Intracranial Parenchymal Mesenchymal Chondrosarcoma: a Case Report
CASE REPORT
Intracranial Parenchymal Mesenchymal Chondrosarcoma: a Case Report
HY Lo1, DCW Tang1, KS Ng2, MH So1, JKL Ng1, AWS Au Yeung1, D Cho1
1 Department of Diagnostic and Interventional Radiology, Kwong Wah Hospital, Hong Kong
2 Department of Pathology, Kwong Wah Hospital, Hong Kong
Correspondence: Dr HY Lo, Department of Diagnostic and Interventional Radiology, Kwong Wah Hospital, Hong Kong. Email: hongyiplo@gmail.com
Submitted: 22 Feb 2021; Accepted: 17 May 2021.
Contributors: HYL and DCWT designed the study. HYL, DCWT and KSN acquired the data. HYL and DCWT analysed the data. HYL drafted
the manuscript. All authors critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed
to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: As an editor of the journal, HYL was not involved in the peer review process. Other authors have disclosed no conflicts of interest.
Funding/Support: This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: The patient was treated in accordance with the Declaration of Helsinki. Written informed consent was obtained for all treatment and procedures.
INTRODUCTION
Primary intracranial mesenchymal chondrosarcoma
is a rare entity, with most cases extra-axial. We
present an unusual case of parenchymal mesenchymal
chondrosarcoma where reaching a radiological diagnosis
is a challenge.
CASE REPORT
In December 2015, a 19-year-old man with unremarkable
past health presented with insidious onset of right-side
facial twitching but no focal neurological deficit.
He attended our department for routine magnetic
resonance imaging (MRI) of the brain. He experienced
a brief episode of generalised convulsion during
the examination. The MRI was aborted, and he was
immediately admitted for in-patient care.
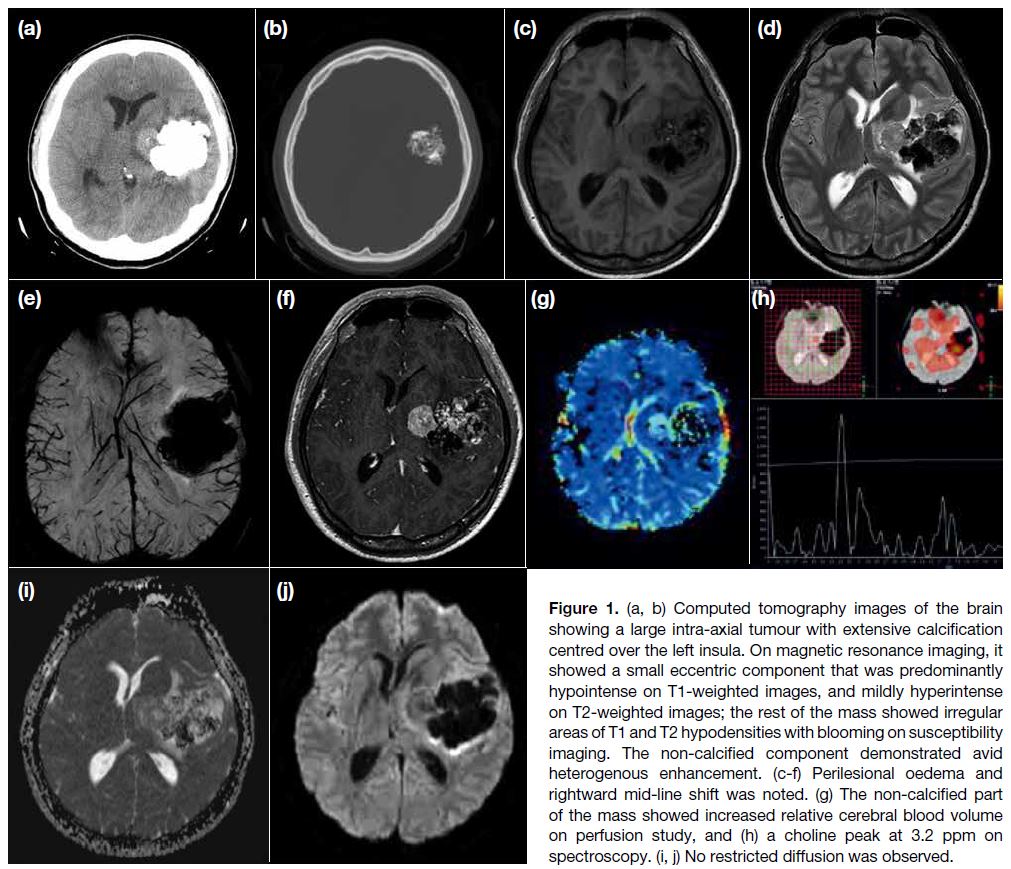
Urgent plain computed tomography scan of the brain
revealed a large intra-axial tumour with extensive
calcification centring over the left insula, with a smaller
eccentric soft tissue component (Figure 1a and b). On
MRI, the mass consisted of a small eccentric component at its medial aspect, predominantly hypointense on T1-weighted images, and mildly hyperintense on T2-weighted
images; the rest of the mass showed irregular areas of T1
and T2 hypodensities with blooming on susceptibility
imaging, corresponding to the calcification on computed
tomography scan. A fair amount of perilesional oedema
