Sonographic Features of Triple-Negative Breast Cancer in an Asian Population
ORIGINAL ARTICLE CME
Sonographic Features of Triple-Negative Breast Cancer in an Asian Population
C Tsoi1, JYS Chan1, HKY Tam2, EHY Hung1, AWH Ng1, HHL Chau1, WCW Chu1
1 Department of Imaging and Interventional Radiology, Prince of Wales Hospital, Hong Kong
2 Department of Radiology, North District Hospital, Hong Kong
Correspondence: Dr C Tsoi, Department of Imaging and Interventional Radiology, Prince of Wales Hospital, Hong Kong. Email: caritatsoi@gmail.com
Submitted: 28 Mar 2021; Accepted: 27 Oct 2021.
Contributors: All authors designed the study. CT, JYSC and HKYT acquired and analysed the data. CT and HKYT drafted the manuscript.
All authors critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study,
approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: As an editor of the journal, WCWC was not involved in the peer review process. Other authors have disclosed no conflicts of interest.
Funding/Support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: This single-centre retrospective study was approved by the Joint Chinese University of Hong Kong–New Territories East Cluster Clinical Research Ethics Committee (Ref: 2021.216).
Acknowledgement: The authors show their appreciation to Ms Min Deng, Research Associate, Department of Imaging and Interventional
Radiology of The Chinese University of Hong Kong for her assistance in statistical analysis in this project.
Abstract
Introduction
Triple-negative breast cancer (TNBC) is well known for its unique clinical and pathological
characteristics. Our study compared the sonographic features of TNBC with those of non-TNBC according to the
sonographic classification system of the American College of Radiology’s Breast Imaging Reporting and Data
System (BI-RADS).
Methods
This was a retrospective study involving sonographic images from 50 patients with TNBC and 52 patients
with non-TNBC diagnosed from 2016 to 2020, which were reviewed by two reviewers simultaneously according to
the fifth edition of BI-RADS and a result was reached by consensus.
Results
TNBCs were significantly associated with higher tumour grade (p < 0.001), higher tumour stage (p = 0.006)
and larger tumour size (p < 0.001). Compared with non-TNBCs, TNBCs had a significantly higher incidence of
the following features: oval or round shape (p = 0.006), microlobulated margin (p = 0.006), parallel orientation
(p = 0.001), posterior acoustic enhancement (p = 0.007), and less architectural distortion (p < 0.001).
Conclusions
TNBCs have their own distinct sonographic features compared with non-TNBCs. Clinicians should
be alert to these features since they mimic a benign lesion but show aggressive clinical behaviours.
Key Words: Breast neoplasms; Triple negative breast cancer
中文摘要
亞洲人群中三陰性乳腺癌的超聲特徵
蔡嘉澄、陳奕璇、譚嘉盈、洪曉義、伍永鴻、周海倫、朱昭穎
引言
三陰性乳腺癌(TNBC)有其獨特的臨床和病理特徵。我們的研究根據美國放射學會乳腺成像報告和數據系統比較TNBC與非TNBC的超聲特徵。
方法
這項回顧性研究納入2016年至2020年診斷的50例TNBC患者和52例非TNBC患者的超聲圖像,並由兩名醫生根據第5版乳腺成像報告和數據系統同時分析並達成共識。
結果
TNBC與更高腫瘤分級(p < 0.001)、更高腫瘤分期(p = 0.006)和腫瘤更大(p < 0.001)顯著相關。與非TNBC相比,TNBC具有以下特徵的發生率顯著更高:橢圓形或圓形(p = 0.006)、微分葉狀邊緣(p = 0.006)、平行面向(p = 0.001)、聲學後部增強(p = 0.007)和更少的架構變形(p < 0.001)。
結論
與非TNBC相比,TNBC有其獨特的超聲特徵。這些特徵類似良性病變但卻表現出侵襲性的生物學行為,因此臨床醫生應對這些超聲特徵保持警惕。
INTRODUCTION
Triple-negative breast cancer (TNBC) is well known
for its unique clinical, radiological and pathological
characteristics. It refers to the distinct subtype of breast
cancer where the three main breast cancer biomarkers,
i.e., oestrogen receptor, progesterone receptor and human
epidermal growth factor receptor 2 (HER2), are absent.[1]
TNBC constitutes 10% to 20% of all newly diagnosed
breast cancers. Affected patients tend to be younger
at diagnosis than those with non-TNBC according to
several population-based cohorts. The incidence of
TNBC is also higher in African Americans.[2] [3]
It is important to distinguish TNBC from other breast
cancers because of its distinct clinical features, including
aggressive tumour behaviour, higher potential for distant
metastases, increased risk of distant recurrence, and
consequent poorer prognosis.[4] On the contrary, TNBCs
tend to share benign imaging features despite their
aggressiveness. Their management options differ to
those for other subtypes of breast cancer because of its
lack of response to hormonal and targeted therapies but
increased chemosensitivity.[5] [6] [7] Therefore, early detection
of these lesions is essential.
