Three-week Cycles of Paclitaxel-Carboplatin Administered Concurrently with Radiotherapy for Inoperable Stage III Non-small Cell Lung Cancer: 10-Year Single-Centre Experience
ORIGINAL ARTICLE CME
Three-week Cycles of Paclitaxel-Carboplatin Administered Concurrently with Radiotherapy for Inoperable Stage III Non-small Cell Lung Cancer: 10-Year Single-Centre Experience
HCY Wong, FMY Lim, ACK Cheng
Department of Oncology, Princess Margaret Hospital, Hong Kong
Correspondence: Dr HCY Wong, Department of Oncology, Princess Margaret Hospital, Hong Kong. Email: henrywong3011@gmail.com
Submitted: 8 Aug 2021; Accepted: 16 Dec 2021.
Contributors: HCYW and FMYL designed the study. HCYW acquired the data. HCYW and FMYL analysed the data. HCYW drafted the
manuscript. All authors critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to
the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: Research data are stored in an institutional repository and will be shared by the corresponding author on reasonable request.
Ethics Approval: This study was approved by the Kowloon West Cluster Research Ethics Committee, Hospital Authority [Ref KW/EX-20-054(146-03)]. The Ethics Committee waived the need for patient consent for this retrospective study.
Abstract
Introduction
We reviewed the efficacy, toxicities, and prognostic factors of two 3-week cycles of paclitaxelcarboplatin
administered concurrently with radiotherapy for treatment of unresectable stage III non-small cell lung
cancer (NSCLC).
Methods
Cases of unresectable stage III NSCLC treated with chemoradiotherapy using paclitaxel 175 mg/m2 and
carboplatin area under the curve=5 on day 1 of a 21-day cycle concurrently with 6 weeks of radiotherapy (60-66 Gy)
from 2007 to 2017 were retrieved.
Results
A total of 65 patients (median age=63 years) were included. At a 29.5-month median follow-up, the median
overall survival was 35.0 months (95% confidence interval [CI]=17.5-52.5 months). Multivariable Cox regression
analyses showed that gross tumour volume (p = 0.001), mean heart dose ≥5 Gy (p = 0.007), and more than four
cycles of chemotherapy administered (p = 0.006) were independent negative prognostic factors. The maximum grade
toxicity was Grade 2 in 27 patients (41.5%), grade 3 in 13 patients (20.0%) and grade 4 in five patients (7.7%). No
grade 5 events were observed. The most common grade 3 or 4 toxicity was neutropenia, which occurred in nine
(13.8%) and five (7.7%) patients, respectively. Three patients (4.6%) had neutropenic fever. Grade ≥2 pneumonitis
and oesophagitis were seen in five (7.7%) and nine (13.8%) patients, respectively.
Conclusion
Two 3-week cycles of paclitaxel-carboplatin given concurrently with radiotherapy for unresectable
stage III NSCLC was well-tolerated, with outcomes comparable to historical data, and fewer hospital visits.
Key Words: Carcinoma, non-small-cell lung; Positron-emission tomography
中文摘要
紫杉醇-卡鉑三週週期及同步放射治療方案治療不宜手術的第III期非小細胞肺癌:十年單中心經驗
黃進業、林美瑩、鄭志堅
引言
回顧每3週為一週期,共兩週期的紫杉醇-卡鉑及同步放射治療用於治療不可切除的第III期非小細胞肺癌的療效、副作用和預後因素。
方法
我們將2007年至2017年期間同步使用紫杉醇-卡鉑(每21天的第1天注射紫杉醇175 mg/m2,卡鉑曲線下面積=5)和6週放射治療(60-66 Gy)醫治不可切除的第III期非小細胞肺癌病例進行回顧性研究。
結果
研究共納入65名患者(年齡中位數63歲)。在29.5個月的中位跟進期中,總存活期中位數為35.0個月(95%置信區間=17.5-52.5個月)。多變量Cox 迴歸分析顯示,腫瘤體積(p = 0.001)、平均心臟劑量≥5 Gy(p = 0.007)和多過四個化療週期(p = 0.006)為獨立的負面預後因素。27例(41.5%)的最大副作用級別為第2級、13例(20.0%)為第3級、5例(7.7%)為第4級,未有發現患者出現第5級副作用。最常見的第3或第4級副作用為嗜中性白血球低下症,分別9例(13.8%)和5例(7.7%)。3例(4.6%)出現嗜中性白血球低下症伴隨發燒。分別有5例(7.7%)和9例(13.8%)出現第2級或以上放射治療引起的肺炎和食道炎。
結論
對於不可切除的第III期非小細胞肺癌,放射治療期間同步使用每3週一次的紫杉醇-卡鉑副作用可接受,效果與文獻數據相若,在醫院接受化療次數也較少。
INTRODUCTION
Concurrent chemoradiotherapy (CCRT) with platinum-based
agents is preferred over a sequential treatment
approach for fit patients with unresectable stage III non-small
cell lung cancer (NSCLC) for optimal survival
rates.[1] [2] However, overall outcomes still remain poor,
with a 5-year overall survival (OS) rate of approximately
15% to 30%.[2] [3] [4] No major recent advances had been
made until the publication of the PACIFIC trial, which
established the role of durvalumab after CCRT.[5]
There have been multiple studies evaluating the efficacy
of different chemotherapy regimens in combination
with radiotherapy (RT) since the 2000s, including
etoposide-cisplatin (EP), weekly paclitaxel-carboplatin
(PC), vinorelbine-cisplatin, and pemetrexed with either
cisplatin or carboplatin.[6] [7] [8] [9] [10] Trials comparing these
regimens were not available until after 2012, and there
has not been a conclusion as to which is superior. For
example, a phase III randomised trial showed a higher
3-year OS with EP compared with weekly PC with an
absolute difference of 15%.[11] In contrast, one meta-analysis
and two other large-scale retrospective analyses showed the opposite effect, with comparable efficacy
using either EP or PC.[12] [13] Earlier guidelines from
The European Society of Medical Oncology (ESMO)
for early unresectable NSCLC published in 2010
recommended that ‘etoposide-cisplatin (or vinblastine or
vinorelbine) and PC both at systemic doses should be
considered as reference regimens’.[14] No recommended
schedule or doses of the agents were suggested.
