Radiological Features of Rhino-Orbital-Cerebral Mucormycosis Complicating COVID-19 Illness: a Pictorial Essay
PICTORIAL ESSAY
Radiological Features of Rhino-Orbital-Cerebral Mucormycosis Complicating COVID-19 Illness: a Pictorial Essay
NS Chauhan1, S Kumar2, P Takkar1, A Sood3
1 Department of Radiodiagnosis, Dr Rajendra Prasad Government Medical College–Tanda, India
2 Department of ENT, Dr Rajendra Prasad Government Medical College–Tanda, India
3 Department of Microbiology, Dr Rajendra Prasad Government Medical College–Tanda, India
Correspondence: Prof NS Chauhan. Department of Radiodiagnosis, Dr Rajendra Prasad Government Medical College–Tanda, India. Email: narvirschauhan@yahoo.com
Submitted: 9 Nov 2021; Accepted: 4 Feb 2022.
Contributors: All authors designed the study and acquired the data. NSC and PT analysed the data. All authors drafted the manuscript, and
critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the
final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: All patients were treated in accordance with the tenets of the Declaration of Helsinki. The patients provided written informed consent for all treatments and procedures.
INTRODUCTION
Coronavirus disease 2019 (COVID-19) infection has
been linked to a myriad of baffling clinical presentations
and complications. Mucormycosis has recently emerged
as an opportunistic life-threatening invasive fungal
infection in patients with COVID-19 infection and
is associated with high mortality rates of 50 to 80%
in patients who develop intra-orbital or intracerebral
disease.[1]
A number of underlying factors are thought to contribute to this high mortality including pre-existing diabetes, over-enthusiastic steroid administration, immunosuppressive
therapy, systemic immune alterations due to COVID-19,
and the critical health of patients that mandates oxygen
support and a prolonged hospital stay.[1] [2] [3] [4]
Diabetes mellitus is considered an independent risk factor
for mucormycosis infection and also associated with
severe disease progression in COVID-19 infections.[5] The
underlying reason for this predisposition is the presence
of a hyperinflammatory state, delayed interferon gamma response and decreased counts of CD4+ and CD8+ cells.
It is speculated that SARS-CoV-2 infection alters innate
immunity by affecting CD4+ and CD8+ T cells counts
and this state of lymphopenia with immune dysregulation
may facilitate development of invasive fungal infection.[6]
Mucormycosis is a broad term used for angioinvasive
diseases due to infection with fungi belonging to the
Mucorales order. It is chiefly caused by Rhizopus,
Mucor, Rhizomucor, Lichtheimia and Cunninghamella
species with Rhizopus being the most commonly
implicated (Figure 1a and b).[5] [6] Spores of mucormycosis
are widespread in nature, occurring ubiquitously in the
air, soil, organic matter and food. They may exist as
commensals in the nasal mucosa of healthy individuals
but later germinate in the paranasal sinuses when an
individual is immunosuppressed.
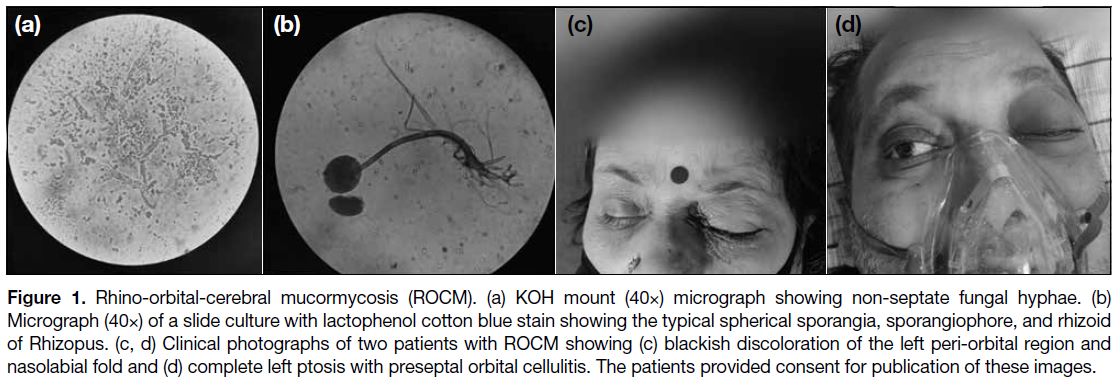
Figure 1. Rhino-orbital-cerebral mucormycosis (ROCM). (a) KOH mount (40×) micrograph showing non-septate fungal hyphae. (b)
Micrograph (40×) of a slide culture with lactophenol cotton blue stain showing the typical spherical sporangia, sporangiophore, and rhizoid
of Rhizopus. (c, d) Clinical photographs of two patients with ROCM showing (c) blackish discoloration of the left peri-orbital region and
nasolabial fold and (d) complete left ptosis with preseptal orbital cellulitis. The patients provided consent for publication of these images.
The hallmarks of mucormycosis include aggressive
and rapid hyphal invasion of tissues, vascular mycotic
infiltration, vasculitis, thrombosis and cerebral infarction.
Clinically the patient can present with black eschar in the nasal cavity/hard palate, nasal blockade, proptosis,
chemosis, ptosis, ophthalmoplegia and facial pain
(Figure 1c and d).[1] Intracranial extension is heralded by the onset of headache and neurological signs and
symptoms.
Radiological investigation is crucial to assess the extent
of disease and associated complications. The current
radiological description of rhino-orbital-cerebral
mucormycosis (ROCM) in concurrent COVID-19
illness is very limited.[3] [7] [8] The spectrum of imaging
characteristics of ROCM in COVID-19 patients is
similar to that classically described for ROCM in non-COVID affected immunocompromised individuals. This
diagnosis should be strongly considered in patients with
COVID-19 infection and suggestive imaging findings,
especially when there is corroborative evidence of a
concurrent diabetes, steroid administration or need for
prolonged intensive care.
In this review, using an anatomical approach, we
illustrate the magnetic resonance imaging (MRI) and
computed tomography (CT) findings of ROCM from
a cluster of microbiologically proven cases diagnosed
in our tertiary care hospital in patients admitted with
reverse transcription polymerase chain reaction–proven
COVID-19 illness.