Breast ultrasonography is the most common imaging for
women with clinical or mammographically suspicious breast lesions. It is particularly heavily relied on in
young women and in Asians with dense breasts.
Evaluation of breast lesions is standardised according
to the sonographic classification system of the Breast
Imaging Reporting and Data System (BI-RADS) of the
American College of Radiology (ACR) that provides
predefined terminology to describe dominant features of
breast lesions.[8]
The main purpose of our study was to identify
distinguishing sonographic features of TNBC compared
with non-TNBC, as ultrasound is the main investigation
applied in our local population with dense breasts.
Various studies have described the unique radiological
features of TNBC compared with non-TNBC[9] [10] [11] [12] [13] [14] [15] [16] [17] [18] but
with variable results. We performed this retrospective
study to evaluate the sonographic features of TNBC
according to BI-RADS’s ultrasound classification and
compare them with those of non-TNBC in an ethnically
Asian population. We sought to determine whether the
previously reported features of TNBC are applicable in
our locality.
METHODS
This is a single-centre retrospective study. Patients who attended the Department of Radiology, North District
Hospital, New Territories, Hong Kong from 2016 to
2020 were reviewed.
Patients
Patients were referred to the Department of Radiology of North District Hospital for imaging of
specific breast-related complaints such as palpable breast
mass, breast pain or suspicious mammographic findings.
Sonographic examinations are performed as part of our
routine practice and service of our breast imaging centre.
All sonographically visible lesions with subsequent
biopsy performed were documented in a centralised
database within our department. We regularly performed
follow-up and documented pathological results of all
biopsied lesions. Non-TNBC was defined as a tumour
with at least one of the three biomarkers (oestrogen
receptor, progesterone receptor or HER2 receptor)
positive. The most recent pathologically confirmed
TNBC lesions (n = 50) and non-TNBC lesions (n = 52)
were used for the study, dating back from July 2020. The
included TNBC lesions had their diagnostic sonographic
examination performed between January 2016 and May
2020, and non-TNBC lesions between April 2020 and
July 2020. Lesions with incomplete information about
receptor status were excluded.
Sonography Examination
The sonographic examinations were performed by
radiologists with at least 5 years’ experience in breast
imaging. All ultrasound examinations were performed
with a GE Logiq E9 equipped with an ML6-15D linear
transducer (6-15 MHz). All patients underwent bilateral
whole breast and axillae sonography.
All lesions were evaluated by conventional ultrasound.
All images were captured in two planes, along the longest
axis of the lesion and orthogonal to it. Three dimensions
of the lesion were measured along the longest axis,
perpendicular to the first measurement, and from the
view orthogonal to the first image. After ultrasound
examination, all lesions with suspicious imaging features
were subjected to ultrasound-guided biopsy, either in the
same session or within the next 2 weeks. At least three
cores of tissue were obtained from each lesion during
the biopsy.
Pathological Examinations
All pathological and immunohistochemical examinations
were performed at the breast centre under North District Hospital.
Oestrogen receptor, progesterone receptor, and
HER2 levels were determined by
immunohistochemistry according to a standardised
institutional protocol. Additional fluorescence in situ
hybridisation was performed to detect possible gene amplification and HER2 positivity with score ≥2. Scores
of 1 or 0 were defined as HER2 negative. Lesions with
negative results for all tests were classified as TNBC.
Histological grade was reported only in excisional
surgical specimens.
Image Analysis
Two reviewers with 3 years’ and 4 years’ experience in
breast imaging reviewed images simultaneously on a
picture archiving and communication system. Evaluation
was based on the sonographic classification system of
ACR BI-RADS Atlas Fifth Edition[8] and by consensus.
The two reviewers were blinded to the pathology results.
Statistical Analysis
Statistical analysis was performed using SPSS (Windows
version 26.0; IBM Corp, Armonk [NY], United States).
