Spontaneous Regression of Subdural Haematoma Due to Redistribution in a Young Child: a Case Report
CASE REPORT
Spontaneous Regression of Subdural Haematoma Due to Redistribution in a Young Child: a Case Report
V Rangankar, P Ajmera
Dr D.Y. Patil Medical College, Hospital and Research Centre, DPU, Pune, India
Correspondence: Dr P Ajmera, Dr D.Y. Patil Medical College, Hospital and Research Centre, DPU, Pune, India. Email: pranavajmera@gmail.com
Submitted: 24 Feb 2021; Accepted: 3 Jun 2021.
Contributors: All authors designed the study, acquired and analysed the data, drafted the manuscript, and critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: The patient was treated in accordance with the tenets of the Declaration of Helsinki. The patient’s mother (guardian) provided written informed consent for all treatments and procedures.
INTRODUCTION
Acute subdural haematoma refers to the presence of
blood between the dura mater and outer layer of the
arachnoid mater. It occurs in almost 21% of all severe
traumatic brain injuries and almost 11% of mild and
moderate injuries. Mortality ranges from 30% to 90%.[1]
The possible mechanisms of injury leading to subdural
haematoma include trauma, rupture of cortical bridging
veins, damage to the surface cortical vasculature,
bleeding from underlying injury to the brain parenchyma,
intracranial hypotension and defective anticoagulation,
among which trauma is the most common cause in
children. Various factors related to a poor outcome
in such patients include low Glasgow Coma Scale
(GCS) score on admission, sluggishly reactive pupils,
hypotension and bilaterality of the haematoma.[1] [2]
Management of subdural haematoma varies depending
on the neurological status and radiological parameters,
for example, thickness of bleed and mass effect leading
to midline shift and herniation. The general consensus
is that in all cases of haematoma with thickness >10 mm or midline shift >5 mm, drainage of the
collection is required irrespective of GCS score. Cases of
lesser severity can be conservatively managed. Surgical
