclavicular, lower cervical
lymph nodes for vaccination at deltoid muscle), and at
the contralateral deltoid (or other injected) muscle and
draining lymph nodes. Positive lymph node and deltoid
muscle uptake was defined as having an SUVmax ≥1.5 ×
that of its contralateral counterpart.[4] [5] [7] [8] The short axis of
each lymph node was measured on the CT images.
Statistical Analysis
Categorical data are expressed as frequency and
percentage. Continuous data are presented as mean ±
standard deviation if they are normally distributed;
otherwise, they are expressed as median (range). An
unpaired Student’s t test was used to compare means
between groups for normally distributed data; a nonparametric
Mann-Whitney U test was used to compare
non-normally distributed data. The Chi-square test
was used to compare proportions between groups
(e.g., comparing proportions of patients with first
dose of vaccine only between Sinovac and BioNTech-Fosun groups). All statistical tests were carried out
using commercial software (MedCalc version 20.019;
MedCalc Software, Ostend, Belgium). Graphs were
composed using commercial software (Excel version
16.43, Microsoft, Redmond [WA], US). A p value of
<0.05 was considered statistically significant.
RESULTS
Patient Characteristics
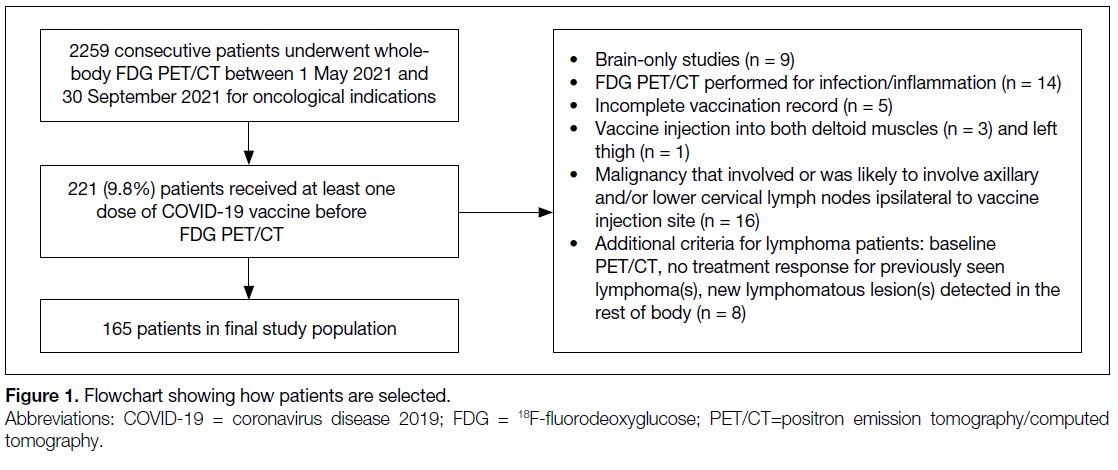
Among 2259 consecutive cases undergoing whole-body
FDG PET/CT during the study period, 221 (9.8%) had
received at least one dose of COVID-19 vaccine before
PET/CT. Figure 1 shows the numbers of cases meeting
exclusion criteria. A total of 165 cases were finally
included in the study population (mean age, 60.8 ± 11.1
years; 45.5% women). Eighty-seven patients (52.7%)
had received Sinovac inactivated vaccine, and the other
78 were vaccinated with BioNTech-Fosun mRNA vaccine. In all, 24.8% of subjects (41/165) had received
one vaccine dose, and 75.2% of subjects (124/165) had
received two doses of vaccine. The median time interval
between vaccination and FDG PET/CT was 41.0 days
(range, 2-176). Details of patient demographics are
illustrated in Table 1.