The Student’s t test was used for continuous data and
comparison of means. Sonographic features of TNBC
and non-TNBC were compared by Pearson’s Chi squared
tests for categorical data. A statistical significance level
of p < 0.05 was used for all tests.
RESULTS
Demographic and Histopathological Findings
The results of demographic and histopathological
findings are summarised in Table 1. The mean age of the subjects in the TNBC group and non-TNBC group
was similar.
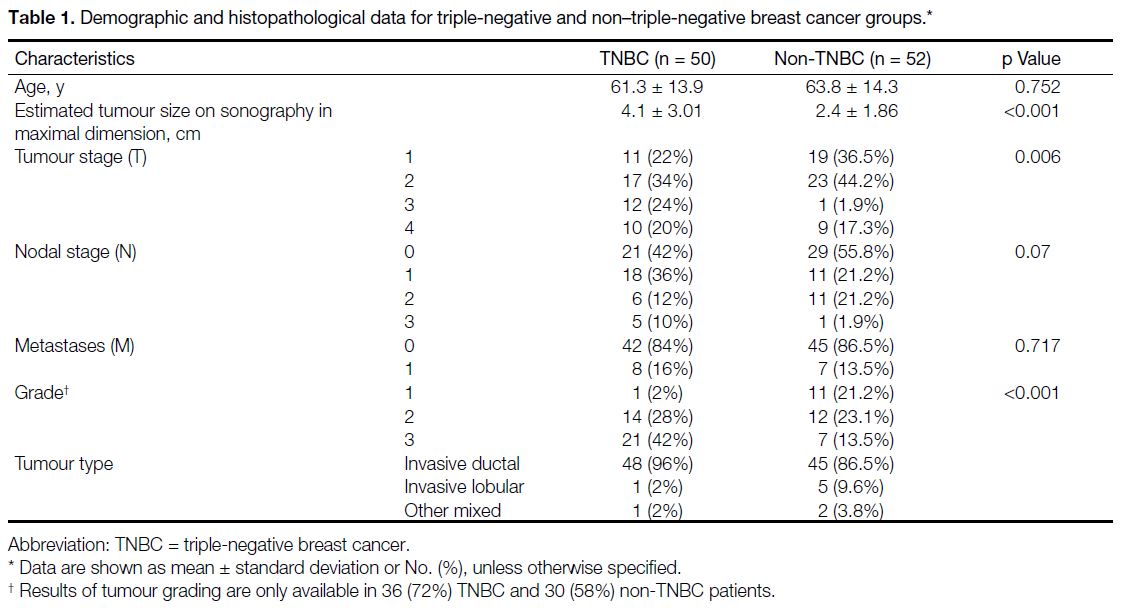
Table 1. Demographic and histopathological data for triple-negative and non–triple-negative breast cancer groups.
The mean tumour size represented by the largest
dimension estimated by sonography was significantly
larger in the TNBC group compared with the non-TNBC
group (4.1 cm vs. 2.4 cm; p < 0.001).
Regarding the tumour, node, and metastasis staging, the
TNBC group had a significantly higher tumour (T) stage
(p = 0.006) and a tendency to higher nodal (N) stage, (p = 0.07) at diagnosis. There was no significant
difference between the two groups in presence of distant
metastases (M) [p = 0.717]. Regarding the differentiation
of the tumours, results were available for 36 TNBC
(72%) and 30 (58%) non-TNBC lesions. TNBCs were
more likely to be poorly differentiated (Grade 3) than
non-TNBCs (42% vs. 13.5%; p < 0.001). There was
no significant difference between the two groups for
histological subtype of breast cancer.
Sonographic Features
The results of sonographic features of TNBC and non-TNBC groups are summarised in Table 2.