SINUS DISEASE
Mucormycosis appears as opacification of the paranasal
sinuses to a variable extent. The distribution of sinus
disease is mostly as pansinusitis (62.5%) or multisinus
disease (37.5%) with a left (37.5%) or right-sided
predominance (37.5%). The ethmoid sinus is universally affected (100%) and the maxillary sinuses in most (80%)
cases.
On CT there is isodense or mixed iso/hypodense
attenuation soft tissue opacification or mucosal thickening
with either a non-enhancing or mildly enhancing pattern.[9]
The MRI imaging findings in sinonasal disease are
variable and all sequences such as T2-weighted (T2W),
T1-weighted (T1W), T2 fat saturated, and post-contrast
T1W fat saturated, should be interpreted in combination.
This is particularly important in early-stage disease that
is confined to the paranasal sinuses. The signal intensity
of the fungal mucosal thickening on T2W images will
depend on the proportion of fungal elements that impart
a low T2W signal due to paramagnetic effects of iron and
manganese and the proportion of necrotic elements that
produce a hyperintense signal. On unenhanced T1W,
the signal intensity is mostly hypointense or isointense
(Figure 2). In a study by Therakathu et al,[9] the percentage
distribution of cases displaying varying T2 signals was
hypointense/isointense in 37%; heterogenous signal
(mixed hypointense and hyperintense areas) in 32% and
uniform hyperintense signal in 32%. The signal intensity
on T1W was hypointense in all cases. On post-contrast
images, the enhancement patterns were non-enhancing/rim enhancing in 36%, heterogeneously enhancing in
36% and homogenously enhancing in 29%.[9]
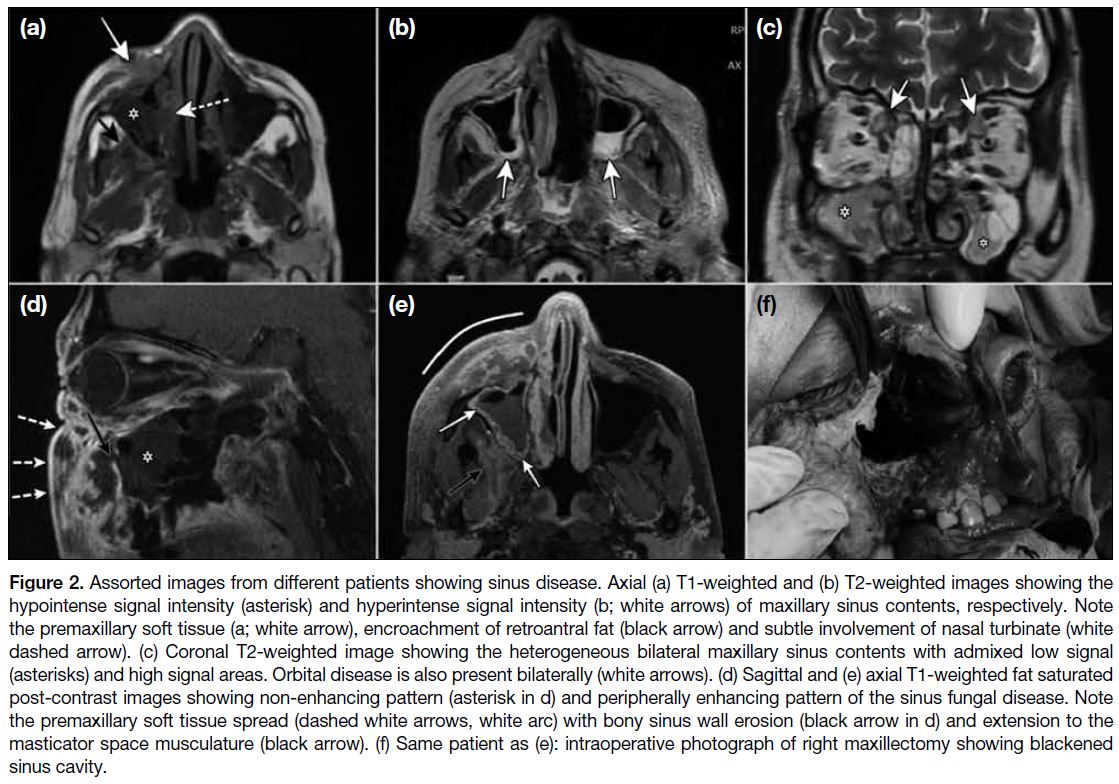
Figure 2. Assorted images from different patients showing sinus disease. Axial (a) T1-weighted and (b) T2-weighted images showing the
hypointense signal intensity (asterisk) and hyperintense signal intensity (b; white arrows) of maxillary sinus contents, respectively. Note
the premaxillary soft tissue (a; white arrow), encroachment of retroantral fat (black arrow) and subtle involvement of nasal turbinate (white
dashed arrow). (c) Coronal T2-weighted image showing the heterogeneous bilateral maxillary sinus contents with admixed low signal
(asterisks) and high signal areas. Orbital disease is also present bilaterally (white arrows). (d) Sagittal and (e) axial T1-weighted fat saturated
post-contrast images showing non-enhancing pattern (asterisk in d) and peripherally enhancing pattern of the sinus fungal disease. Note
the premaxillary soft tissue spread (dashed white arrows, white arc) with bony sinus wall erosion (black arrow in d) and extension to the
masticator space musculature (black arrow). (f) Same patient as (e): intraoperative photograph of right maxillectomy showing blackened
sinus cavity.
With its superior soft tissue contrast, MRI may also
reveal surrounding bone marrow infiltration in early-stage
disease before erosive changes are apparent on
CT. Involvement of retro-antral fat should be diligently
looked for both on CT and MRI as it is an early sign of
soft tissue infiltration.