From 2007, our institution adopted paclitaxel 175 mg/m2 and carboplatin area under the curve [AUC]=5
administered on day 1 of a 21-day cycle as one option
given concurrently with RT for inoperable stage III
NSCLC because of its convenient schedule requiring
only one in-patient day in the chemotherapy centre
every cycle. The chemotherapy component is well-described
for palliation in advanced NSCLC[15] [16] [17] but
data combining the chemotherapy as part of CCRT for
unresectable stage III disease are limited. Movsas et al[18]
established the safety of this regimen with RT using
paclitaxel 175 mg/m2 and carboplatin AUC=5 in a dose
escalation study in 2001. A small retrospective study of
43 patients (15 patients given PC, 28 patients given EP) in China showed that there was no statistical difference
in response rates, progression-free survival (PFS), or OS
compared with the EP group.[19]
This study aimed to review the survival outcomes,
toxicities, and prognostic factors of giving 3-week
cycles of PC during RT and compare with another cohort
receiving EP at our institution.
METHODS
Patients
The case cohorts were identified from the list of NSCLC
patients treated with RT from the treatment planning
system from January 2007 to April 2017. Inclusion
criteria were diagnosis of inoperable stage III (restaged
based on the TNM American Joint Committee on Cancer
7th edition) NSCLC treated with CCRT of curative
intent with 3-week cycles of PC. Histological diagnosis
was preferred, but radiological diagnosis with a positive
positron emission tomography–computed tomography
(PET-CT) scan was allowed if obtaining histology was
not feasible. Patients were excluded if no chemotherapy
was administered concurrently with RT and/or total RT
dose was <60 Gy. Patients administered more than two
cycles of chemotherapy before RT were considered
as having received sequential treatment and were also
excluded. Cases were included if they had received at
least one of their cycles of chemotherapy beginning on
day 1 of RT. A case cohort with the same inclusion and
exclusion criteria but receiving EP was identified for
comparison. This study was approved by the local clinical
research ethics committee with permission waived
due to its retrospective nature. The STROBE reporting
guidelines were implemented in this manuscript.
Procedures
RT was administered 5 times per week (Monday to Friday
with weekend rests) in 2 Gy fractions using 6 to 15 MV
photons. Free-breathing contrast-enhanced simulation
computed tomography (CT) with 5 mm thickness was
acquired with a scan range from neck to the upper
abdomen including the entire liver. All treatments were
administered using three-dimensional conformal RT.
Dose constraints were as follows: lung V20 <30%, whole
heart <40 Gy, spinal cord Dmax <45 Gy, and oesophagus
V55 <30% or mean oesophageal dose <34 Gy. RT doses
of 60 to 66 Gy were prescribed to the planning target
volume (PTV). Contouring of treatment volumes was
done on simulation contrast CT and assisted by PET
imaging if available. Gross tumour volume (GTV) was
defined as the primary tumour and any involved regional lymph nodes with short axis >1 cm on diagnostic CT
or fluorodeoxyglucose avid on pretreatment PET-CT.
Clinical target volume (CTV) was defined as the GTV
with a 0.6- to 0.8-cm margin for the primary tumour
and was the same as the GTV for the regional involved
lymph nodes. CTV of the primary tumour was trimmed
off from the chest wall and vertebral bodies unless there
was tumour involvement. Elective nodal irradiation was
not given. PTV of the primary tumour was defined as the
CTV with a 1.0 cm margin axially and 1.0 to 1.5 cm in
the superior-inferior direction to account for respiratory
motion. PTV of the lymph nodes was defined as the CTV
with a 1.0 cm margin. PTV coverage was achieved if
95% of the PTV was covered by 95% of the prescribed
dose. Treatment verification was carried out with online
kV portal images on the first day, mid-treatment, and,
if needed, as determined by the treating radiotherapist
at any time during treatment without the use of fiducial
markers.
Chemotherapy consisted of either PC regimen
(paclitaxel 175 mg/m2 and carboplatin AUC=5 given
every 3 weeks on day 1 of the cycle) or EP regimen
(etoposide 100 mg/m2 and cisplatin 30 mg/m2 days
every 3 weeks on days 1 to 3 of the cycle). Patients
were scheduled to receive a total of four cycles of
chemotherapy, in which two were concurrent with RT.
A maximum of up to six chemotherapy cycles were
allowed at the treating clinicians’ discretion. RT had
to commence concurrently with the first three cycles
of chemotherapy. No prophylactic granulocyte-colony
stimulating factor was used. In the case of Grade 3 or
4 toxicities, chemotherapy was withheld until toxicities
improved to Grade 1 or less and a 25% dose reduction
was applied to the subsequent cycle. Chemotherapy
was stopped if toxicities failed to return to Grade 1 or
the treating clinician decided that the risks of further
chemotherapy outweighed the benefits. Paclitaxel
infusion was given over 3 hours after premedication
with intravenous dexamethasone 20 mg, intravenous
chlorpheniramine 10 mg, an H2 blocker (oral or
intravenous), and standard anti-emetics, followed by
carboplatin infusion given over 30 minutes.
Assessments
Baseline clinical, serological, and pathological parameters
within 4 weeks prior to the first cycle of chemotherapy
were documented. Clinical parameters including TNM
stage based on the American Joint Committee on Cancer
7th edition, Eastern Cooperative Oncology Group
performance status, smoking history, use of PET-CT for staging, medical co-morbidities, and serological levels
of haemoglobin, platelets, neutrophils, and albumin. RT
and dosimetric details including treatment dose, GTV
(cc), mean lung dose, mean oesophageal dose, and mean
heart dose were retrieved from the treatment planning
system. Treatment-related toxicities were graded based
on Common Terminology Criteria for Adverse Events
(CTCAE) version 4.0.
Patients were followed up every 3 to 4 months in the
first year, every 6 months in the second to third year
and 6 to 8 months in the fourth and fifth year, then once
every year. Routine follow-up assessments included
assessment of symptoms and signs of recurrence, Eastern
Cooperative Oncology Group performance status,
and CTCAE grading of adverse events. Plain chest
radiograph was done at every follow-up visit, while CT
scans were acquired within the first year after treatment
and if clinically indicated as determined by the treating
physician. Response was assessed using the RECIST
(Response Evaluation Criteria in Solid Tumors) criteria.
Statistical Analyses
Baseline characteristics and dosimetric parameters were
compared with the Chi-squared test or Fisher’s exact
test for categorical variables and Student’s t test for
continuous variables between patients with or without
high-grade treatment-related toxicities. PFS was defined
as the duration from the commencement of the first cycle
of chemotherapy to the time when there was radiological
or clinical evidence of disease progression or patient death.
OS was calculated from the time of commencement of
the first cycle of chemotherapy to the time of death. PFS
and OS were estimated using the Kaplan–Meier method.
Patients lost to follow-up were censored. Cox regression
analysis was used to determine the prognosticators for
PFS and OS. Statistically significant parameters in the
simple analysis were included in the multivariable Cox
regression analysis to determine independent prognostic
factors. The toxicities of the EP and PC case cohorts
were compared using Chi-squared or Fisher’s exact
tests. Median PFS and OS of the cohorts were estimated
with the Kaplan–Meier method and compared with log
rank tests. Statistical significance was set at p < 0.05.