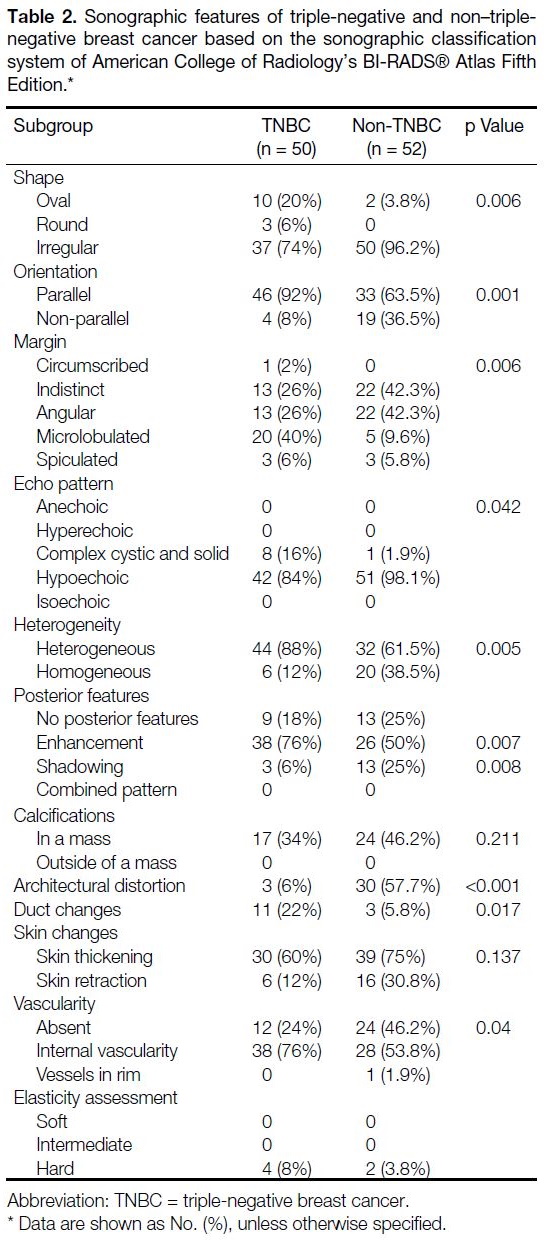
Table 2. Sonographic features of triple-negative and non–triplenegative
breast cancer based on the sonographic classification
system of American College of Radiology’s BI-RADS® Atlas Fifth Edition.
Shape and Orientation
TNBCs were more likely than non-TNBCs to be oval
(20% vs. 3.8%) or round (6% vs. 0%; Figure 1) [p =
0.006], and of parallel orientation (92% vs. 63.5%; p =
0.001).
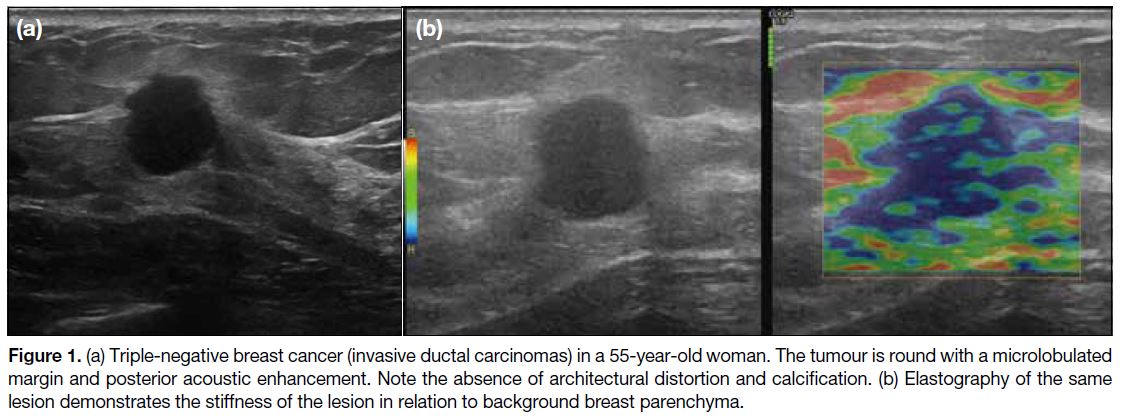
Figure 1. (a) Triple-negative breast cancer (invasive ductal carcinomas) in a 55-year-old woman. The tumour is round with a microlobulated
margin and posterior acoustic enhancement. Note the absence of architectural distortion and calcification. (b) Elastography of the same
lesion demonstrates the stiffness of the lesion in relation to background breast parenchyma.
Margins
Lesions were classified as either circumscribed or non-circumscribed
in margin. Circumscribed margin was
defined as the presence of an abrupt line surrounding
the entire lesion from the background parenchyma. If
the lesion was non-circumscribed, its margin was further
classified as indistinct, angular, microlobulated or
spiculated.[8] There was a significantly higher incidence of
microlobulated margins (40% vs. 9.6%) [Figures 2 3 4],
and significantly lower incidence of indistinct (26% vs.
42.3 %) and angular margins (26% vs. 42.3%) in TNBC
group compared with non-TNBC group (p = 0.006).