Figure 1. Flowchart showing how patients are selected
Table 1. Summary of patient demographic and clinical characteristics
18F-fluorodeoxyglucose-avid Lymph Nodes
Ipsilateral to COVID-19 Vaccination
Overall, 26.7% (44/165; 29 women and 15 men) of
cases had positive uptake in axillary and lower cervical
lymph nodes ipsilateral to the vaccine injection site. A
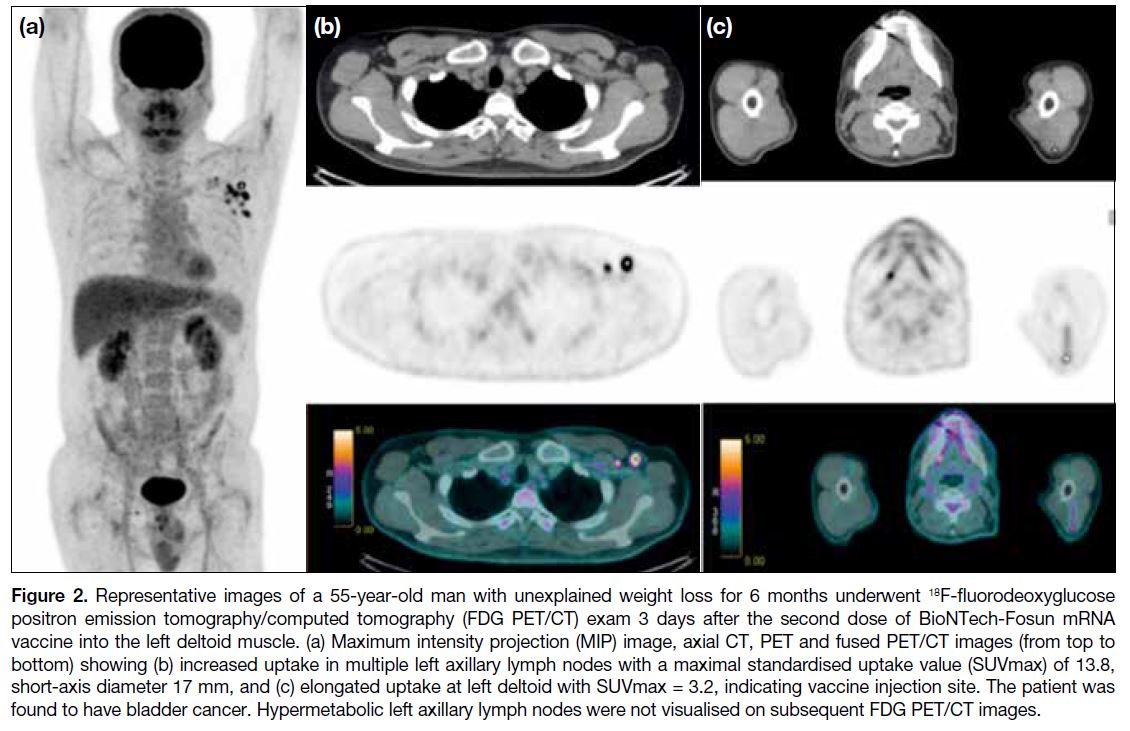
representative case is shown in Figure 2. The median
SUVmax of VAHL was 3.2 (range, 1.7-13.8). Most
of them were of normal size (88.6% were <10 mm;
median, 7 mm; range, 3-17 mm). The number of FDGavid
lymph nodes ranged from 1-15 (median, 3). VAHL
was most frequently seen in ipsilateral axillary level I
nodes (52.1%, 86/165; while 27.3% (45/165) and 2.4%
(4/165) were detected in ipsilateral axillary levels II
and III, respectively. In all, 20.5% of cases (9/44) also
showed positive ipsilateral extra-axillary nodal uptake
(including interpectoral [located between the pectoralis
major and pectoralis minor muscles], infraclavicular,
supraclavicular, lower cervical, and mediastinal regions).
Figure 2. Representative images of a 55-year-old man with unexplained weight loss for 6 months underwent 18F-fluorodeoxyglucose
positron emission tomography/computed tomography (FDG PET/CT) exam 3 days after the second dose of BioNTech-Fosun mRNA
vaccine into the left deltoid muscle. (a) Maximum intensity projection (MIP) image, axial CT, PET and fused PET/CT images (from top to
bottom) showing (b) increased uptake in multiple left axillary lymph nodes with a maximal standardised uptake value (SUVmax) of 13.8,
short-axis diameter 17 mm, and (c) elongated uptake at left deltoid with SUVmax = 3.2, indicating vaccine injection site. The patient was
found to have bladder cancer. Hypermetabolic left axillary lymph nodes were not visualised on subsequent FDG PET/CT images.