options include craniotomy with or without bone flap
removal.[3] [4]
In cases where conservative management is sought,
spontaneous resolution may take weeks to months,
depending on the size of bleed. Few cases in the
literature describe rapid spontaneous resolution. We
describe a young girl in whom subdural haematoma
sufficiently large on initial scans to warrant surgical
intervention showed a significant decrease in size with
redistribution to other sites, simultaneously linked to
rapid improvement in neurological status.
CASE PRESENTATION
In January 2021, a 5-year-old girl was admitted to our emergency department after a fall while playing. She
became disoriented and lost consciousness over the
course of 30 minutes, after which she also developed
tonic, posturing of the limbs that lasted for a few minutes but was not associated with deviation of the angle
of the mouth or frothing at the mouth. Neurological
examination revealed a GCS score of 10/15.
Subsequently, the patient was referred to the radiology
department for computed tomography (CT) scan of
head. The CT scan performed nearly 60 minutes after
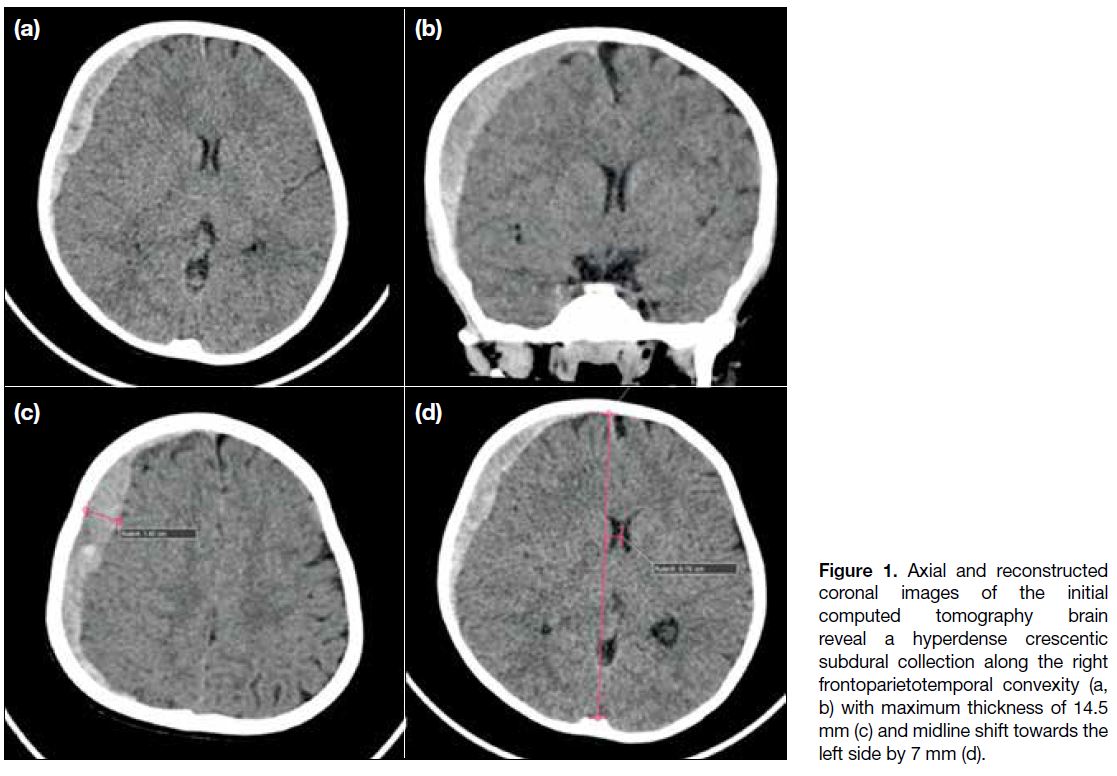
her injury revealed a large concavo-convex (crescentic),
extra-axial hyperdense collection overlying the right
frontoparietal cortex, measuring approximately
12.5 cm anteroposteriorly, 10 cm craniocaudally and
14.5 mm in maximum thickness (Figure 1). There was
an associated midline shift of 7 mm with uncal and
subfalcine herniation to the left side. The underlying
cerebral sulci and the right lateral ventricle were effaced
due to compression by the subdural haematoma. There
was a small extension of the haematoma into the anterior
interhemispheric fissure but no damage to the cranial
bones or sign of cerebral contusion.
Figure 1. Axial and reconstructed coronal images of the initial computed tomography brain reveal a hyperdense crescentic subdural collection along the right frontoparietotemporal convexity (a, b) with maximum thickness of 14.5 mm (c) and midline shift towards the left side by 7 mm (d).