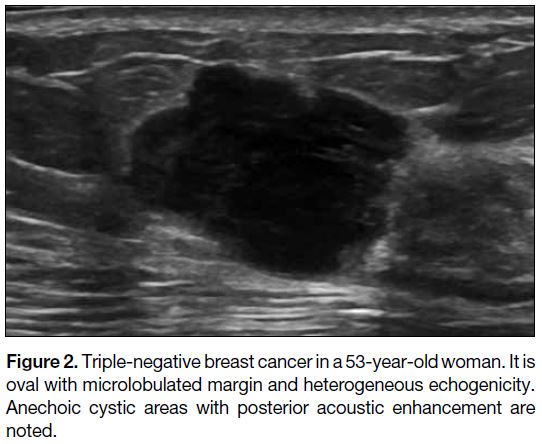
Figure 2. Triple-negative breast cancer in a 53-year-old woman. It is
oval with microlobulated margin and heterogeneous echogenicity.
Anechoic cystic areas with posterior acoustic enhancement are
noted.
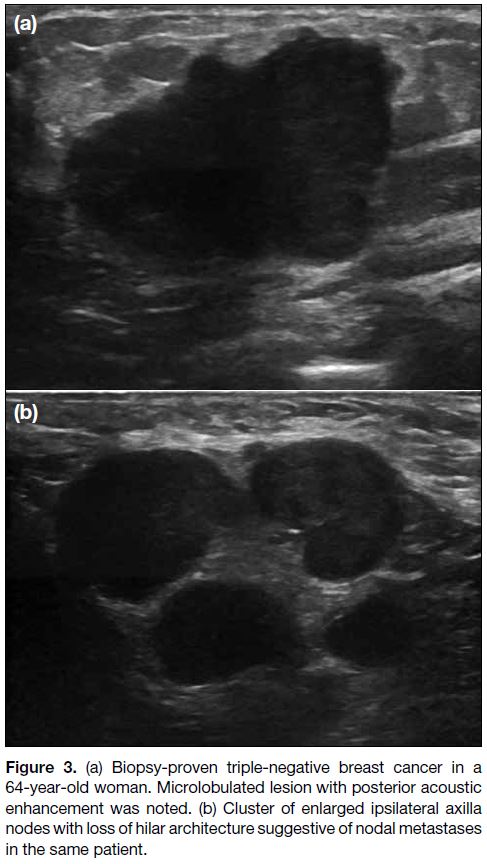
Figure 3. (a) Biopsy-proven triple-negative breast cancer in a
64-year-old woman. Microlobulated lesion with posterior acoustic
enhancement was noted. (b) Cluster of enlarged ipsilateral axilla
nodes with loss of hilar architecture suggestive of nodal metastases
in the same patient.
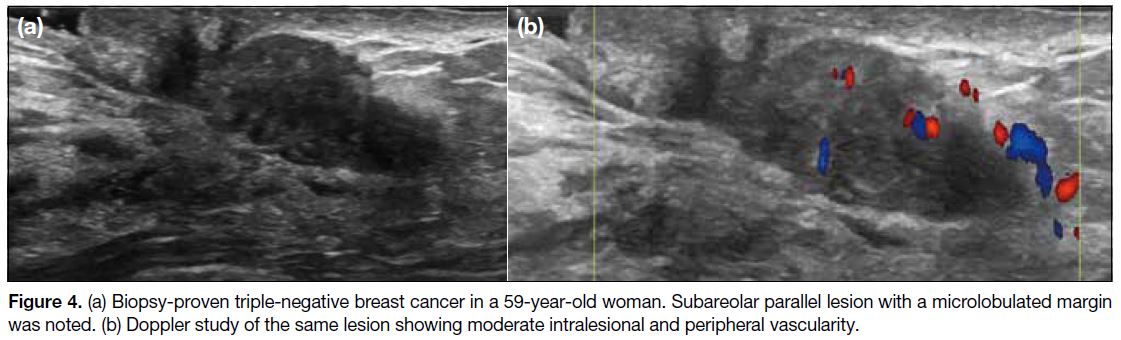
Figure 4. (a) Biopsy-proven triple-negative breast cancer in a 59-year-old woman. Subareolar parallel lesion with a microlobulated margin
was noted. (b) Doppler study of the same lesion showing moderate intralesional and peripheral vascularity.
Echo Pattern and Posterior Acoustic Features
TNBCs were more likely than non-TNBCs to be complex
cystic and solid (16% vs. 1.9%; p = 0.042) [Figure 5] and heterogeneous (88% vs. 61.5%; p = 0.005) in
appearance. They were also more likely to have posterior
acoustic enhancement (76% vs. 50%; p = 0.007) and less
posterior acoustic shadowing (6% vs. 25%; p = 0.008).