NASAL CAVITY DISEASE
Nasal cavity disease (Figure 3) manifests as soft tissue
opacification and/or inflammatory fluid on CT. The
middle turbinate is most frequently affected and may
show destructive or ulcerative changes. On MRI, a black
turbinate sign or a black mucosal sign has been described
and refers to non-enhancing mucosa of the nasal
turbinate or sinuses respectively on post-contrast MRI. It
is considered a feature of early Mucormycosis.[10] [11]
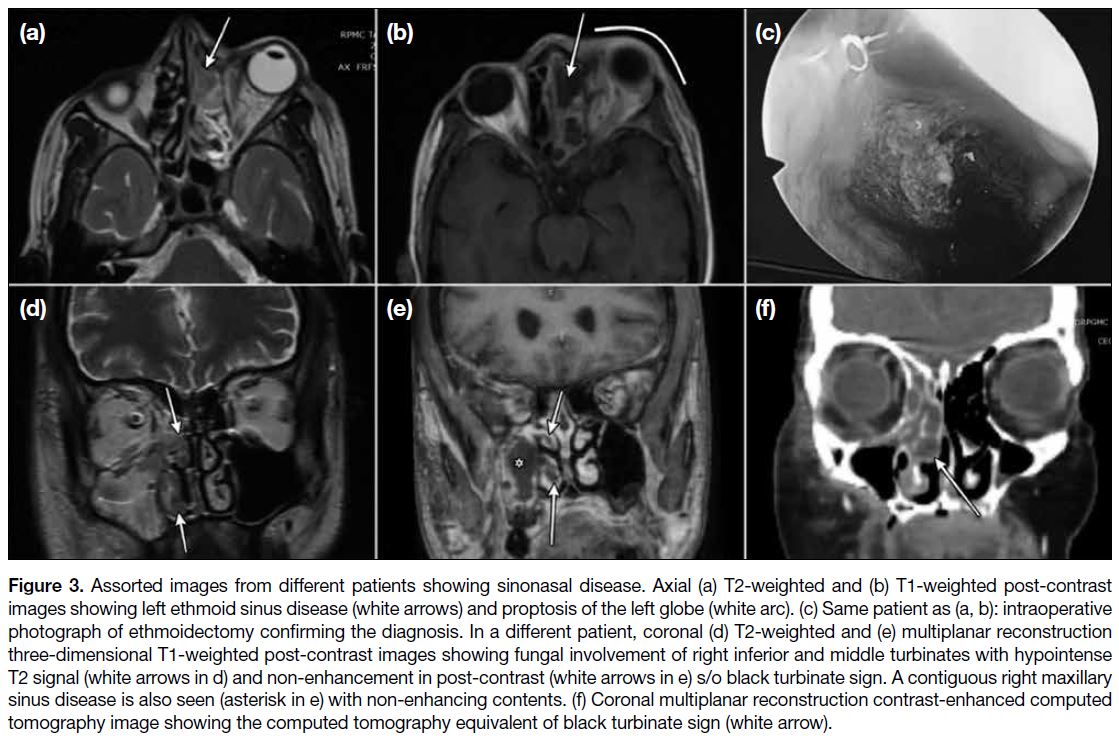
Figure 3. Assorted images from different patients showing sinonasal disease. Axial (a) T2-weighted and (b) T1-weighted post-contrast
images showing left ethmoid sinus disease (white arrows) and proptosis of the left globe (white arc). (c) Same patient as (a, b): intraoperative
photograph of ethmoidectomy confirming the diagnosis. In a different patient, coronal (d) T2-weighted and (e) multiplanar reconstruction
three-dimensional T1-weighted post-contrast images showing fungal involvement of right inferior and middle turbinates with hypointense
T2 signal (white arrows in d) and non-enhancement in post-contrast (white arrows in e) s/o black turbinate sign. A contiguous right maxillary
sinus disease is also seen (asterisk in e) with non-enhancing contents. (f) Coronal multiplanar reconstruction contrast-enhanced computed
tomography image showing the computed tomography equivalent of black turbinate sign (white arrow).
EXTRASINUS SPREAD
Non orbital extrasinus spread (Figure 4) can occur in the
face, masticator space, premaxillary area, infra-temporal
or temporal fossa, retroantral fat, pterygopalatine fossa,
or skull base. It is evidenced as fat stranding or soft
tissue extension with similar imaging characteristics to
intrasinus soft tissue.[9] Although there may be erosive
bony destruction in some cases, it is worth noting
that in the majority, the extrasinus spread takes place
without bony involvement, suggesting a perivascular
or perineural route of spread. Extrasinus disease in association with new onset facial/orbital swelling and
visual complaints in a COVID-19 patient is a red flag for
possible invasive fungal disease.
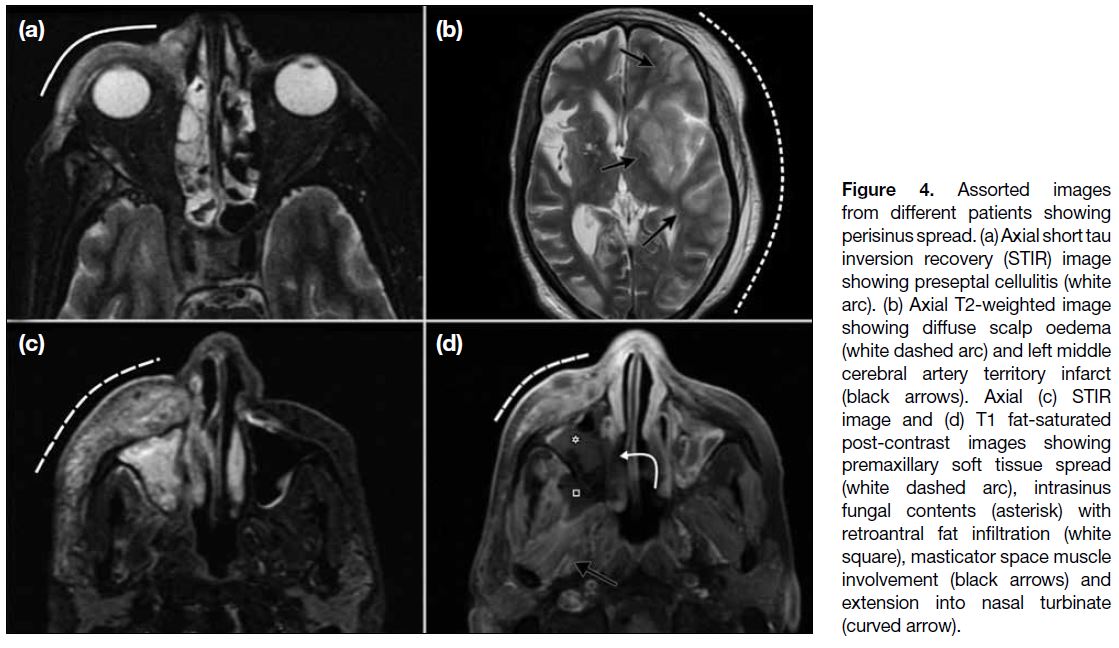
Figure 4. Assorted images
from different patients showing perisinus spread. (a) Axial short tau inversion recovery (STIR) image showing preseptal cellulitis (white arc). (b) Axial T2-weighted image showing diffuse scalp oedema (white dashed arc) and left middle cerebral artery territory infarct (black arrows). Axial (c) STIR image and (d) T1 fat-saturated post-contrast images showing premaxillary soft tissue spread (white dashed arc), intrasinus fungal contents (asterisk) with retroantral fat infiltration (white square), masticator space muscle involvement (black arrows) and extension into nasal turbinate (curved arrow).