On the basis of the GCS score and CT findings,
neurosurgical intervention was planned and routine
blood investigations performed. The patient was commenced on intravenous 3% hypertonic saline. After
an hour, the young girl began to regain consciousness
and GCS score improved slightly to 11/15. New CT
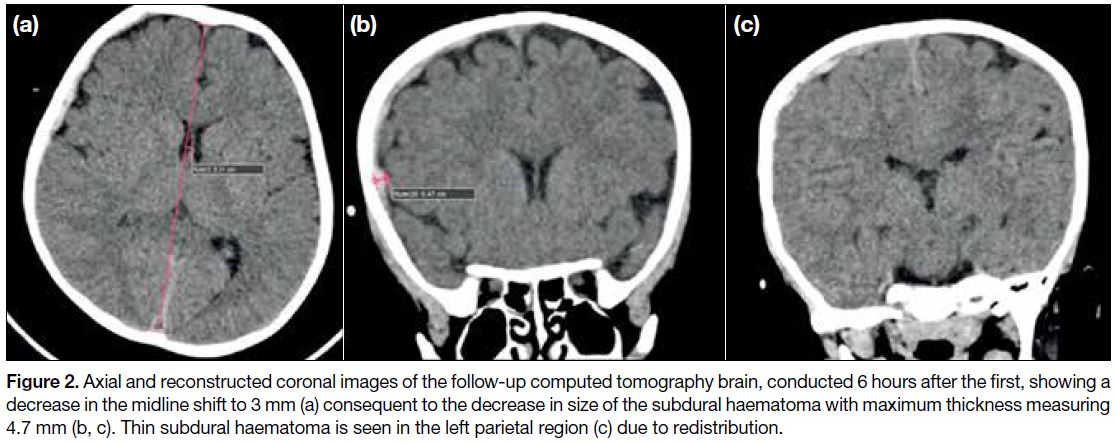
scan 6 hours after the first revealed highly unexpected
findings. The anteroposterior extent of the crescentic
collection had reduced to 9 cm, and the thickness had
shrunk by almost two thirds to nearly 5.8 mm with a now
reduced midline shift of 3 mm (Figure 2). A separate
thin new subdural collection of thickness 3.5 mm was
noted in the left high-parietal region. Another new
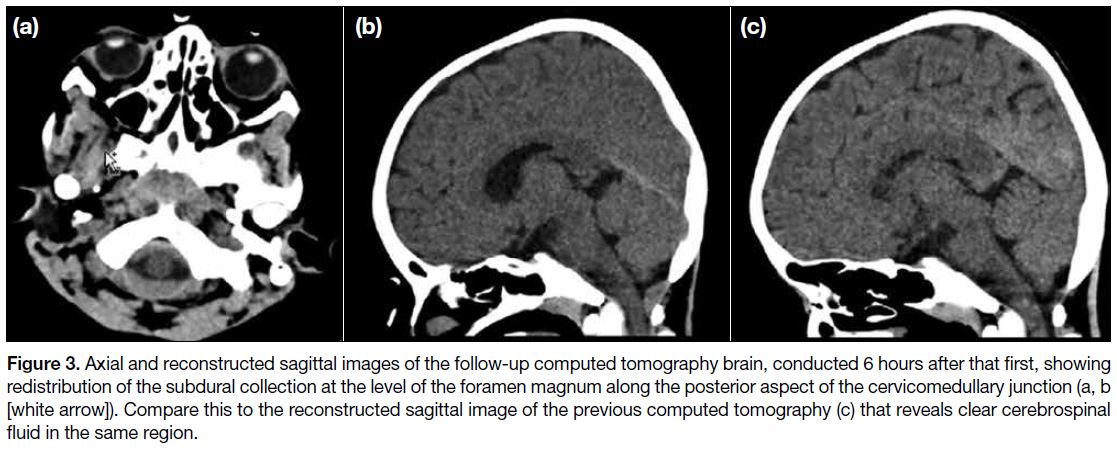
subdural collection was noted at the cervicomedullary
junction, indenting the posterior subarachnoid space but
not compressing the cervicomedullary junction (Figure 3). A smaller subdural collection was noted extending
into the posterior interhemispheric fissure and tentorium
cerebelli. Essentially, the large collection over the right
cerebral cortex had become decompressed and been
redistributed. Clinically too, the patient’s GCS score had
improved to 13/15.
Figure 2. Axial and reconstructed coronal images of the follow-up computed tomography brain, conducted 6 hours after the first, showing a
decrease in the midline shift to 3 mm (a) consequent to the decrease in size of the subdural haematoma with maximum thickness measuring
4.7 mm (b, c). Thin subdural haematoma is seen in the left parietal region (c) due to redistribution.
Figure 3. Axial and reconstructed sagittal images of the follow-up computed tomography brain, conducted 6 hours after that first, showing
redistribution of the subdural collection at the level of the foramen magnum along the posterior aspect of the cervicomedullary junction (a, b [white arrow]). Compare this to the reconstructed sagittal image of the previous computed tomography (c) that reveals clear cerebrospinal
fluid in the same region.