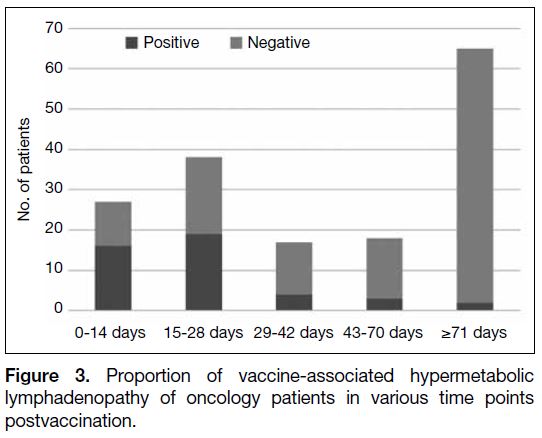
The proportion of VAHL at various time intervals
following vaccination is shown in Figure 3. The greatest
percentage of vaccinated cases with VAHL was seen
within first 2 weeks (59.3%), and there was still a
significantly larger proportion of cases with VAHL
within the first 4 weeks after vaccination compared with those beyond 4 weeks after vaccination (p < 0.001).
In total, 11.4% of cases (5/44) with FDG-avid axillary
lymph nodes were found >6 weeks after vaccination.
Figure 3. Proportion of vaccine-associated hypermetabolic
lymphadenopathy of oncology patients in various time points
postvaccination
In a subgroup analysis of cases undergoing FDG PET/CT
exam within 87 days following vaccination (because
no benign VAHL was seen 87 days after vaccination), BioNTech-Fosun mRNA vaccine was significantly
associated with a higher incidence (p < 0.001) and larger
number (p = 0.038) of FDG-avid ipsilateral lymph nodes
compared with Sinovac inactivated vaccine, but there
was no significant association with size (p = 0.142) or
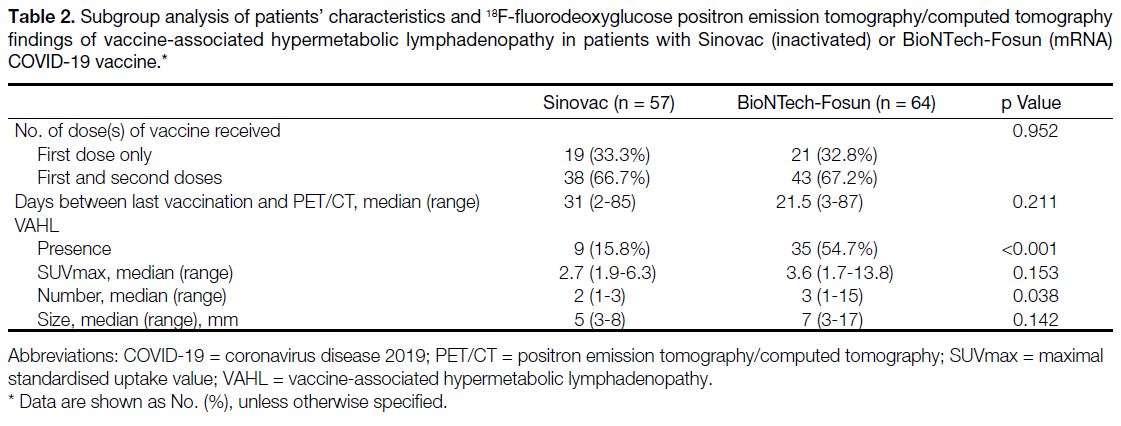
SUVmax (p = 0.153) [Table 2]. In addition, VAHL was
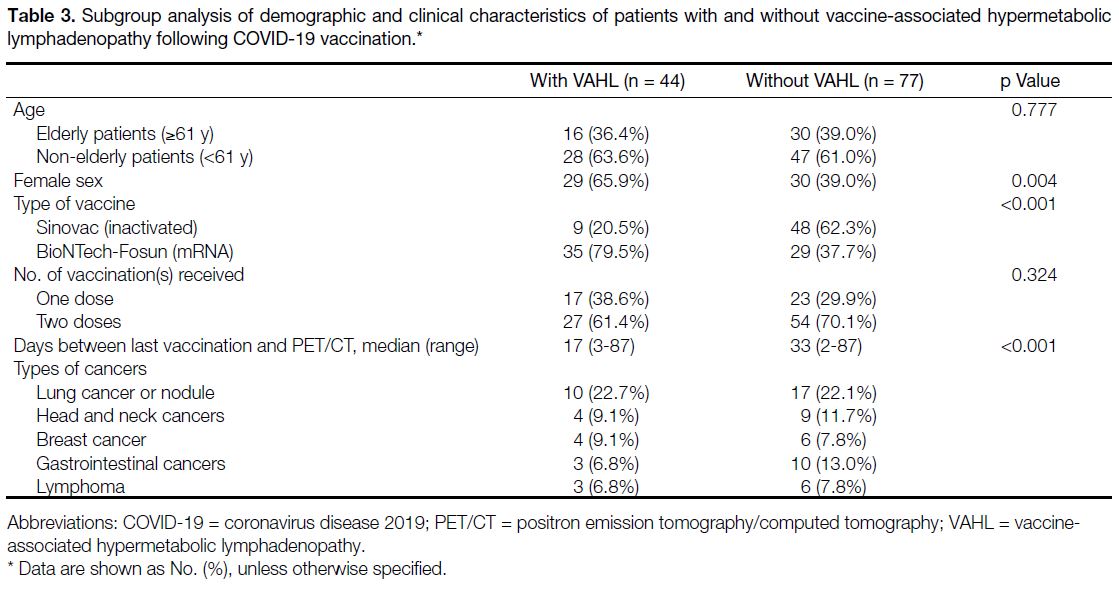
more frequently seen in women (p = 0.004) [Table 3].
There was no statistically significant difference in the
incidence, SUVmax, short-axis diameter or number of
VAHL nodes between those cases that had received
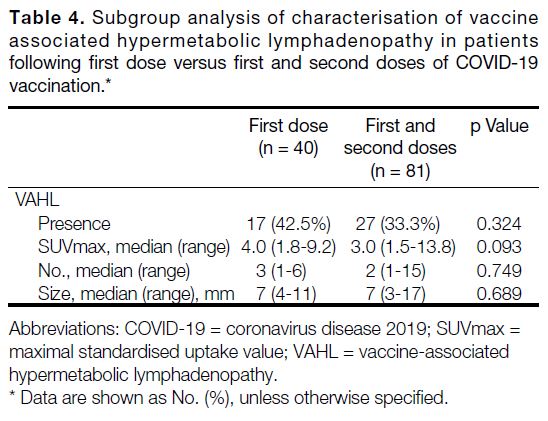