with mild rightward mid-line shift was noted (Figure 1c to e). The non-calcified component demonstrated
avid heterogenous enhancement, with an increased
relative blood volume on perfusion study (Figure 1f to g). There was an elevated choline (Cho) peak at
3.2 ppm on spectroscopy, with elevated Cho:creatine
and Cho:N-acetylaspartate ratios (Figure 1h). No
restricted diffusion was observed (Figure 1i to j). For
the calcified part, there was only very low signal and the
perfusion and spectroscopy pattern were not interpretable.
The initial suspicion was that of a high-grade glioma,
such as an astrocytoma or oligodendroglioma, less likely
a germ cell tumour.
Figure 1. (a, b) Computed tomography images of the brain
showing a large intra-axial tumour with extensive calcification
centred over the left insula. On magnetic resonance imaging, it
showed a small eccentric component that was predominantly
hypointense on T1-weighted images, and mildly hyperintense
on T2-weighted images; the rest of the mass showed irregular
areas of T1 and T2 hypodensities with blooming on susceptibility
imaging. The non-calcified component demonstrated avid
heterogenous enhancement. (c-f) Perilesional oedema and
rightward mid-line shift was noted. (g) The non-calcified part
of the mass showed increased relative cerebral blood volume
on perfusion study, and (h) a choline peak at 3.2 ppm on
spectroscopy. (i, j) No restricted diffusion was observed.
The patient underwent surgical excision 2 days later.
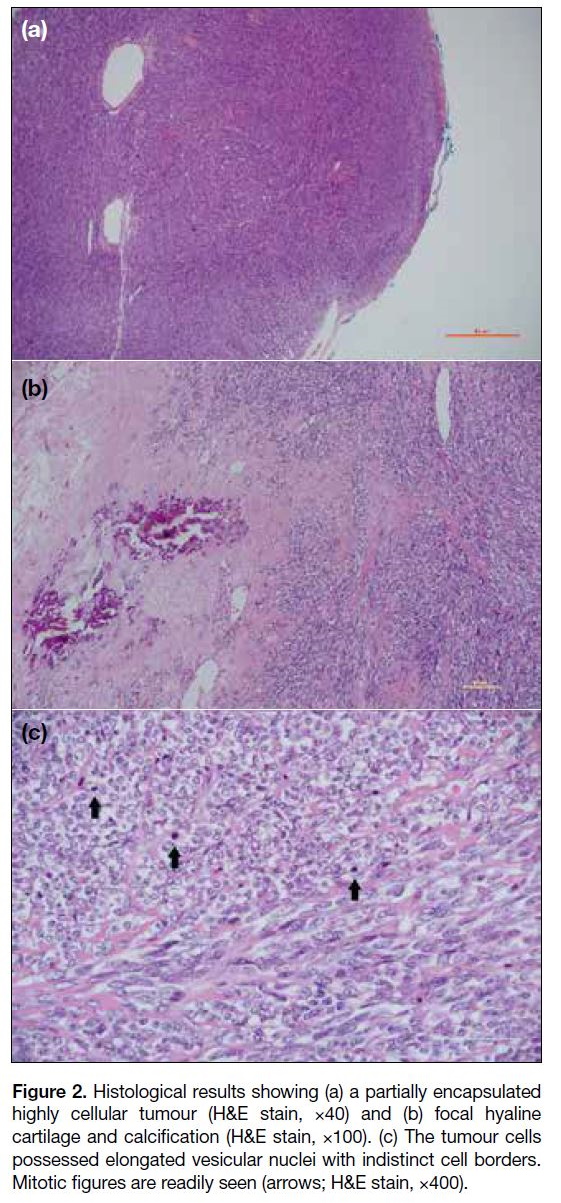
Intra-operatively, a largely calcified left insula tumour with some soft tissue and fibrous component was
identified. Histology showed a partially encapsulated
tumour with high cellularity and components of hyaline
cartilage and calcification. Mitotic figures were readily
seen (Figure 2). Reverse transcription polymerase chain
reaction confirmed the presence of fusion gene product
of mesenchymal chondrosarcoma.
Figure 2. Histological results showing (a) a partially encapsulated
highly cellular tumour (H&E stain, ×40) and (b) focal hyaline
cartilage and calcification (H&E stain, ×100). (c) The tumour cells
possessed elongated vesicular nuclei with indistinct cell borders.
Mitotic figures are readily seen (arrows; H&E stain, ×400).
The recovery period was unremarkable. He underwent a
course of radiotherapy and remained free of recurrence
with no gross neurological deficits at 5-year follow-up