ORBITAL SPREAD
Orbital spread (Figure 5) commonly occurs through
the medial orbital wall and nasolacrimal duct. We
found preseptal and/or orbital cellulitis to be a constant
feature with involvement of extraconal fat, intraconal fat
and the orbital apex as well as the extraocular muscle
cone to varying degrees. Oedematous thickening of the
extraocular muscle manifests as hyperintense signal
intensity of muscle on fluid-sensitive MRI sequences
with post-gadolinium enhancement. Medial, inferior,
and superior recti muscles are more commonly affected,
putatively due to their proximity to the lamina papyracea
that is the main route of spread. Sometimes there may be
cone-shaped deformation of the ocular globe posteriorly,
known as the ‘Guitar pick sign’, with consequent
increased intra-orbital pressure and compromised
vision.
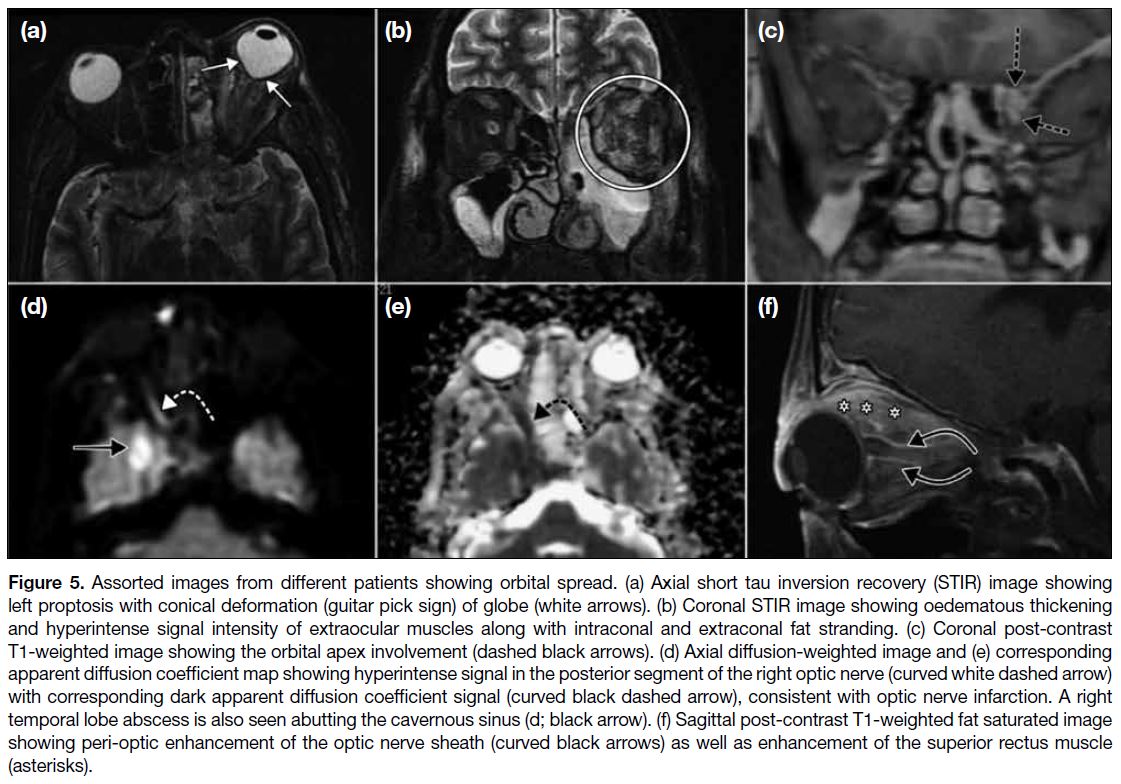
Figure 5. Assorted images from different patients showing orbital spread. (a) Axial short tau inversion recovery (STIR) image showing
left proptosis with conical deformation (guitar pick sign) of globe (white arrows). (b) Coronal STIR image showing oedematous thickening
and hyperintense signal intensity of extraocular muscles along with intraconal and extraconal fat stranding. (c) Coronal post-contrast
T1-weighted image showing the orbital apex involvement (dashed black arrows). (d) Axial diffusion-weighted image and (e) corresponding
apparent diffusion coefficient map showing hyperintense signal in the posterior segment of the right optic nerve (curved white dashed arrow)
with corresponding dark apparent diffusion coefficient signal (curved black dashed arrow), consistent with optic nerve infarction. A right
temporal lobe abscess is also seen abutting the cavernous sinus (d; black arrow). (f) Sagittal post-contrast T1-weighted fat saturated image
showing peri-optic enhancement of the optic nerve sheath (curved black arrows) as well as enhancement of the superior rectus muscle
(asterisks).