the first dose only and those that had received first and
second doses (Table 4).
Table 2. Subgroup analysis of patients’ characteristics and 18F-fluorodeoxyglucose positron emission tomography/computed tomography
findings of vaccine-associated hypermetabolic lymphadenopathy in patients with Sinovac (inactivated) or BioNTech-Fosun (mRNA)
COVID-19 vaccine.
Table 3. Subgroup analysis of demographic and clinical characteristics of patients with and without vaccine-associated hypermetabolic
lymphadenopathy following COVID-19 vaccination
Table 4. Subgroup analysis of characterisation of vaccine
associated hypermetabolic lymphadenopathy in patients
following first dose versus first and second doses of COVID-19
vaccination
DISCUSSION
Overall, 26.7% of subjects showed hypermetabolic
ipsilateral lymph nodes. Within 2 weeks after
vaccination, VAHL was seen in 59.3% of patients.
Such findings were postulated to be associated with
the inflammatory response to vaccine components
at the inoculation site and draining lymph nodes.9
Indeed, VAHL was previously reported after vaccines
against other pathogens (such as influenza virus,
human papillomavirus, and pneumococcus), and
was also observed in various non-FDG PET exams (e.g., 68Ga-DOTATATE, 18F- or 68Ga-PSMA).[4] [10] [11]
VAHL can become enlarged,[2] [3] [12] as in 11.4% of cases in
our study, which could not be readily distinguished from
nodal metastases based on CT alone. Follow-up scan
showed that such enlargement did not persist.