examination.
DISCUSSION
Chondrosarcoma is a malignant bone tumour characterised by the production of chondroid matrix.
There are four pathological subtypes: conventional
chondrosarcoma, mesenchymal chondrosarcoma,
clear cell chondrosarcoma and de-differentiated
chondrosarcoma, with the latter two subtypes being
exceedingly rare as intracranial tumour.[1]
Intracranial chondrosarcoma usually affect individuals
45 to 49 years of age, with no gender preference.[2]
Nonetheless the mesenchymal subtype, as in our case,
tends to affect younger patients in their 20s.[3]
The majority of intracranial mesenchymal
chondrosarcoma, in contrast to the classic subtypes, are
less frequently found at the skull base.[1] [4] [5] Instead, the most common location is the craniospinal meninges.[6] [7]
In one previous study by Wang et al,[8] all included cases
had a dural attachment. A sole intra-axial location is rare.
Radiographically the diagnosis can be challenging. On
CT, it is often calcified and a characteristic ring and
arc configuration may be observed.[9] [10] When extra-axial,
as in most cases, it can mimic a meningioma or haemangiopericytoma; as an intra-axial mass,
the differential includes an oligodendroglioma,
ganglioglioma and vascular malformation. On MRI,
owing to the calcified matrix, it often displays an
internal foci of low T1/2 signal with blooming on
susceptibility imaging, while the soft tissue components
show a heterogenous enhancement. There are currently
limited data on MRI perfusion study and spectroscopy
in intracranial mesenchymal chondrosarcoma. Some
previous cases suggest a hypovascular pattern for the
tumour.[9] [11] Nonetheless this was not fully compatible in
our case. The presence of Cho peak can be observed in
many malignant bone and soft tissue tumours,[12] and is
non-specific for the diagnosis. In a rare case of intracranial
myxoid chondrosarcoma, an N-acetyl aspartate peak was
noted, presumably due to the myxoid component.[13]
Mesenchymal chondrosarcoma is considered a more
aggressive subtype, with an increased tendency for
local and distant recurrences.[14] [15] Unfortunately, due to
its infrequent occurrence, there is no well-established
treatment protocol, and the use of adjuvant chemo- and
radio-therapy remains controversial.[1] [6] [10] However, a
more aggressive and individualised multidisciplinary
approach should always be considered in view of the
worse prognosis.