All statistical analyses were performed using the SPSS
(Windows version 21.0; IBM Corp, Armonk [NY], US).
RESULTS
Case Characteristics
A total of 65 cases (median age 63 years, range 45-74) who received 3-week cycles of PC with RT were analysed (Table 1). Molecular tests were done on all
non-squamous cell cancers except for four patients
because of inadequate tissue. One patient with squamous
cell carcinoma also received molecular tests. Only a
small number of patients had epidermal growth factor
receptor or anaplastic lymphoma kinase genomic
aberrations (16.9%). PET-CT was available in 73.8% of
patients as part of the staging workup. The distribution
of staging was similar among stage IIIA (53.8%) and
stage IIIB (46.2%). Most patients received four cycles
of chemotherapy (83.1%) in total. Almost all patients
(96.9%) received 60 Gy of concurrent RT and 41.5%
(n = 27) had RT started concurrent with the first two
cycles of chemotherapy.
Table 1. Baseline characteristics
Treatment Delivery and Acute Toxicities
The majority of cases (89.2%) received RT treatment
without interruption, with 96.9% completing the planned
number of chemotherapy cycles. Dose reduction was
required in 40.0% of cases and deferral of chemotherapy
was required in 36.9%.
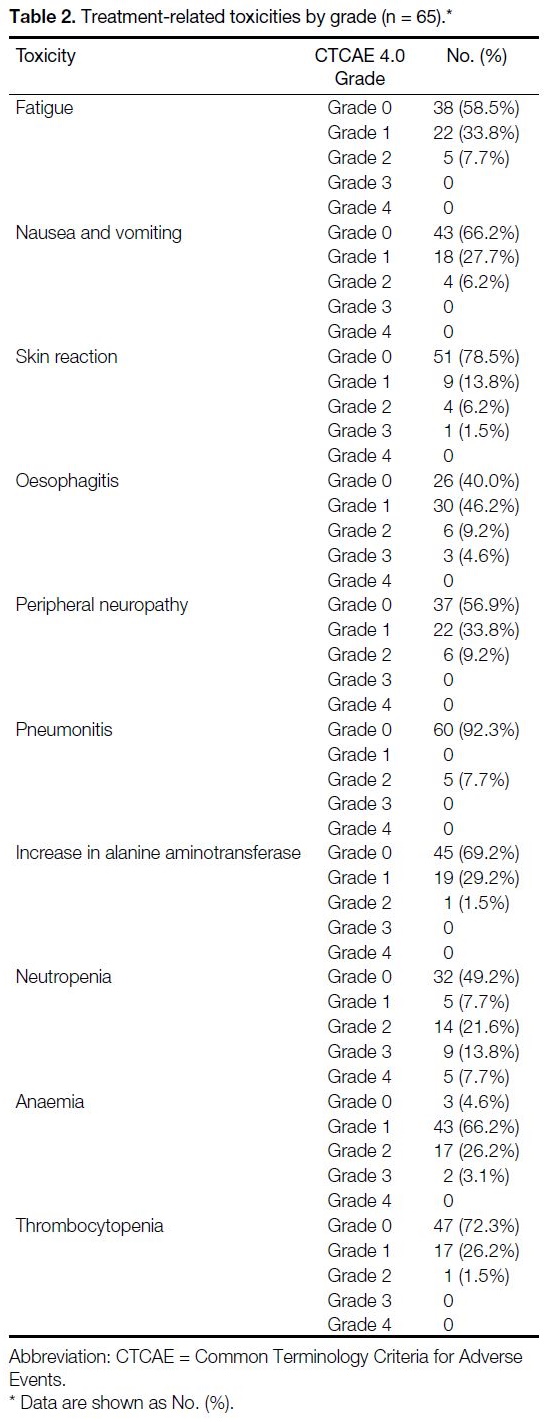
The maximum grade toxicity observed per patient was
grade 1 in 20 (30.8%) patients, grade 2 in 27 (41.5%),
grade 3 in 13 (20.0%) and grade 4 in five (7.7%);
there were no grade 5 toxicities observed (Table 2).
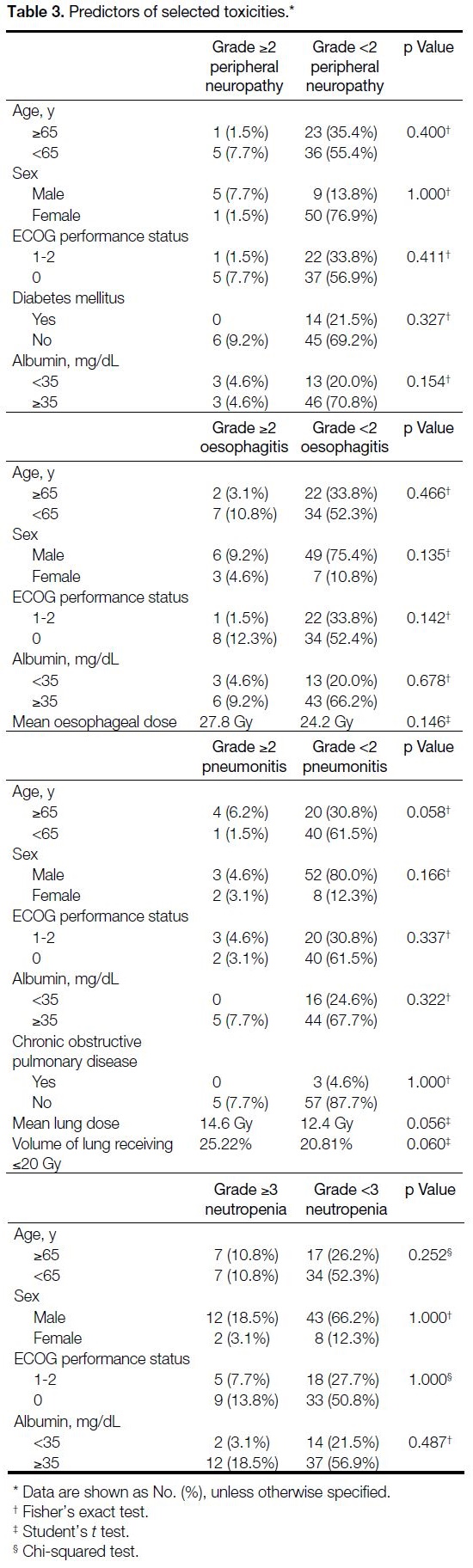
No pretreatment clinical characteristics or dosimetric
parameters were associated with higher-grade toxicities
(Table 3).