Considering the new findings, a more conservative
approach was adopted and the child was kept under
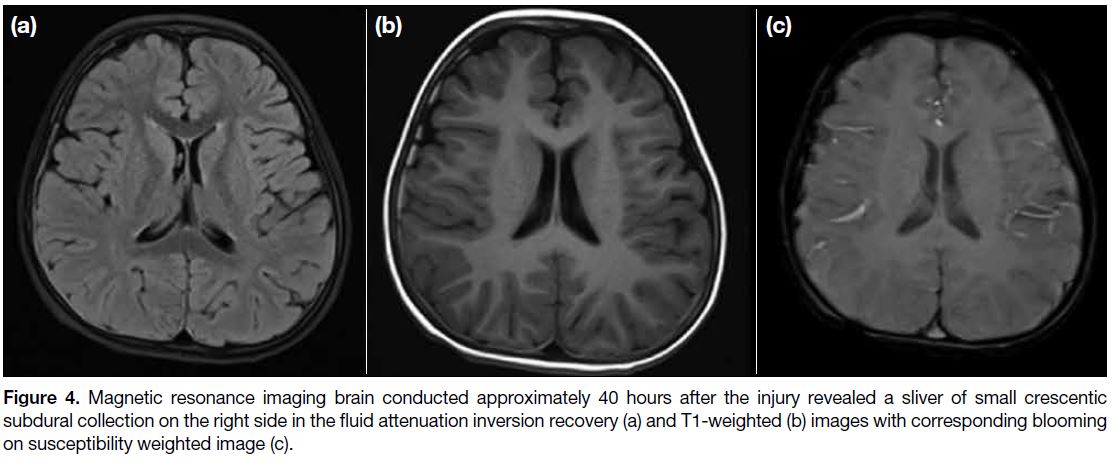
observation with regular monitoring of GCS score. An evaluation was sought by magnetic resonance imaging
of the brain 40 hours after the first CT and subsequently
revealed a small collection of maximum thickness 3.6 mm
along the right frontotemporal region (Figure 4).
Magnetic resonance angiography was also performed
to exclude any vascular aetiology such as aneurysm and
was normal.
Figure 4. Magnetic resonance imaging brain conducted approximately 40 hours after the injury revealed a sliver of small crescentic
subdural collection on the right side in the fluid attenuation inversion recovery (a) and T1-weighted (b) images with corresponding blooming on susceptibility weighted image (c).
DISCUSSION
Patients with traumatic subdural haematoma and poor
GCS score on admission, as in our case, are considered
candidates for craniotomy and drainage of haematoma to
prevent further brain damage and reduce morbidity and
risk of mortality.[3] [5] However, spontaneous improvement
in symptomatology and associated reduction in
haematoma size mandates a more conservative approach.
The first case of spontaneous resolution of subdural
haematoma was documented in Tokyo in 1986 by Nagao
et al.[6] Many cases have since been reported although
few over such a short period of time, between serial
CT scans. Even fewer such cases have been reported in
children, less than 10.
Two principal mechanisms are ascribed to spontaneous
resolution of haematoma, both of which involve
redistribution of blood at their core rather than actual
regression. One is associated with intrinsic redistribution
that involves acute swelling of the brain. The other,
an extrinsic one, is linked to a dural or arachnoid tear
that results in redistribution of blood to other intra- or
extra-cranial spaces, thereby releasing pressure from the
primary site.[7] [8] The mixing of blood with cerebrospinal fluid and subsequent washout serves to dilute the bleed
and carry it into the subarachnoid, subdural or spinal
subdural space.[9] [10] The presence of cerebral atrophy with
prominent subarachnoid spaces may help the dilution of
subdural haematoma.[11] Rarely, the haematoma can drain
into the subgaleal or diploic space in the presence of skull
fracture and associated dural injury.[12] In our case, there
was redistribution of subdural bleed to newer sites, to the
interhemispheric fissure and also to the spinal subdural
space, evidenced by the presence of blood in the spinal
space at the cervicomedullary junction. Nonetheless
there was no subarachnoid extension of the haemorrhage.
Various factors have been linked to and studied in
resolving acute subdural haematoma,[10] [13] [14] namely
instances where subdural haematoma is located at the
frontotemporoparietal, frontotemporal, or frontoparietal
convexities; where a patient presents with transient
deterioration in neurological status with subsequent
improvement[15]; or a patient prescribed anticoagulants
where the lack of clotting factors prevented formation
of a platelet plug and thus enabled redistribution.[16] Other
observations in similar cases have included sustained
trauma with GCS score >8; and presence of a band of
low density between the haematoma and the interior
table of the skull bone.[10] The extent of their significance
has not been completely determined.
Our patient presented with various factors favouring
spontaneous resolution — frontal location of the bleed,
thickness of the haematoma close to the mean thickness
seen in other cases with spontaneous resolution of subdural haematoma. The subdural haematoma was
located over the right frontoparietal cortex, with thickness
of 14.3 mm at admission. The patient had a low GCS
score at admission, which subsequently improved over
a period of 6 hours with redistribution of the subdural
bleed and consequent spontaneous resolution with
symptomatic improvement. The patient was able to be
managed conservatively and avoided major surgery and
associated risks.