The definitions of positive draining lymph node and
deltoid uptake after vaccination in the current study
(i.e. having a SUVmax ratio of ≥1.5 between ipsilateral
to contralateral reference sites) were used previously
by other research groups for COVID-19 and influenza
vaccines.[4] [5] [7] [8] Compared with other criteria (e.g., using blood pool SUVmax or surrounding background
as reference),[2] [3] [6] these criteria were relatively more
quantitative, reproducible and took into account that
lymphadenopathy contralateral to vaccine injection is
extremely rare. Lim et al[13] reviewed 67 published reports
and found that only four out of 3072 cases (0.13%)
showed lymphadenopathy outside ipsilateral axillary
and cervical regions (including contralateral axilla).
There are other criteria of lymph node or deltoid uptake
in the literature as mentioned above[2] [3] [6] and caution
should be taken when comparing results of different
studies.
Although many studies focused on VAHL in the
ipsilateral axillary region, our findings showed
that 20.5% of patients had ipsilateral extra-axillary
lymph nodal uptake, which should not be ignored by
interpreting physicians. Furthermore, in spite of several
recommendations advising to delay nonurgent imaging
4 to 6 weeks after the recent dose of vaccination,[12] [14] [15]
Eshet et al[5] demonstrated that VAHL was still found
in 29% of subjects 7 to 10 weeks after the second dose
of vaccination. Similarly, we also observed 11.4% of
patients having VAHL beyond 6 weeks after vaccination
(the longest duration was 87 days) in the current study.
Therefore, a delay of 4 to 6 weeks of a nonurgent scan
is useful but cannot completely avoid the occurrence of
VAHL.
Compared with mRNA COVID-19 vaccines, there are
fewer studies about VAHL following other types of
COVID-19 vaccines.[7] [16] The current study illustrated
that BioNTech-Fosun mRNA vaccine was associated
with a higher incidence of VAHL compared with the
Sinovac inactivated vaccine, which was similar to the
results recently reported in Turkish patients (incidence
of 9.9% for CoronaVac and 37.5% for BioNTech).[7] It
can be explained by the fact that mRNA vaccines have
inherently greater immunogenicity based on laboratory
and clinical data.[17] Moreover, a higher incidence of
VAHL was also observed in female patients. Such sex-related
differences have been noted previously,[18] and
could be explained by genetic factors as well as the
immunostimulatory effect of oestrogens.[19]
From the results of the present study, 26.7% of subjects showed hypermetabolic ipsilateral regional lymph nodes after COVID-19 vaccination, suggesting that VAHL is
common and distinguishing VAHL from true metastatic
lymphadenopathy is important to avoid additional
testing, unnecessary biopsy and even alteration of
therapy. CT alone is not adequate, since some of these
lymph nodes are enlarged (11.4% of cases in our study)
and could not be readily differentiated from nodal
metastases. Obtaining a detailed vaccination history
before tracer injection is useful, which should at least
include all the items recommended by the Society of
Nuclear Medicine and Molecular Imaging COVID-19
Task Force and the Scientific Expert Panel of the journal
Radiology: injection site(s), date(s) of vaccination and
type(s) of vaccine.[12] [14] Information on injection site(s)
helps interpreting physicians determine which lymph
nodes are more likely to be related to vaccine infection.