Table 2. Treatment-related toxicities by grade (n = 65).
Table 3. Predictors of selected toxicities
Treatment Outcomes
Overall Survival
At a median follow-up of 29.5 months (interquartile
range=13.3-56.3), the median OS was 35.0 months
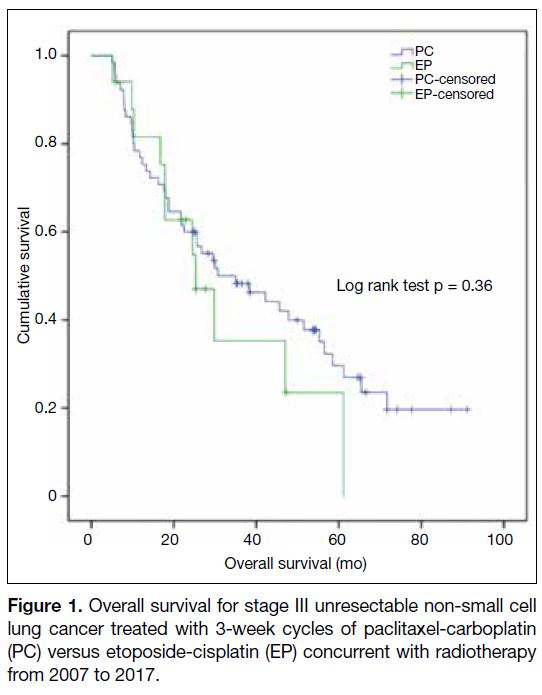
(95% confidence interval [CI] = 17.5-52.5) [Figure 1].
The 1-, 3- and 5-year OS rates were 76.9%, 48.3% and
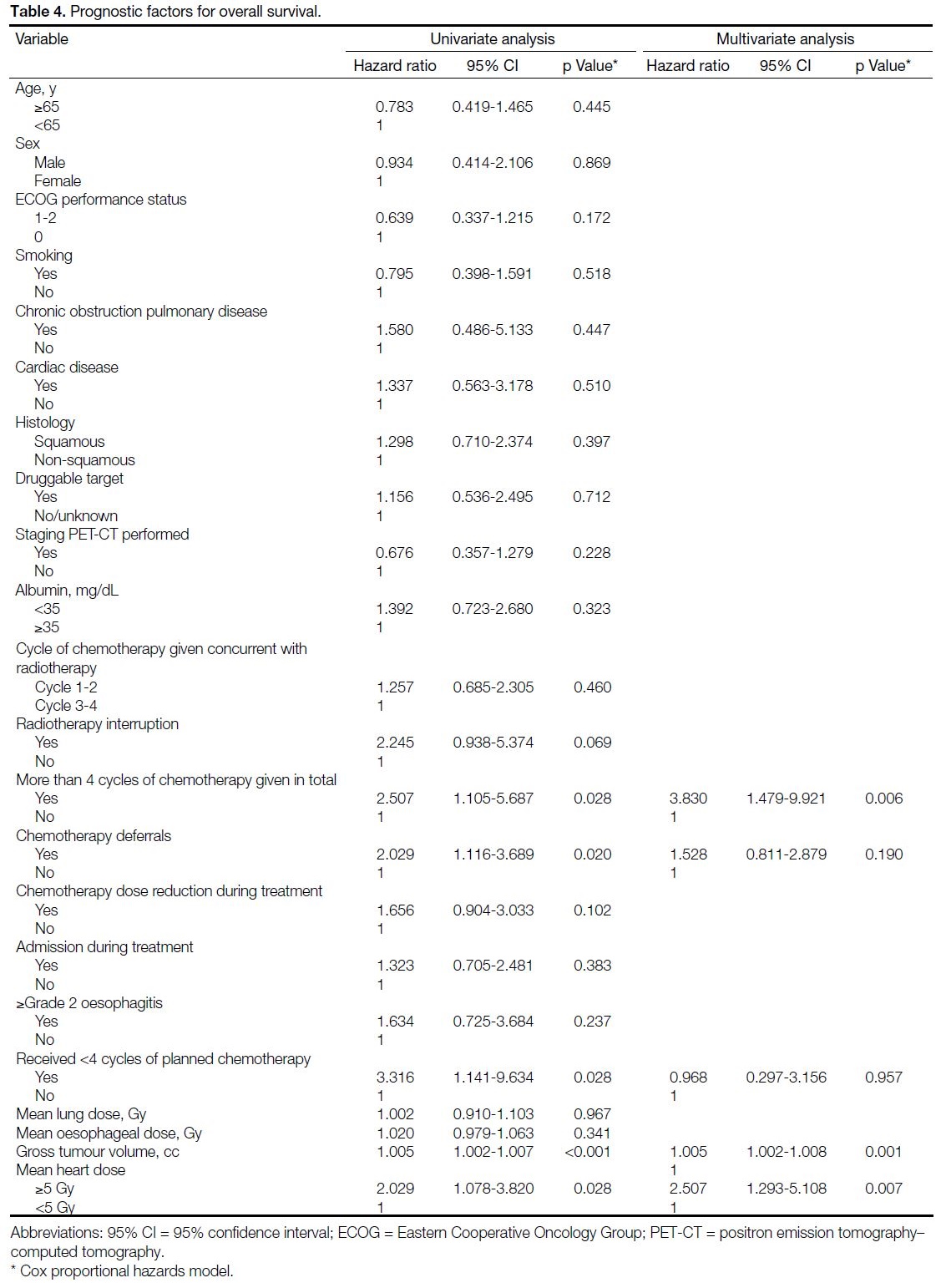
29.7%, respectively. Simple analysis identified five
parameters associated with poorer OS: GTV, mean heart
dose ≥5 Gy, more than four cycles of chemotherapy
administered, omission of planned chemotherapy cycles,
and chemotherapy deferrals. Multivariable regression
analyses showed that GTV (hazard ratio [HR] = 1.005,
95% CI = 1.002-1.008; p = 0.001), mean heart dose
≥5 Gy (HR = 2.507, 95% CI = 1.293-5.108; p = 0.007),
and more than four cycles of chemotherapy administered
(HR = 3.830, 95% CI = 1.479-9.921; p = 0.006) were
independent prognostic parameters (Table 4).
Figure 1. Overall survival for stage III unresectable non-small cell
lung cancer treated with 3-week cycles of paclitaxel-carboplatin
(PC) versus etoposide-cisplatin (EP) concurrent with radiotherapy
from 2007 to 2017.