Ischaemic optic nerve infarction has been described
rarely in orbital mucormycosis and is probably due to
vascular invasion by the Mucor fungus. The posterior
intra-orbital segment of the optic nerve is mostly affected
and the nerve displays a hyperintense diffusion-weighted
imaging signal, and a corresponding hypointensity
on apparent diffusion coefficient maps with non-enhancement
on post-contrast images.[12]
A circular enhancement around the optic nerve may
be observed on contrast-enhanced MRI. This is
nonetheless a rarely reported observation in ROCM and
may be indicative of perineuritis or direct optic nerve
infiltration.[9] [13]
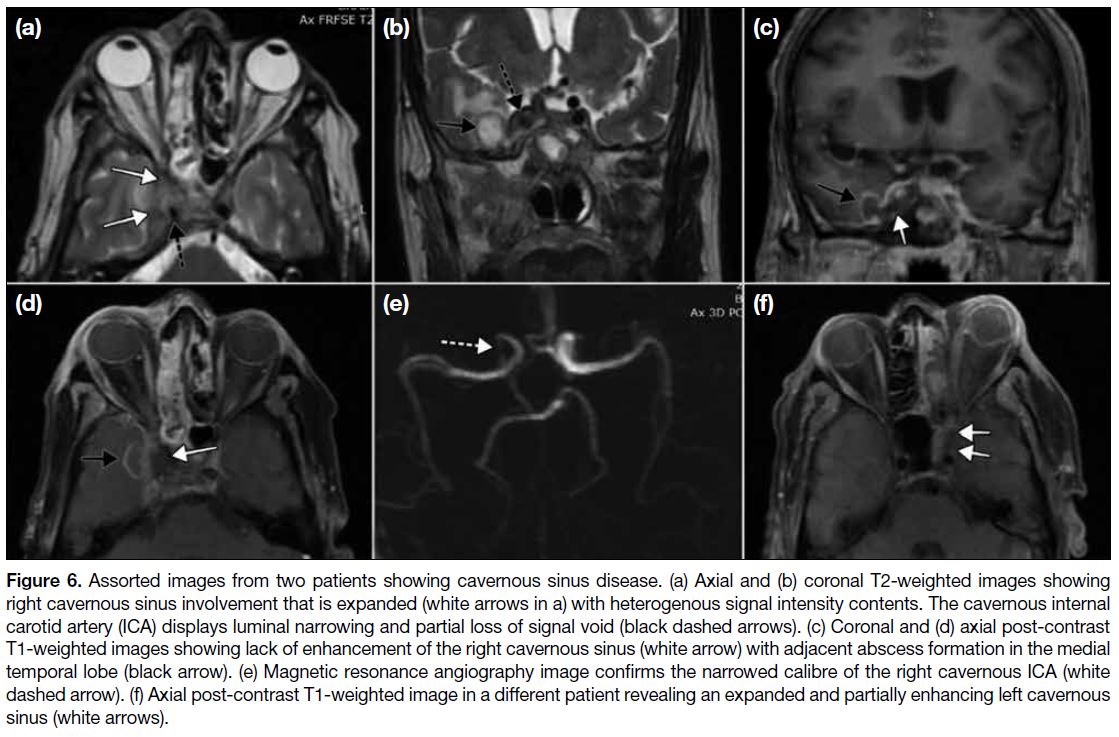
CAVERNOUS SINUS INVOLVEMENT
Cavernous sinus (Figure 6) involvement is better
assessed on MRI owing to its superior soft tissue contrast.
It may manifest as a hypointense intrasinus signal
intensity on T1W; mixed hypo/hyper signal on T2W; or non-enhancement/inhomogeneous enhancement
in post-gadolinium images with or without features of
sinus expansion. It is vital to assess the flow void of the
cavernous segment of the internal carotid artery for any
signal loss or narrowing of calibre. Internal carotid artery
stenosis in the setting of cavernous sinus involvement
may be indicative of infectious arteritis and predates a
more sinister complication of thrombosis with cerebral
infarction in later stages.
Figure 6. Assorted images from two patients showing cavernous sinus disease. (a) Axial and (b) coronal T2-weighted images showing
right cavernous sinus involvement that is expanded (white arrows in a) with heterogenous signal intensity contents. The cavernous internal
carotid artery (ICA) displays luminal narrowing and partial loss of signal void (black dashed arrows). (c) Coronal and (d) axial post-contrast
T1-weighted images showing lack of enhancement of the right cavernous sinus (white arrow) with adjacent abscess formation in the medial
temporal lobe (black arrow). (e) Magnetic resonance angiography image confirms the narrowed calibre of the right cavernous ICA (white
dashed arrow). (f) Axial post-contrast T1-weighted image in a different patient revealing an expanded and partially enhancing left cavernous
sinus (white arrows).