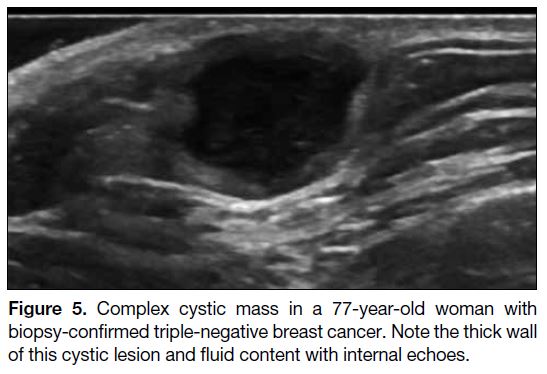
Figure 5. Complex cystic mass in a 77-year-old woman with
biopsy-confirmed triple-negative breast cancer. Note the thick wall
of this cystic lesion and fluid content with internal echoes.
Architectural Distortion, Duct Changes and Skin
Changes
Architectural distortion was less common in TNBC
group (6% vs. 57.7%, p < 0.001), but more ductal changes
(22% vs. 5.8%, p = 0.017) were observed. There was
no significant difference in the presence of skin changes
between the two groups.
Vascularity, Calcification, and Elasticity
Assessment
TNBCs tended to be more vascular than non-TNBCs,
of which most showed internal vascularities (76%
vs. 53.8%; p = 0.04). There was a tendency for less
calcification in TNBCs but the result was not significant
(34% vs. 46.2%; p = 0.211). Also, data for elastography were only available
for four TNBC lesions and two non-TNBC lesions.
All were stiff.
DISCUSSION
The results of our study revealed that the patient’s age at diagnosis for TNBC and non-TNBC was similar,
although not statistically significant (p = 0.752). This
is contrary to previous studies in which TNBC patients
were usually younger at diagnosis.[11] [12] [13] [17] [18] [19] The difference
could be due to the different ethnicity of subjects, i.e.,
only Asians were included in our study. The tumour (T) stage and histological grade were both higher in TNBC
group with a tendency towards higher nodal (N) stage, suggesting more aggressive disease at diagnosis. Early
detection of this aggressive subtype of breast cancer
therefore has an important prognostic implication.
Accurate sonographic detection and subsequent guided
biopsy are vital to early tumour identification.
Previous meta-analyses[19] have shown that TNBC lacks
the typical malignant sonographic features of breast
cancer, including features of irregular shape, non-circumscribed
margin, non-parallel orientation, posterior
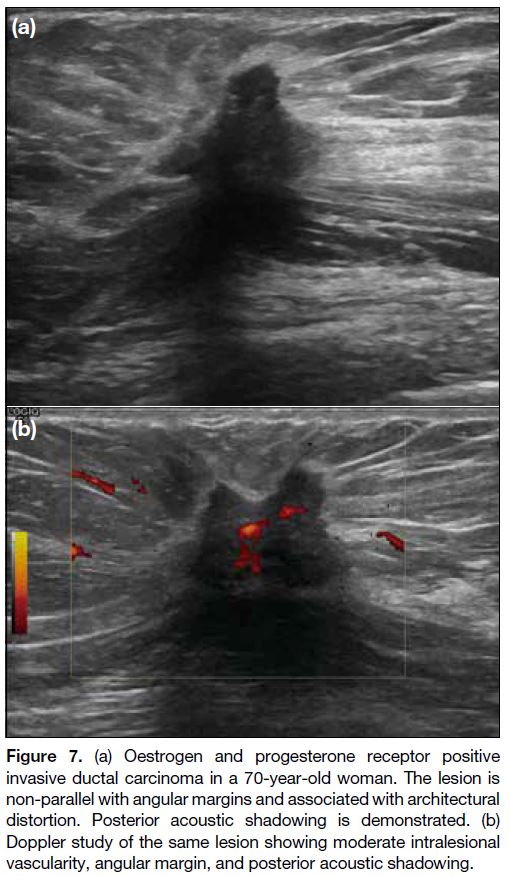
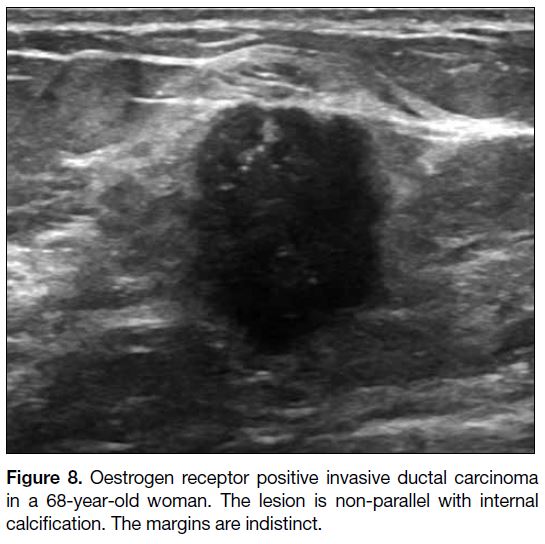
acoustic shadowing, and microcalcification (Figures 6 7 8).