Table 4. Prognostic factors for overall survival
Progression-free Survival
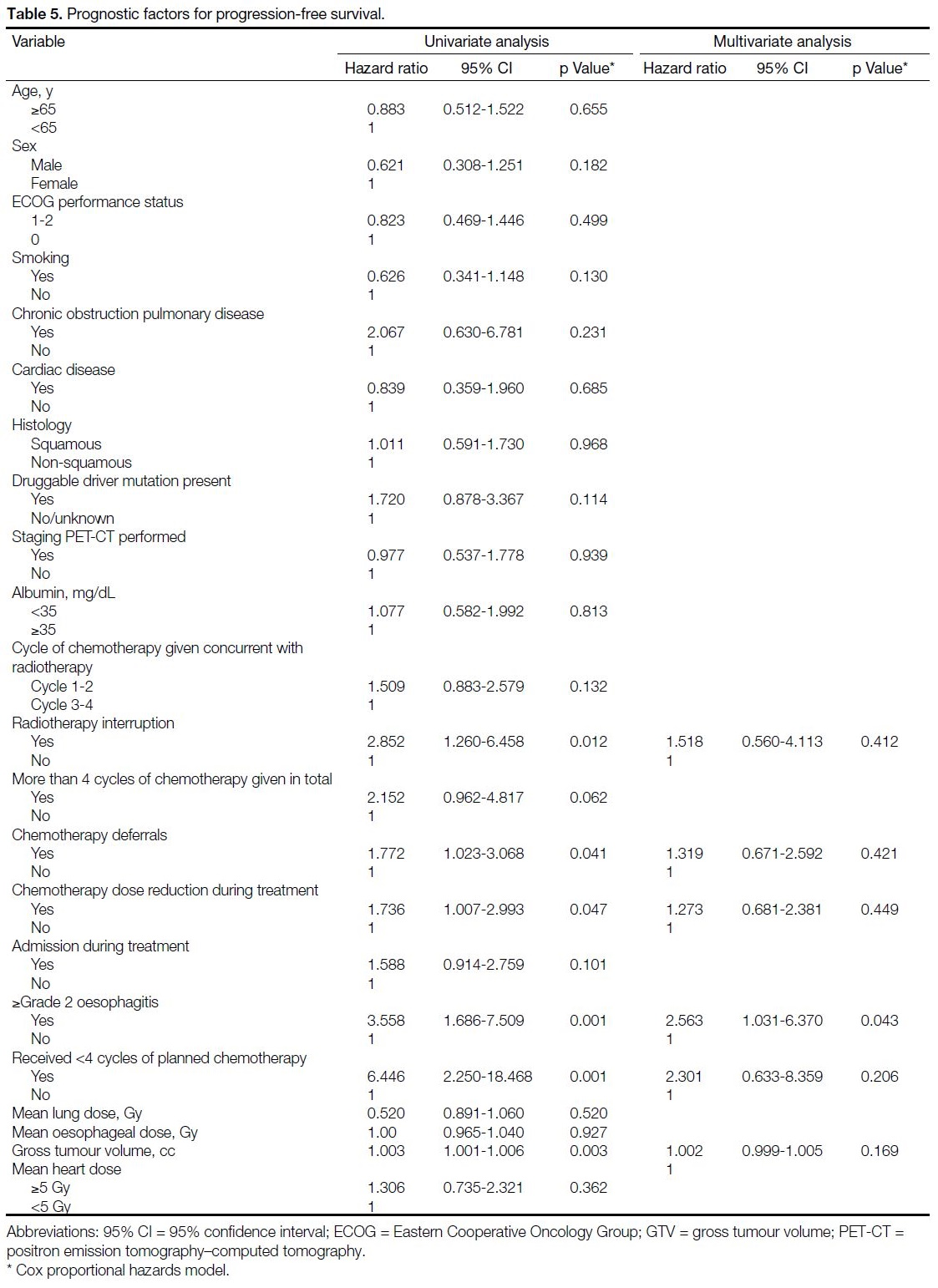
The median PFS for this cohort was 12.2 months
(95% CI = 9.0-15.4) [Figure 2]. The 1-, 3-, and 5-year PFS rates were 52.3%, 19.5% and 12.7%, respectively.
RT interruption, chemotherapy deferrals, chemotherapy
dose reduction, grade 2 oesophagitis or above, less than
four cycles of chemotherapy completed, and GTV were
noted to be prognostic factors in simple analysis, while
grade 2 oesophagitis or above was the only independent
adverse factor for PFS (HR = 2.563, 95% CI = 1.031-
6.370; p = 0.043) [Table 5].
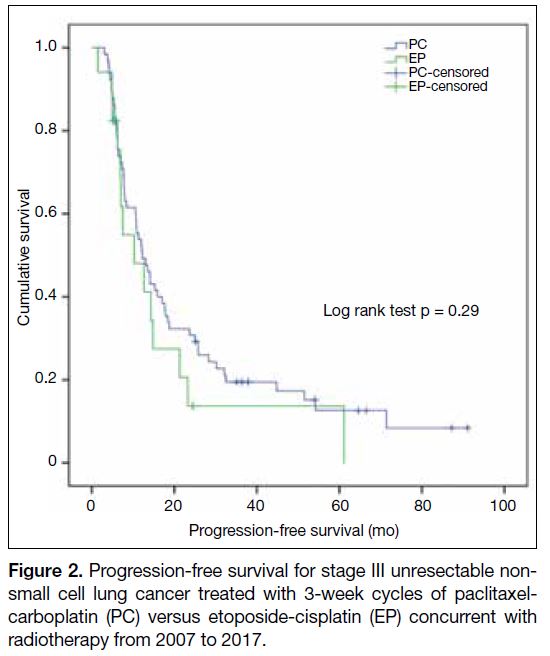
Figure 2. Progression-free survival for stage III unresectable nonsmall
cell lung cancer treated with 3-week cycles of paclitaxelcarboplatin
(PC) versus etoposide-cisplatin (EP) concurrent with
radiotherapy from 2007 to 2017.
Table 5. Prognostic factors for progression-free survival.