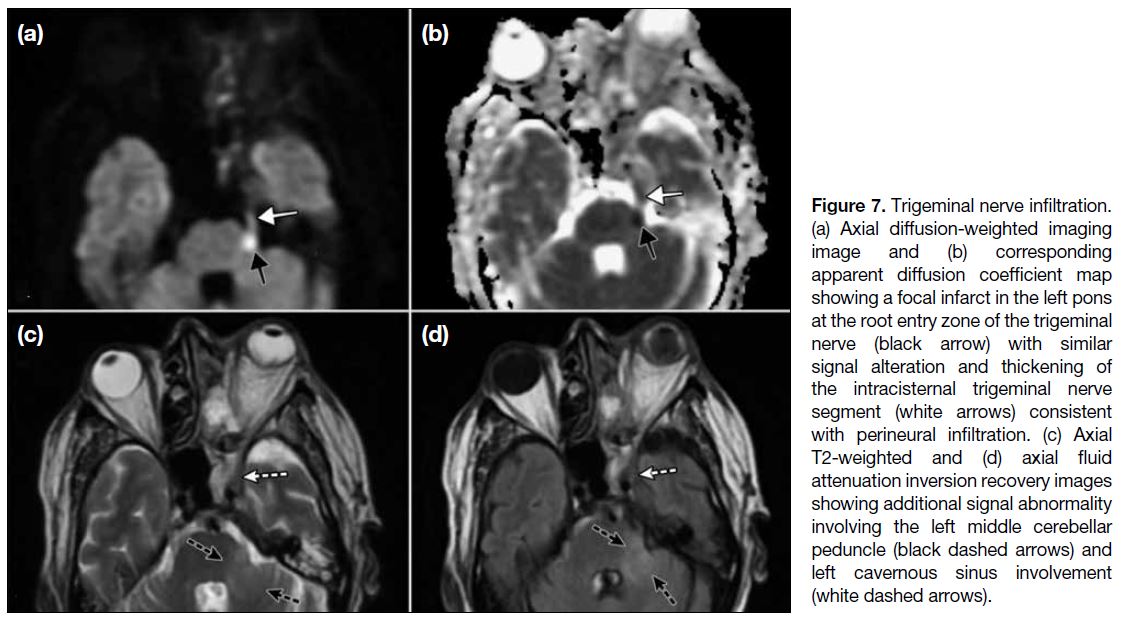
PERINEURAL SPREAD
Perineural spread (Figure 7) from the cavernous
sinus along the cisternal trigeminal nerve course is
exceptionally uncommon in ROCM.[14] On MRI there is
thickening with enhancement of the cisternal segment of
the nerve that may extend to the pons around the root
entry zone. Pontine infiltration may result in infarction,
presumably due to associated arteritis or pontine abscess
formation with their typical respective MRI imaging
manifestations.
Figure 7. Trigeminal nerve infiltration. (a) Axial diffusion-weighted imaging image and (b) corresponding apparent diffusion coefficient map showing a focal infarct in the left pons at the root entry zone of the trigeminal nerve (black arrow) with similar signal alteration and thickening of the intracisternal trigeminal nerve segment (white arrows) consistent with perineural infiltration. (c) Axial T2-weighted and (d) axial fluid attenuation inversion recovery images showing additional signal abnormality involving the left middle cerebellar peduncle (black dashed arrows) and left cavernous sinus involvement (white dashed arrows).
CENTRAL NERVOUS SYSTEM
INVOLVEMENT
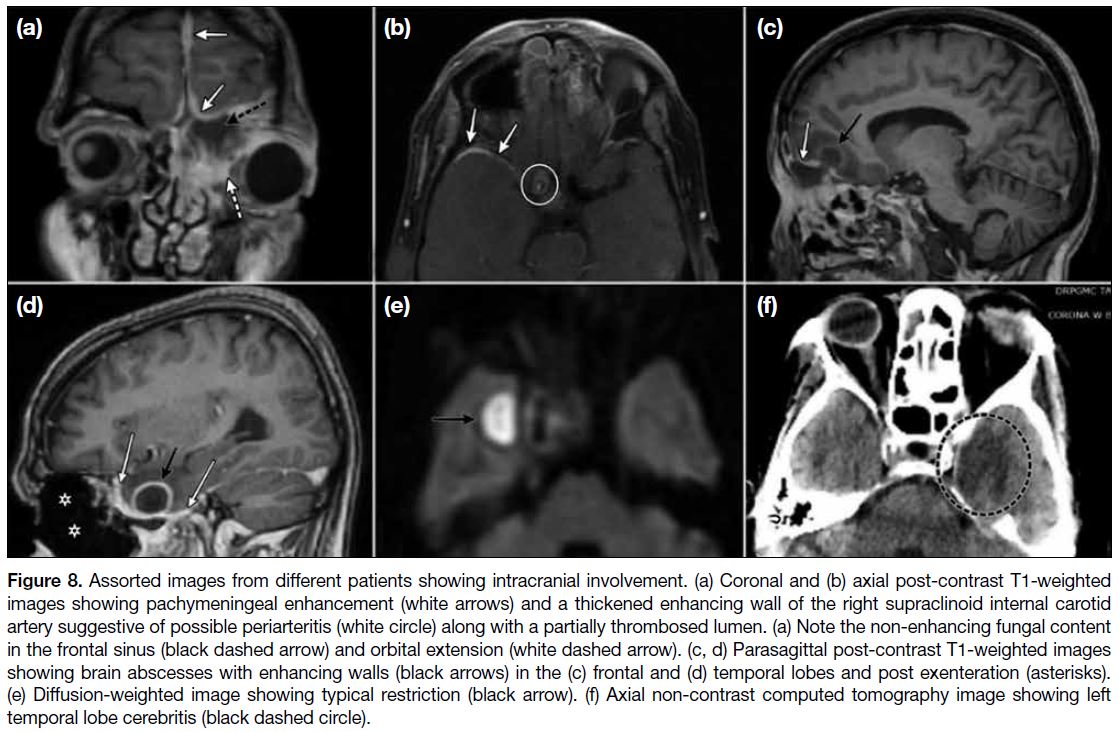
Intracerebral extension (Figures 8 and 9) is an ominous
development. It may present as cerebritis, cerebral
abscess, meningeal enhancement, extradural collections
or ischaemia with typical neuroimaging features
ascribed to these entities. The imaging appearances
of infarcts depend on their acuteness, with the acute/early subacute stage infarctions appearing hypodense
on CT and restricting on diffusion-weighted imaging.
Parenchymal fungal abscesses resemble the pyogenic
ones and show peripheral rim enhancement on contrast
enhanced CT/MRI with centrally restricted diffusion.
Meningeal enhancement can be dural or leptomeningeal
and is better delineated on T1 post-contrast MRI images.
Figure 8. Assorted images from different patients showing intracranial involvement. (a) Coronal and (b) axial post-contrast T1-weighted
images showing pachymeningeal enhancement (white arrows) and a thickened enhancing wall of the right supraclinoid internal carotid
artery suggestive of possible periarteritis (white circle) along with a partially thrombosed lumen. (a) Note the non-enhancing fungal content
in the frontal sinus (black dashed arrow) and orbital extension (white dashed arrow). (c, d) Parasagittal post-contrast T1-weighted images
showing brain abscesses with enhancing walls (black arrows) in the (c) frontal and (d) temporal lobes and post exenteration (asterisks).