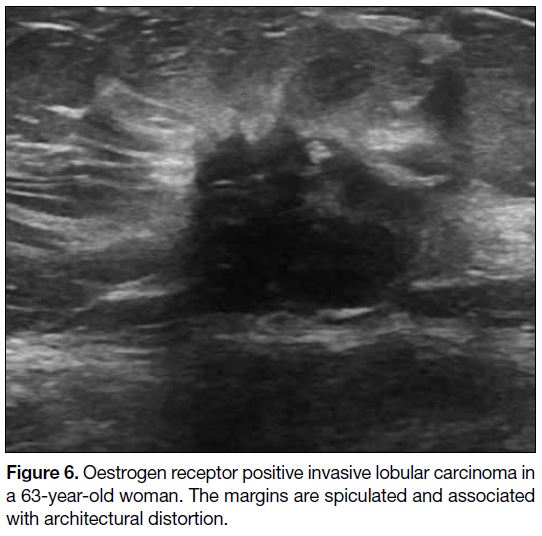
Figure 6. Oestrogen receptor positive invasive lobular carcinoma in
a 63-year-old woman. The margins are spiculated and associated
with architectural distortion.
Figure 7. (a) Oestrogen and progesterone receptor positive
invasive ductal carcinoma in a 70-year-old woman. The lesion is
non-parallel with angular margins and associated with architectural
distortion. Posterior acoustic shadowing is demonstrated. (b)
Doppler study of the same lesion showing moderate intralesional
vascularity, angular margin, and posterior acoustic shadowing.
Figure 8. Oestrogen receptor positive invasive ductal carcinoma
in a 68-year-old woman. The lesion is non-parallel with internal
calcification. The margins are indistinct.
In our study, TNBCs were significantly more likely to be
parallel in orientation, associated with posterior acoustic
enhancement and with a lack of architectural distortion
compared with non-TNBC breast cancers. Although
similar to non-TNBCs in that both subtypes of cancers
are most commonly irregular in shape, the prevalence
of oval or round shape was still higher in TNBC group
than non-TNBC group (Table 2 and Figure 1). This is in
concordance with previous studies.[9] [10] [11] [12] [13] [15] [16] [17] [20] The above
sonographic features are usually regarded as benign
features in ACR BI-RADS, in contrast to the aggressive
nature of this subtype of tumour. The relatively benign
sonographic appearance of TNBC can probably be
explained by their rapid cellular proliferation and
therefore reduced likelihood of sufficient time to induce
stromal reactions,[20] with a consequent typical growth
pattern of a ‘pushing border’ in the absence of infiltration.
Fortunately, there are distinctive features that allow
TNBC to be differentiated from benign lesions such
as fibroadenoma. In our study, the incidence of a
circumscribed appearance in TNBC was lower than
that reported elsewhere.[20] The sonographic features
of margin were diverse and included microlobulated,
indistinct, angular and spiculated, of which the incidence
of microlobulated margins was highest (Figures 2 3 4).
This is in accordance with some previous studies[10] [11] [15]
although others also reported ill-defined margin as the
most commonly occurring margin.[20] The microlobulated
margin is a useful sonographic feature to distinguish
TNBC from benign lesions, and this appearance is again
explained by the pushing margin phenomenon.
TNBC has a significantly more heterogeneous echo
pattern than non-TNBC. This could be partially explained
by the larger size of TNBC lesions in our cohort, where
lesion matrix could be more easily evaluated than in
smaller-sized non-TNBC lesions that usually appear
homogeneous in echogenicity at their early stage. For
the same reason, a significantly higher incidence of
intralesional vascularity on Doppler ultrasound could be
identified in the larger-sized TNBC lesions than the non-TNBC lesions.