CONCLUSION
The present case describes the rare occurrence of
spontaneous regression of subdural haematoma with
redistribution of blood to other sites and symptomatic
improvement. Close monitoring of a patient with subdural
haematoma by GCS score and serial non-contrast CT
brain can help identify spontaneous regression and avoid
the need for surgical intervention.
PATIENT PERSPECTIVE
In the child's words, “I know I'm at a hospital right now [well oriented] and I feel better now. Though I still feel
some pain over my head but I want to go home now”.
REFERENCES
1. Prahaladu P, Satyavara Prasad K, Rajasekhar B, Satyanarayana
Reddy K. Clinical study of acute subdural haematoma — a level
I trauma care centre experience. Int J Res Med Sci. 2017;5:857-62. Crossref
2. Atalay T, Ak H, Gülsen I, Karacabey S. Risk factors associated
with mortality and survival of acute subdural haematoma: A
retrospective study. J Res Med Sci. 2019;24:27.
3. Bullock MR, Chesnut R, Ghajar J, Gordon D, Hartl R, Newell DW,
et al. Surgical management of acute subdural haematomas. Crossref
4. Feliciano CE, De Jesús O. Conservative management outcomes
of traumatic acute subdural haematomas. P R Health Sci J.
2008;27:220-3.
5. Petridis AK, Dörner L, Doukas A, Eifrig S, Barth H, Mehdorn M.
Acute subdural haematoma in the elderly; clinical and CT factors
influencing the surgical treatment decision. Cent Eur Neurosurg.
2009;70:73-8. Crossref
6. Nagao T, Aoki N, Mizutani H, Kitamura K. Acute subdural haematoma with rapid resolution in infancy: case report. Neurosurgery. 1986;19:465-7. Crossref
7. Öğrenci A, Ekşi MŞ, Koban O, Karakuş M. Spontaneous rapid resolution of acute subdural haematoma in children. Childs Nerv Syst. 2015;31:2239-43. Crossref
8. Kundra SN, Kundra R. Extracranial redistribution causing rapid spontaneous resolution of acute subdural haematoma. Neurol India. 2005;53:124. Crossref
9. Gelsomino M, Awad AJ, Gerndt C, Nguyen HS, Doan N,
Mueller W. Mechanism for the rapid spontaneous resolution of
an acute subdural haematoma and transformation into a subdural
hygroma. World Neurosurg. 2018;115:282-4. Crossref
10. Zhuang Z, Luo J, Ou C, Chen B, Liu B. The clinical and CT features of rapid spontaneous resolution of traumatic acute
subdural haematoma: A retrospective study of 14 cases. Brain Inj.
2015;29:1239-45. Crossref
11. Park JY, Moon KS, Lee JK, Jeung KW. Rapid resolution of acute
subdural haematoma in child with severe head injury: a case report.
J Med Case Rep. 2013;7:67. Crossref
12. Berker M, Gulsen S, Ozcan OE. Ultra rapid spontaneous resolution
of acute posttraumatic subdural haematomas in patient with
temporal linear fracture. Acta Neurochir (Wien). 2003;145:715-7. Crossref
13. Fujimoto K, Otsuka T, Yoshizato K, Kuratsu J. Predictors of rapid
spontaneous resolution of acute subdural haematoma. Clin Neurol
Neurosurg. 2014;118:94-7. Crossref
14. Chaudhary N, Krivosheya D, Small E, Hsia C, Ng W, Leung A.
Rapid resolution of acute subdural haematoma in a coagulopathic
patient. Can J Neurol Sci. 2013;40:599-600. Crossref
15. Horikoshi T, Naganuma H, Fukasawa I, Uchida M, Nukui H.
Computed tomography characteristics suggestive of spontaneous
resolution of chronic subdural haematoma. Neurol Med Chir
(Tokyo). 1998;38:527-32. Crossref
16. Bae HJ, Lee SB, Yoo DS, Huh PW, Lee TG, Cho KS. Rapid spontaneous resolution of acute subdural haematoma in a patient with liver cirrhosis. Korean J Neurotrauma. 2014;10:134-6. Crossref