(e) Diffusion-weighted image showing typical restriction (black arrow). (f) Axial non-contrast computed tomography image showing left
temporal lobe cerebritis (black dashed circle).
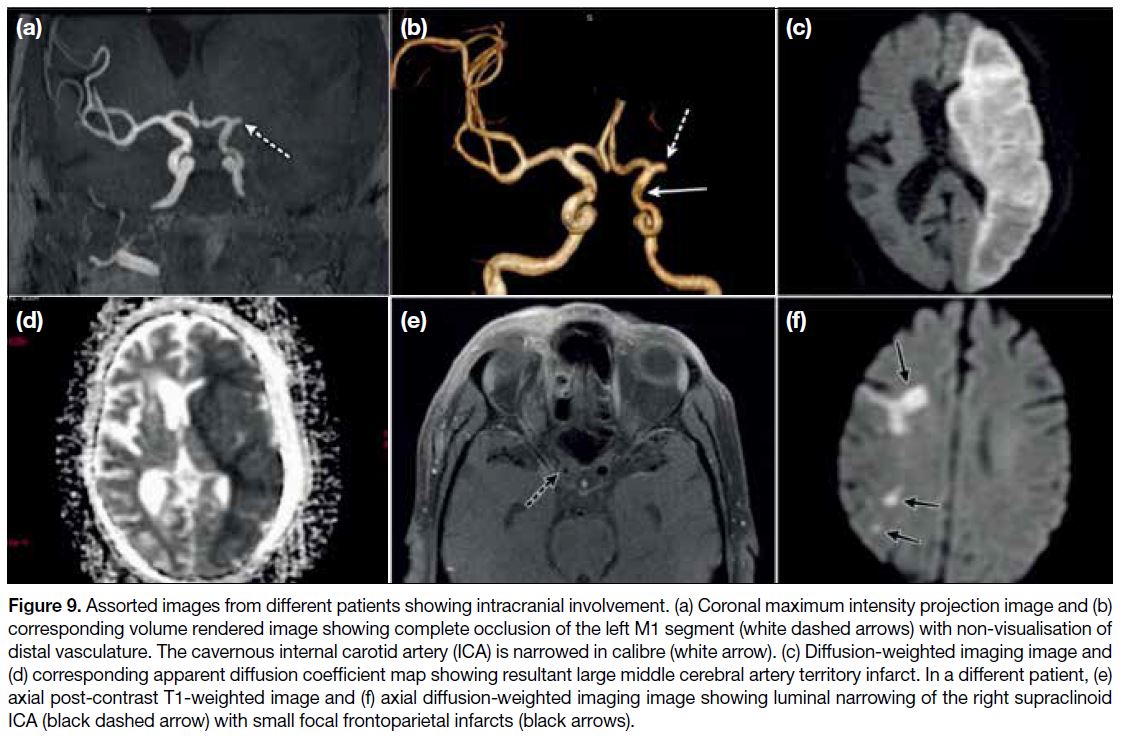
Figure 9. Assorted images from different patients showing intracranial involvement. (a) Coronal maximum intensity projection image and (b)
corresponding volume rendered image showing complete occlusion of the left M1 segment (white dashed arrows) with non-visualisation of
distal vasculature. The cavernous internal carotid artery (ICA) is narrowed in calibre (white arrow). (c) Diffusion-weighted imaging image and
(d) corresponding apparent diffusion coefficient map showing resultant large middle cerebral artery territory infarct. In a different patient, (e)
axial post-contrast T1-weighted image and (f) axial diffusion-weighted imaging image showing luminal narrowing of the right supraclinoid
ICA (black dashed arrow) with small focal frontoparietal infarcts (black arrows).
OSSEOUS INVOLVEMENT
Bony involvement (Figure 10) can occur in 40% of
ROCM cases and is better demonstrated on CT. The
involvement can be as erosions, lytic destruction or
thinning with rarefaction and may involve the paranasal
bony sinus perimeter or adjacent bony structures such
as the pterygoid plates, zygomatic arch and skull base.[9]
Erosion of thin lamina papyracea is associated with orbital spread while occasionally erosion of the maxillary
alveolus may cause oro-antral fistulisation. Bony
erosions, evaluated in combination with other suggestive
imaging findings, can be a useful in the diagnosis of
invasive fungal sinusitis.[15]
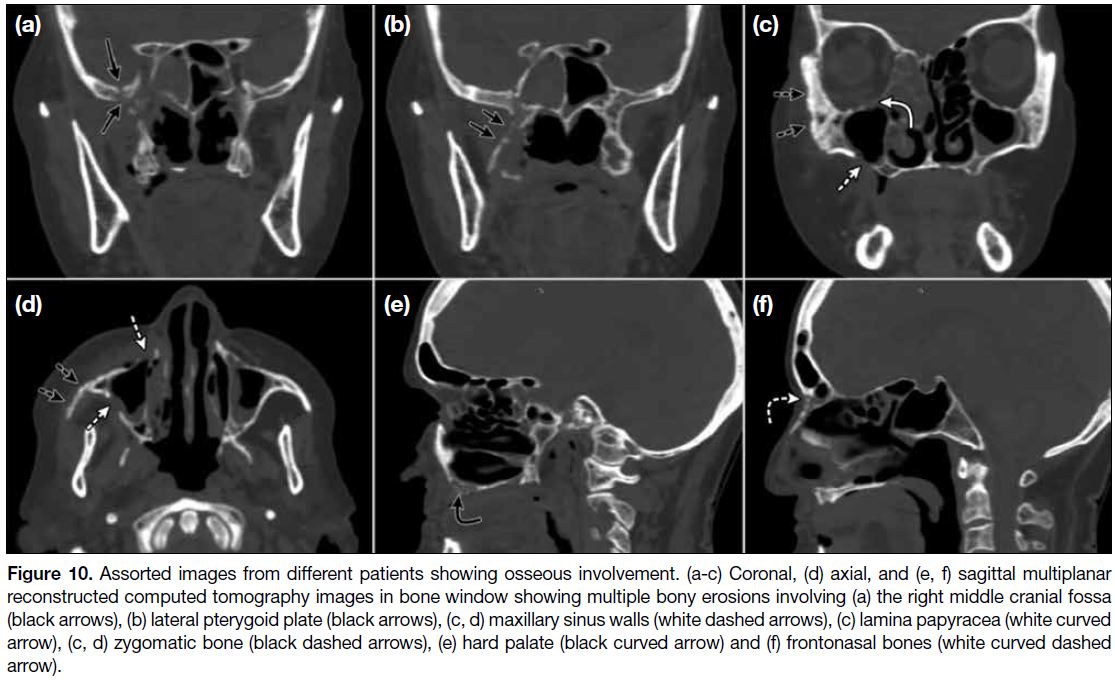
Figure 10. images from different patients showing osseous involvement. (a-c) Coronal, (d) axial, and (e, f) sagittal multiplanar
reconstructed computed tomography images in bone window showing multiple bony erosions involving (a) the right middle cranial fossa
(black arrows), (b) lateral pterygoid plate (black arrows), (c, d) maxillary sinus walls (white dashed arrows), (c) lamina papyracea (white curved
arrow), (c, d) zygomatic bone (black dashed arrows), (e) hard palate (black curved arrow) and (f) frontonasal bones (white curved dashed
arrow).