Comparison with Historical Cohort of Patients
Treated with Etoposide-Cisplatin
There were no differences in the baseline characteristics
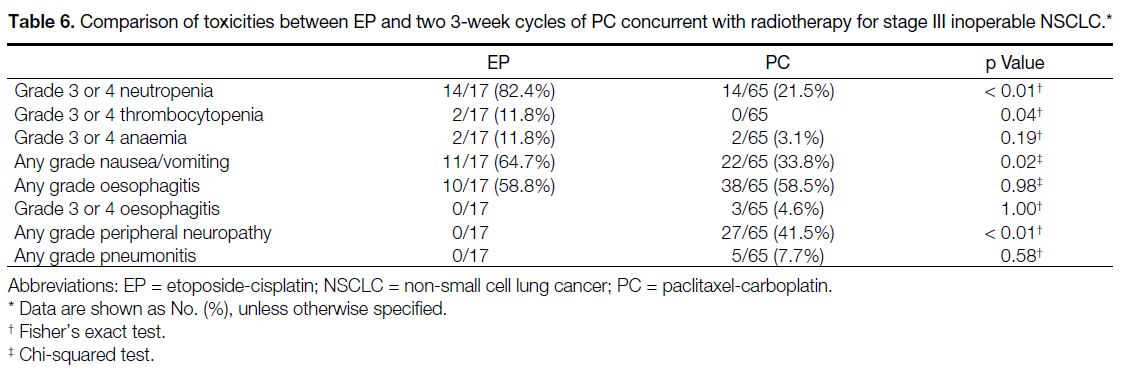
between the PC and EP case cohorts (Table 1). Compared
with the PC case cohort, the 17 patients who received EP
had significantly more grade 3 or 4 neutropenia (EP 82.4%
vs. PC 21.5%; p < 0.01), grade 3 or 4 thrombocytopenia
(EP 11.8% vs. PC 0.0%, p = 0.04), any grade nausea or
vomiting (EP 64.7% vs. PC 33.8%; p = 0.02), but less
of any grade of peripheral neuropathy (EP 0.0% vs. PC
41.5%; p < 0.01). There were no significant differences
between any grade pneumonitis, grade 3 or 4 anaemia,
and any or grade 3 or 4 oesophagitis between the two
regimens (Table 6). There were also no statistically
significant differences in the median PFS (EP 10.3
months, 95% CI = 9.0-15.4 months vs. PC 12.2 months,
95% CI = 3.3-17.3 months; p = 0.29) and median OS (EP
25.3 months, 95% CI = 14.0-36.6 months vs. PC 35.0
months, 95% CI = 17.5-52.5 months; p = 0.36).
Table 6. Comparison of toxicities between EP and two 3-week cycles of PC concurrent with radiotherapy for stage III inoperable NSCLC
DISCUSSION
The optimal choice of chemotherapy for CCRT remains
debatable for unresectable stage III NSCLC. However,
there are limited data on the efficacy and toxicities of the
convenient schedule of PC given every 3 weeks in the
existing literature. To our knowledge, this is the largest
review of using this regimen for this disease stage.
Results of our 3-week cycle PC cohort (median OS
35.0 months, 3-year OS 48.3%, 5-year OS 29.7%) are
comparable to data reported using other regimens. In the
RTOG 0617 study,[20] patients treated with weekly PC
concurrent with standard dose RT (60 Gy) had a median
survival of 28.7 months, and a 5-year OS of 32.1%. In the
control arm of the PACIFIC trial, patients had a median
survival of 29.1 months and a 3-year OS of 43.5%.[21]
Although statistically significant conclusions cannot be
drawn across trials, our similar survival results suggest
that PC given every 3 weeks is a feasible alternative.
Treatment with this regimen was well-tolerated in our
study. Grade ≥2 pneumonitis and Grade ≥3 oesophagitis
occurred in <10% of patients. Moreover, no grade 5
toxicities were observed. Treatment interruption due to
intercurrent illness or adverse events occurred in 10.8%
of patients, which was less than the 19.7% in the RTOG
0617 trial.[20] This is of particular advantage in the era of
maintenance durvalumab, where timely administration
of the drug after patients have recovered from acute
toxicities of CCRT is crucial.
Neutropenia was the most common grade 3 or 4 toxicity
in our 3-week cycle PC cohort, occurring in 21.5% of
patients, but only 4.6% had neutropenic fever. This was
similar to the grade 3 or 4 neutropenia rate of 24% in
the standard dose RT arm of the RTOG 0617 trial.[20]
Prophylactic granulocyte colony-stimulating factor may
be indicated to prevent chemotherapy delays and dose
reductions, which were negative prognostic factors for
worse PFS at univariate analysis.
Comparison with the historical cohort of EP at our
institution showed that patients treated with 3-week
cycles of PC had no significant difference in survival
outcomes but less grade 3 or 4 neutropenia and
thrombocytopenia, which suggests 3-week cycles of PC
is a safer regimen compared with EP. These comparisons
should be interpreted with caution due to the imbalance
in patient numbers between the two cohorts. Besides,
the 3-week cycle EP regimen used in our institution is
different from the 4-week cycle used in other randomised
trials (etoposide 50 mg/m2 days 1-5, cisplatin 50 mg/m2 day 1 and 8 every 4 weeks).[6] [11] [12]
Analysing dosimetric parameters revealed that a mean
heart dose of ≥5 Gy was an independent negative
prognostic factor for OS. In the RTOG 0617 study, heart
V40 was associated with worse OS after adjusting for
other prognostic factors.[23] Retrospective analyses also
demonstrated that there is a continuous increase in risk
of cardiac events with each Gy increase in mean heart
dose.[24] [25] Our results confirmed the association between
high cardiac radiation exposure and worse OS.
Strategies have to be constructed to reduce cardiac dose in order to lower cardiac complications and related
deaths, as NSCLC patients have better survival from nonchemotherapeutic systemic treatment options,
which can carry cardiac toxicities, e.g., prolonged QT
interval from crizotinib and osimertinib and immune-related
myocarditis from immunotherapy.[26] The use
of four-dimensional CT and intensity-modulated RT
for treatment planning, which has been shown to
reduce cardiac dose,[23] [27] [28] should be reviewed to see
whether clinical benefit can be derived from those
techniques.
In our series, all patients were designated to receive
four cycles of chemotherapy, with two given with RT.
An additional two cycles after the fourth was allowed
depending on patient’s fitness, response and tolerance to
treatment, and presence of any poor prognostic factors,
e.g., large tumours, N3 disease and neuroendocrine
component. The worse OS in patients receiving
more than four cycles of chemotherapy suggests that
continuing chemotherapy beyond four cycles does not
alter the poor prognosis of these patients. Instead, they
should be considered for adjuvant durvalumab as soon as
possible after recovering from CCRT based on the recent
PACIFIC trial.[5]
The ongoing coronavirus disease 2019 pandemic has
changed how oncologic care is delivered, as cancer
patients are more vulnerable to infections and its
complications. ESMO highlights minimising hospital
attendance with alternative treatment schedules as an
important strategy in preventing this highly contagious
virus.[29] PC given every 3 weeks has the merit of only
requiring 1 day of admission in 3 weeks, compared with
6 days of admission for the SWOG 9019 EP regimen[6]
and 3-day admissions for weekly PC every 3 weeks. The
fewer doses of high-dose dexamethasone (20 mg once
every 3 weeks) required for premedication compared with the weekly regimen (10 mg once every week)
reduces the risk of immunosuppression.