Date(s) of vaccination is important because VAHL is
transient and wanes with time. Knowing which type
of vaccine has been injected is useful since incidence
of VAHL differs among type of vaccine; for instance,
our study showed that BioNTech-Fosun mRNA vaccine
was associated with higher incidence and larger number
of VAHL compared with Sinovac inactivated vaccine. Nevertheless, vaccination history should not be based
on pre-injection questionnaire alone, since patients
may not remember accurately, and some details may be
omitted. Details of vaccination history should be readily
found in medical records and electronic vaccination
records, which can be accessed using smartphones (in
some countries or regions where it is feasible). Such
vaccination history should include injection site(s) apart
from date(s) and type(s) of vaccination.[12] [14] Furthermore,
efforts should be made to avoid administration of
vaccine on the side ipsilateral to the primary or suspected
malignancy. First and second doses should be given in
the same arm.[12] [14] [15] Promotion of awareness of referring
physicians and patients and proper training of healthcare
professionals who provide COVID-19 vaccination are
possible measures to implement. Last but not least,
scheduling of FDG PET/CT for a certain period after
the last vaccination can allow for VAHL to resolve. The
National Comprehensive Cancer Network COVID-19
Vaccination Advisory Committee suggests a delay of
4 to 6 weeks,[15] which is based on the recommendation
of the Society of Breast Imaging.[20] On the other hand,
the Scientific Expert Panel of the journal Radiology
recommended a postponement of at least 6 weeks,
because the preliminary experience of the panel members
showed that the some enlarged lymph nodes persisted
even after 4 weeks.[12] The present study provided
further evidence that such recommendations can also be applied to hypermetabolic lymphadenopathy seen on
FDG PET/CT, since 79.5% (35/44) and 88.6% (39/44)
of subjects demonstrated hypermetabolic lymph
nodes within 4 weeks and 6 weeks postvaccination,
respectively. Because women experience VAHL more
commonly than men, and breast cancer is a common
type of cancer among women, it may be necessary to
inject breast cancer patients in the lower extremity, or at
least in deltoid contralateral to the site of primary tumour.
Given that the healthcare system is currently facing
enormous strain due to the COVID-19 pandemic, some
of the above measures to tackle FDG-avid lymph nodes
may not be readily implemented and require cooperation
of a multidisciplinary team.
There were several limitations in the present study. First, it was a retrospective single-centre study design. Second,
recall bias of vaccination history existed. Furthermore,
the study was conducted in a relative short study period.
Follow-up FDG PET/CT of two subjects with VAHL
demonstrated interval metabolic resolution/improvement
of previously hypermetabolic lymph nodes. For the
other cases, however, there was no biopsy or follow-up
imaging to support the benignity of VAHL; follow-up or
even biopsy of enlarged/hypermetabolic lymph nodes is
necessary to confirm that VAHL was benign.
CONCLUSION
In summary, in view of emergence of new variants of
the coronavirus, recommendation of additional booster
shot as well as lowering the age limit of vaccination,
the vaccination rate will continue to rise, and awareness
of COVID-19 vaccine-associated imaging findings
is important for interpreting physicians. In the present
study, we found that 26.7% of subjects showed positive
ipsilateral lymph node uptake more frequently with
BioNTech-Fosun mRNA vaccine and in women. Some
of the reactive nodes were enlarged and persisted beyond
6 weeks, which could not be readily distinguished from
malignant lesions based on CT alone. Therefore, advising
patients to get vaccination contralateral to primary
tumour or in the lower extremity, proper vaccination
history documentation, and recognition of co-occurrence
of positive deltoid uptake with VAHL may help avoid
misinterpretation.
REFERENCES
1. Chan WL, Ho YH, Wong CK, Choi HC, Lam KO, Yuen KK, et al.
Acceptance of COVID-19 vaccination in cancer patients in Hong
Kong: approaches to improve the vaccination rate. Vaccines
(Basel). 2021;9:792. Crossref
2. Bernstine H, Priss M, Anati T, Turko O, Gorenberg M,
Stenmetz AP, et al. Axillary lymph nodes hypermetabolism after
BNT162b2 mRNA COVID-19 vaccination in cancer patients
undergoing 18F-FDG PET/CT: a cohort study. Clin Nucl Med.
2021;46:396-401. Crossref
3. Cohen D, Krauthammer SH, Wolf I, Even-Sapir E. Hypermetabolic
lymphadenopathy following administration of BNT162b2 mRNA
Covid-19 vaccine: Incidence assessed by [18F]FDG PET-CT and
relevance to study interpretation. Eur J Nucl Med Mol Imaging.
2021;48:1854-63. Crossref
4. Eifer M, Tau N, Alhoubani, Y, Kanana N, Domachevsky L,
Shams J, et al. COVID-19 mRNA vaccination: age and immune
status and its association with axillary lymph node PET/CT Uptake.
J Nucl Med. 2021;63:134-9. Crossref
5. Eshet Y, Tau N, Alhoubani Y, Kanana N, Domachevsky L, Eifer M.
Prevalence of increased FDG PET/CT axillary lymph node uptake
beyond 6 weeks after mRNA COVID-19 vaccination. Radiology.