CONCLUSION
As an exceedingly rare entity with confusing imaging
findings, intracranial parenchymal mesenchymal
chondrosarcoma is undoubtedly a challenging
radiological diagnosis. It may mimic a high-grade glioma
as illustrated in our case.
REFERENCES
1. Ma X, Meng G, Wang K, Li D, Wang L, Li H, et al. The
differences between intracranial mesenchymal chondrosarcoma and
conventional chondrosarcoma in clinical features and outcomes.
World Neurosurg. 2019;122:e1078-82. Crossref
2. Jones JC, Habboub G, Das P, Lang M, Colby S, Volovetz J, et al.
Cranial chondrosarcomas: descriptive epidemiology from the years
2001 to 2014 in the United States. J Neurol Surg B Skull Base.
2018;79(S 01):S1-188. Crossref
3. Frezza AM, Cesari M, Baumboer D, Biau D, Bielack S, Campanacci DA, et al. Mesenchymal chondrosarcoma: prognostic factors and outcome in 113 patients. A European musculoskeletal
Oncology Society study. Eur J Cancer. 2015;51:374-81. Crossref
4. Kathiravel Y, Finnis ND. Primary falcine chondrosarcoma. J Clin
Neurosci. 2008;15:1406-9. Crossref
5. Bingaman KD, Alleyne CH Jr, Olson JJ. Intracranial extraskeletal
mesenchymal chondrosarcoma: case report. Neurosurgery.
2000;46:207-11. Crossref
6. Shabani S, Kaushal M, Kaufman B, Knipstein J, Lawlor MW,
Lew S, et al. Intracranial extraskeletal mesenchymal
chondrosarcoma: case report and review of the literature of reported cases in adults and children. World Neurosurg. 2019;129:302-10. Crossref
7. Chen JY, Hsu SS, Ho JT. Extraskeletal intracranial mesenchymal
chondrosarcoma: case report and literature review. Kaosiung J Med
Sci. 2004;20:240-6. Crossref
8. Wang K, Ma XJ, Guo TX, Wang L, Li D, Hao SY, et al. intracranial
mesenchymal chondrosarcoma: report of 16 cases. World
Neurosurg. 2018;116:e691-8. Crossref
9. Nishita K, Law M, Cha S, Zagzag D. Conventional and perfusion
MR imaging of parafalcine chondrosarcoma. AJNR Am J
Neuroradiol. 2003;24:245-8.
10. Bhatt AA, Campeau N, Black DF. Primary intracranial extraskeletal
chondrosarcoma. Appl Radiol. 2017;46:32-4.
11. Kojima D, Beppu T, Saura H, Sato Y, Fujiwara S, Ogasawara K.
Apparent diffusion coefficient and arterial spin labeling perfusion of conventional chondrosarcoma in the parafalcine region: a case
report. Radiol Case Rep. 2018;13:220-4. Crossref
12. Zampa V, Roselli G, Beltrami G. MRI of bone tumors: advances in diagnosis and treatment assessment. Imaging Med. 2010;2:325-40. Crossref
13. Kumaran SP, Assis ZA, Viswamitra S, Ghosal N, Narayanam SK.
N-acetyl aspartate peak in extra-axial extraosseous chondrosarcoma
of the brain on MRI: Unravelling a diagnostic dilemma. Neurol
India. 2016;64:176-8. Crossref
14. Nakashima Y, Unni KK, Shives TC, Swee RG, Dahlin DC.
Mesenchymal chondrosarcoma of bone and soft tissue. A review
of 111 cases. Cancer. 1986;57:2444-53. Crossref
15. Hassounah M, Al-Mefty O, Akhtar M, Jinkins JR, Fox JL. Primary
cranial and intracranial chondrosarcoma. A survey. Acta Neurochir
(Wien). 1985;78:123-32. Crossref