Another chemotherapy regimen which would reduce day
admissions is the pemetrexed-platinum combination,
similarly, only requiring one infusion every 3 weeks.
The PROCLAIM trial demonstrated that there was no
statistically significant survival difference between
pemetrexed-cisplatin and EP with RT, but the former
was associated with fewer Grade 3 or 4 events, including
neutropenia.[30] PC given every 3 weeks has two potential
advantages compared with the pemetrexed-platinum
combination. First, pemetrexed-platinum requires
slightly more dexamethasone as premedication (4 mg
twice daily for 3 days for a total of 24 mg every 3 weeks).
Besides, as intramuscular vitamin B12 injections and
daily folic acid supplementation are required, drug
compliance may be an issue for some patients.
In the era of precision oncology, there are emerging data
regarding use of neoadjuvant oral targeted treatment
before surgery for borderline operable stage III-N2
tumours with driver mutations.[31] [32] This strategy is also
favourable during the pandemic as patients can receive
treatment at home and avoid multi-day RT treatment.
However, many questions still have to be answered in
prospective studies before routine application of this
approach, including the total duration of perioperative
treatment, patient selection, and its benefit can be
compared with definitive CCRT.
A major limitation of this study is that it is a single-centre
retrospective study, and hence prone to selection bias.
Inoperable stage III lung cancer is a heterogeneous group
with varying treatment strategies employed depending on
the size and location of tumours as well as patient’s age,
performance status and co-morbidities. In our cohort,
>60% of our patients had performance status score 0
and most did not have significant cardiopulmonary co-morbidities.
Therefore, patients may have had better
tolerance of treatment and hence a better prognosis.
Moreover, as intensity-modulated RT technique was
not used for treatment planning, tumours with poorer
prognostic factors, for example, larger bulky disease,
contralateral nodal involvement, or close proximity
to critical organs like the spinal cord were excluded as
dosimetric limits cannot be met with three-dimensional
conformal technique for a dose of 60 Gy. These two
reasons could have resulted in better treatment outcomes
in our cohort.
Another limitation is lack of consistency in progress
imaging interval for response assessment due to resource
constraints. Only 31 patients (47.7%) had a follow-up
CT or PET-CT within the first 6 months after CCRT.
Patients with asymptomatic disease progression or with
extrathoracic metastases not detected on a follow-up chest
radiograph were possibly missed. Therefore, the PFS
may have been overestimated. On the other hand, only a
small number of patients in the cohort had brain imaging
for staging prior to treatment (magnetic resonance
imaging: two patients [3.1%], contrast-enhanced CT:
seven patients [10.8%]). Small asymptomatic brain
metastases could have been missed for some patients and
hence underestimated the PFS.
In summary, PC given every 3 weeks concurrently with
RT is a well-tolerated option for stage III inoperable
NSCLC with comparable outcomes to those of other
chemotherapy regimens reported in the literature. Its
schedule, with fewer day admissions for chemotherapy
infusions, may be an advantage during the ongoing
coronavirus disease pandemic. Future studies should
evaluate whether this regimen in combination with more
sophisticated RT techniques (e.g., intensity-modulated
RT, four-dimensional CT) could further improve the
therapeutic ratio of treatment in this group of patients in
the era of consolidative durvalumab.
REFERENCES
1. Curran WJ Jr, Paulus R, Langer CJ, Komaki R, Lee JS, Hauser S,
et al. Sequential vs concurrent chemoradiation for stage III nonsmall
cell lung cancer: randomized phase III trial RTOG 9410. J
Natl Cancer Inst. 2011;103:1452-60. Crossref
2. Aupérin A, Le Péchoux C, Rolland E, Curran WJ, Furuse K,
Fournel P, et al. Meta-analysis of concomitant versus sequential
radiochemotherapy in locally advanced non–small-cell lung cancer.
J Clin Oncol. 2010;28:2181-90. Crossref
3. Spina R, Chu SY, Chatfield M, Chen J, Tin MM, Boyer M.
Outcomes of chemoradiation for patients with locally advanced
non-small-cell lung cancer. Intern Med J. 2013;43:790-7. Crossref
4. Gandara DR, Chansky K, Albain KS, Gaspar LE, Lara PN Jr,
Kelly K, et al. Long-term survival with concurrent chemoradiation
therapy followed by consolidation docetaxel in stage IIIB non–small-cell lung cancer: a phase II southwest oncology group study
(S9504). Clin Lung Cancer. 2006;8:116-21. Crossref
5. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R,
et al. Durvalumab after chemoradiotherapy in stage III non–small-cell
lung cancer. N Engl J Med. 2017;377:1919-29. Crossref
6. Albain KS, Crowley JJ, Turrisi AT 3rd, Gandara DR, Farrar WB,
Clark JI, et al. Concurrent cisplatin, etoposide, and chest
radiotherapy in pathologic stage IIIB non–small-cell lung cancer:
a southwest oncology group phase II study, SWOG 9019. J Clin
Oncol. 2002;20:3454-60. Crossref
7. Belani CP, Choy H, Bonomi P, Scott C, Travis P, Haluschak J,
et al. Combined chemoradiotherapy regimens of paclitaxel and carboplatin for locally advanced non–small-cell lung cancer: a
randomized phase II locally advanced multi-modality protocol. J
Clin Oncol. 2005;23:5883-91. Crossref
8. Naito Y, Kubota K, Nihei K, Fujii T, Yoh K, Niho S, et al.
Concurrent chemoradiotherapy with cisplatin and vinorelbine for
stage III non-small cell lung cancer. J Thorac Oncol. 2008;3:617-22. Crossref
9. Surmont V, Smit EF, de Jonge M, Aerts JG, Nackaerts K,
Vernhout R, et al. Pemetrexed and cisplatin with concurrent
radiotherapy for locally advanced non-small cell and limited disease
small cell lung cancer: Results from 2 phase I studies. Lung Cancer.
2010;69:302-6. Crossref
10. Xu Y, Ma S, Ji Y, Sun X, Jiang H, Chen J, et al. Concomitant
chemoradiotherapy using pemetrexed and carboplatin for
unresectable stage III non-small cell lung cancer (NSCLC):
Preliminary results of a phase II study. Lung Cancer. 2011;72:327-32. Crossref
11. Liang J, Bi N, Wu S, Chen M, Lv C, Zhao L, et al. Etoposide and
cisplatin versus paclitaxel and carboplatin with concurrent thoracic
radiotherapy in unresectable stage III non-small cell lung cancer: a
multicenter randomized phase III trial. Ann Oncol. 2017;28:777-83. Crossref
12. Steuer CE, Behera M, Ernani V, Higgins KA, Saba NF, Shin DM,
et al. Comparison of concurrent use of thoracic radiation with either
carboplatin-paclitaxel or cisplatin-etoposide for patients with stage
III non–small-cell lung cancer: a systematic review. JAMA Oncol.