Treatment of ROCM is prompt surgical debridement
of the involved area and administration of intravenous
antifungal agents. Liposomal amphotericin B is the
drug of choice with posaconazole an alternative in
cases of resistance/intolerance. The overall prognosis
is grim with mortality at 50 to 80%. Presence of brain
invasion is associated with even higher mortality rates
exceeding 80% but an aggressive surgical approach can
be rewarding and has been associated with improved
outcome.[1]
CONCLUSION
Opportunistic infections such as ROCM complicating
COVID-19 illness in a susceptible patient is a relatively
new and dangerous phenomena. It carries a high mortality
rate if intracranial complications develop and early
identification with expeditious surgical intervention is
pivotal in improving the morbidity and mortality.
Radiologists are an important cog in the wheel of
multidisciplinary management by virtue of their ability
to provide a speedy, accurate diagnosis and elucidate the
surgical roadmap.
Radiological diagnosis can be made with a high degree
of confidence if attention is paid to the presence of
suggestive imaging findings, especially in the subset
of patients with COVID-19 who are diabetic or have a
history of steroid use or prolonged oxygen or ventilatory
support. Radiologists must also be aware of rare
manifestations of ROCM such as optic nerve infarction
and perineural trigeminal nerve spread.
REFERENCES
1. Sharma S, Grover M, Bhargava S, Samdani S, Kataria T. Post
coronavirus disease mucormycosis: a deadly addition to the
pandemic spectrum. J Laryngol Otol. 2021;135:442-7. Crossref
2. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y et al.
Epidemiological and clinical characteristics of 99 cases of 2019
novel coronavirus pneumonia in Wuhan, China: a descriptive study.
Lancet. 2020;395:507-13. Crossref
3. Mehta S, Pandey A. Rhino-orbital mucormycosis associated with
COVID-19. Cureus. 2020;12:e10726. Crossref
4. Garg D, Muthu V, Sehgal IS, Ramachandran R, Kaur H, Bhalla A,
et al. Coronavirus disease (Covid-19) associated mucormycosis
(CAM): case report and systematic review of literature.
Mycopathologia. 2021;186:289-98. Crossref
5. Jeong W, Keighley C, Wolfe R, Lee WL, Slavin MA, Kong DC,
et al. The epidemiology and clinical manifestations of mucormycosis: a systematic review and meta-analysis of case
reports. Clin Microbiol Infect. 2019;25:26-34. Crossref
6. Montaño DE, Voigt K. Host immune defense upon fungal infections
with mucorales: pathogen-immune cell interactions as drivers of
inflammatory responses. J Fungi (Basel). 2020;6:173. Crossref
7. Alekseyev K, Didenko L, Chaudhry B. Rhinocerebral mucormycosis
and COVID-19 pneumonia. J Med Cases. 2021;12:85-9. Crossref
8. Saldanha M, Reddy R, Vincent MJ. Title of the article: paranasal
mucormycosis in COVID-19 patient. Indian J Otolaryngol Head
Neck Surg. 2021 Apr 22. Epub ahead of print. Crossref
9. Therakathu J, Prabhu S, Irodi A, Sudhakar SV, Yadav VK, Rupa V.
Imaging features of rhinocerebral mucormycosis: a study of 43
patients. Egypt J Radiol Nucl Med. 2018;49:447-52. Crossref
10. Raab P, Sedlacek L, Buchholz S, Stolle S, Lanfermann H.
Imaging patterns of rhino-orbital-cerebral mucormycosis in
immunocompromised patients: when to suspect complicated
mucormycosis. Clin Neuroradiol. 2017;27:469-75. Crossref
11. Safder S, Carpenter JS, Roberts TD, Bailey N. The “Black
Turbinate” sign: An early MR imaging finding of nasal
mucormycosis. AJNR Am J Neuroradiol. 2010;31:771-4. Crossref
12. Mathur S, Karimi A, Mafee MF. Acute optic nerve infarction
demonstrated by diffusion-weighted imaging in a case of
rhinocerebral mucormycosis. AJNR Am J Neuroradiol.
2007;28:489-90.
13. Chen IW, Lin CW. Rhino-orbital-cerebral mucormycosis. CMAJ.
2019;191:E450. Crossref
14. McLean FM, Ginsberg LE, Stanton CA. Perineural spread
of rhinocerebral mucormycosis. AJNR Am J Neuroradiol.
1996;17:114-6.
15. Middlebrooks EH, Frost CJ, De Jesus RO, Massini TC, Schmalfuss
IM, Mancuso AA. Acute invasive fungal rhinosinusitis: a
comprehensive update of CT findings and design of an effective
diagnostic imaging model. AJNR Am J Neuroradiol. 2015;36:1529-35. Crossref