2021;300:E345-7. Crossref
6. Schroeder DG, Jang S, Johnson DR, Takahashi H, Navin PJ,
Broski SM, et al. Frequency and characteristics of nodal and deltoid
FDG and 11C-choline uptake on PET imaging performed after
COVID-19 vaccination. AJR Am J Roentgenol. 2021;217:1206-16. Crossref
7. Sahin O. Hypermetabolic axillary lymphadenopathy on FDG PET/CT Due to COVID-19 vaccination. Selcuk Med J. 2021;37:269-75. Crossref
8. Thomassen A, Lerberg Nielsen A, Gerke O, Johansen A,
Petersen H. Duration of 18F-FDG avidity in lymph nodes after
pandemic H1N1v and seasonal influenza vaccination. Eur J Nucl
Med Mol Imaging. 2011;38:894-8. Crossref
9. Youn H, Hong KJ. Non-invasive molecular imaging of immune cell dynamics for vaccine research. Clin Exp Vaccine Res. 2019;8:89-93. Crossref
10. McIntosh LJ, Bankier AA, Vijayaraghavan GR, Licho R,
Rosen MP. COVID-19 vaccination-related uptake on FDG PET/CT: an emerging dilemma and suggestions for management. AJR
Am J Roentgenol. 2021;217:975-83. Crossref
11. Treglia G, Cuzzocrea M, Muoio B, Elzi L. PET findings after
COVID-19 vaccination: “Keep calm and carry on”. Clin Transl
Imaging. 2021;9:209-14. Crossref
12. Becker AS, Perez-Johnston R, Chikarmane SA, Chen MM,
El Homsi M, Feigin KN, et al. Multidisciplinary recommendations
regarding post-vaccine adenopathy and radiologic imaging:
Radiology Scientific Expert Panel. Radiology. 2021;300:E323-7. Crossref
13. Lim J, Lee SA, Khil EK, Byeon SJ, Kang HJ, Choi JA. COVID-19
vaccine-related axillary lymphadenopathy in breast cancer patients:
Case series with a review of literature. Semin Oncol. 2021;48:283-91. Crossref
14. Society of Nuclear Medicine and Molecular Imaging (SNMMI).
SNMMI statement: the effect of COVID-19 vaccination on FDG
PET/CT. J Nucl Med. 2021;62:30N.
15. National Comprehensive Cancer Network. Recommendations
of the e National Comprehensive Cancer Network® (NCCN®)
Advisory Committee on COVID-19 vaccination and pre-exposure
prophylaxis. Available from: https://www.nccn.org/docs/defaultsource/
covid-19/2021_covid-19_vaccination_guidance_v4-0.pdf?sfvrsn=b483da2b_70. Accessed 7 Dec 2021.
16. Shin M, Hyun CY, Choi YH, Choi JY, Lee KH, Cho YS.
COVID-19 vaccination-associated lymphadenopathy on FDG PET/CT: distinctive features in adenovirus-vectored vaccine. Clin Nucl
Med. 2021;46:814-9. Crossref
17. Mingos M, Howard S, Giacalone N, Kozono D, Jacene H. Systemic
immune response to vaccination on FDG-PET/CT. Nucl Med Mol
Imaging. 2016;50:358-61. Crossref
18. Adin ME, Isufi E, Kulon M, Pucar D. Association of COVID-19 mRNA Vaccine with ipsilateral axillary lymph node reactivity on imaging. JAMA Oncol. 2021;7:1241-2. Crossref
19. Giefing-Kröll C, Berger P, Lepperdinger G, Grubeck-Loebenstein B.
How sex and age affect immune responses, susceptibility to
infections, and response to vaccination. Aging cell. 2015;14:309-21. Crossref
20. Grimm L, Destounis S, Dogan B, Nicholson B, Dontchos, Sonnenblick E, et al. Society of Breast Imaging: SBI recommendations for the management of axillary adenopathy in
patients with recent COVID-19 vaccination. Available from: https://www.sbi-online.org/Portals/0/Position%20Statements/2021/SBI-reco.... Accessed 7 Dec 2021.