2017;3:1120-9. Crossref
13. Santana-Davila R, Devisetty K, Szabo A, Sparapani R, Arce-Lara C,
Gore EM, et al. Cisplatin and etoposide versus carboplatin and
paclitaxel with concurrent radiotherapy for stage III non-small-cell
lung cancer: an analysis of Veterans Health Administration data. J
Clin Oncol. 2015;33:567-74. Crossref
14. Crinò L, Weder W, van Meerbeeck J, Felip E, ESMO Guidelines
Working Group. Early stage and locally advanced (non-metastatic)
non-small-cell lung cancer: ESMO Clinical Practice Guidelines for
diagnosis, treatment and follow-up. Ann Oncol. 2010;21 Suppl
5:v103-15. Crossref
15. Laohavinij S, Maoleekoonpairoj S, Cheirsilpa A, Maneechavakajorn J,
Sirachainant E, Arpornvivat W, et al. Phase II study of paclitaxel
and carboplatin for advanced non-small-cell lung cancer. Lung
Cancer. 1999;26:175-85. Crossref
16. Stathopoulos GP, Veslemes M, Georgatou N, Antoniou D,
Giamboudakis P, Katis K, et al. Front-line paclitaxel-vinorelbine
versus paclitaxel-carboplatin in patients with advanced non-small-
cell lung cancer: a randomized phase III trial. Ann Oncol.
2004;15:1048-55. Crossref
17. Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A,
et al. Paclitaxel–carboplatin alone or with bevacizumab for non–small-cell lung cancer. N Engl J Med. 2006;355:2542-50. Crossref
18. Movsas B, Hudes RS, Schol J, Millenson M, Rosvold E,
Nicolaou N, et al. Induction and concurrent paclitaxel/carboplatin
every 3 weeks with thoracic radiotherapy in locally advanced
non–small-cell lung cancer: an interim report. Clin Lung Cancer.
2001;3:125-32. Crossref
19. Zhao J, Zhang X, Hu K, Wang H, Xu Y, Si X, et al. Outcomes
and toxicity of concurrent radiotherapy with carboplatin/paclitaxel
administrated every three weeks in inoperable advanced non-small cell lung cancer: a retrospective study from a single center [in
Chinese]. Zhongguo Fei Ai Za Zhi. 2016;19:731-7.
20. Bradley JD, Hu C, Komaki RR, Masters GA, Blumenschein GR,
Schild SE, et al. Long-term results of NRG Oncology RTOG 0617:
Standard- versus high-dose chemoradiotherapy with or without
cetuximab for unresectable stage III non–small-cell lung cancer. J
Clin Oncol. 2020;38:706-14. Crossref
21. Gray JE, Villegas A, Daniel D, Vicente D, Murakami S, Hui R,
et al. Three-year overall survival with durvalumab after
chemoradiotherapy in stage III NSCLC — update from PACIFIC.
J Thorac Oncol. 2020;15:288-93. Crossref
22. Wang L, Wu S, Ou G, Bi N, Li W, Ren H, et al. Randomized phase
II study of concurrent cisplatin/etoposide or paclitaxel/carboplatin
and thoracic radiotherapy in patients with stage III non-small cell
lung cancer. Lung Cancer. 2012;77:89-96. Crossref
23. Chun SG, Hu C, Choy H, Komaki RU, Timmerman RD, Schild SE,
et al. Impact of intensity-modulated radiation therapy technique for
locally advanced non–small-cell lung cancer: a secondary analysis
of the NRG oncology RTOG 0617 randomized clinical trial. J Clin
Oncol. 2017;35:56-62. Crossref
24. Wang K, Eblan MJ, Deal AM, Lipner M, Zagar TM, Wang Y, et al.
Cardiac toxicity after radiotherapy for stage III non–small-cell lung
cancer: pooled analysis of dose-escalation trials delivering 70 to
90 Gy. J Clin Oncol. 2017;35:1387-94. Crossref
25. Atkins KM, Rawal B, Chaunzwa TL, Lamba N, Bitterman DS,
Williams CL, et al. Cardiac radiation dose, cardiac disease,
and mortality in patients with lung cancer. J Am Coll Cardiol.
2019;73:2976-87. Crossref
26. Palaskas N, Lopez-Mattei J, Durand JB, Iliescu C, Deswal A.
Immune checkpoint inhibitor myocarditis: pathophysiological
characteristics, diagnosis, and treatment. J Am Heart Assoc.
2020;9:e013757. Crossref
27. Ju X, Li M, Zhou Z, Zhang K, Han W, Fu G, et al. 4D-CT-based
plan target volume (PTV) definition compared with conventional
PTV definition using general margin in radiotherapy for lung cancer
[in Chinese]. Zhonghua Zhong Liu Za Zhi. 2014;36:34-8.
28. Dess RT, Sun Y, Muenz DG, Paximadis PA, Dominello MM,
Grills IS, et al. Cardiac dose in locally advanced lung cancer: results
from a statewide consortium. Pract Radiat Oncol. 2020;10:e27-36. Crossref
29. Curigliano G, Banerjee S, Cervantes A, Garassino MC, Garrido P,
Girard N, et al. Managing cancer patients during the COVID-19
pandemic: an ESMO multidisciplinary expert consensus. Ann
Oncol. 2020;31:1320-35. Crossref
30. Senan S, Brade A, Wang LH, Vansteenkiste J, Dakhil S,
Biesma B, et al. PROCLAIM: Randomized phase III trial of
pemetrexed-cisplatin or etoposide-cisplatin plus thoracic radiation
therapy followed by consolidation chemotherapy in locally
advanced nonsquamous non–small-cell lung cancer. J Clin Oncol.
2016;34:953-62. Crossref
31. Reyes R, Reguart N. Neoadjuvant treatment of stage IIIA-N2 in
EGFR-mutant/ALK-rearranged non-small cell lung cancer. Transl
Lung Cancer Res. 2021;10:607-21. Crossref
32. Sun L, Guo YJ, Song J, Wang YR, Zhang SL, Huang LT, et al.
Neoadjuvant EGFR-TKI therapy for EGFR-mutant NSCLC: a
systematic review and pooled analysis of five prospective clinical
trials. Front Oncol. 2021;10:586596. Crossref