There were also significantly more TNBC lesions that
were complex cystic and solid in echo pattern (Figure 5).
This could be due to a higher tumour grade with
more necrosis and thus fluid in the lesion. The same
phenomenon also accounts for the increased incidence
of posterior acoustic enhancement in TNBC.[21]
Similar to other studies, although the incidence of
calcification was lower in TNBC group than non-TNBC
group in this cohort, it was not a rare feature in either
and the difference was not statistically significant
(Table 2 and Figure 8).[11] [13] [16] The pathological basis of
microcalcification is partial necrosis and local ischaemia.
Nonetheless sonography is not the most optimal tool to
evaluate the presence and morphology of calcifications.
These features are better seen on complementary
mammography. Calcification is not a useful feature to
distinguish between TNBC and non-TNBC.
We observed a higher incidence of duct distension in
TNBC lesions. This feature has not been reported or
evaluated in previous studies. In our cohort, the size of TNBC lesions associated with duct distension was not
significantly different to those without duct distension
(mean diameter 3.26 cm and 4.31 cm for TNBC and non-TNBC groups, respectively). Further studies will
evaluate the relationship between TNBC and duct
changes.
Limitations of Our Study
Our study was limited by the relatively small sample
size. It was a retrospective study and therefore some
parameters were not measured or documented during
examination (e.g., elastography), limiting full evaluation
and comprehensive comparison. The ultrasound images
were evaluated by consensus reading by two reviewers
and therefore inter-observer agreement was not assessed.
Further prospective studies with larger sample size and
evaluation by individual observers may aid in arriving at
more consistent and significant results.
CONCLUSIONS
TNBC has its own distinct sonographic features,
enabling it to be distinguished from its non-TNBC
counterparts. Most of our findings from our local
population echoed those of previous studies. By
identifying the distinguishing sonographic features of
TNBC, radiologists can be alerted to the need for early
biopsy when these features are encountered and reach a
definitive diagnosis.
REFERENCES
1. Gluz O, Liedtke C, Gottschalk N, Pusztai L, Nitz U, Harbeck N.
Triple-negative breast cancer — current status and future
directions. Ann Oncol. 2009;20:1913-27. Crossref
2. Dawood S. Triple-negative breast cancer: epidemiology and management options. Drugs. 2010;70:2247-58. Crossref
3. Howard FM, Olopade OI. Epidemiology of triple-negative breast cancer: a review. Cancer J. 2021;27:8-16. Crossref
4. Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429-34. Crossref
5. Isakoff SJ. Triple-negative breast cancer: role of specific
chemotherapy agents. Cancer J. 2010;16:53-61. Crossref
6. Denkert C, Liedtke C, Tutt A, von Minckwitz G. Molecular
alterations in triple-negative breast cancer — the road to new
treatment strategies. Lancet. 2017;389:2430-42. Crossref
7. Diana A, Franzese E, Centonze S, Carlino F, Della Corte CM,
Ventriglia J, et al. Triple-negative breast cancers: systematic
review of the literature on molecular and clinical features with
a focus on treatment with innovative drugs. Curr Oncol Rep.
2018;20:76. Crossref
8. Mendelson EB, Böhm-Vélez M, Berg WA, Whitman GJ, Feldman MI, Madjar H, et al. ACR BI-RADS®
Ultrasound. In: ACR BI-RADS® Atlas, Breast Imaging Reporting
and Data System, 5th edition. Reston, VA, American College of
Radiology; 2013.
9. Wojcinski S, Soliman AA, Schmidt J, Makowski L, Degenhardt F,
Hillemanns P. Sonographic features of triple-negative and non-triple-
negative breast cancer. J Ultrasound Med. 2012;31:1531-41. Crossref
10. Yang Q, Liu HY, Liu D, Song YQ. Ultrasonographic features of
triple-negative breast cancer: a comparison with other breast cancer
subtypes. Asian Pac J Cancer Prev. 2015;16:3229-32. Crossref
11. Wang D, Zhu K, Tian J, Li Z, Du G, Guo Q, et al. Clinicopathological
and ultrasonic features of triple-negative breast cancers: a
comparison with hormone receptor–positive/human epidermal
growth factor receptor-2-negative breast cancers. Ultrasound Med
Biol. 2018;44:1124-32. Crossref
12. Li Z, Tian J, Wang X, Wang Y, Wang Z, Zhang L, et al. Differences
in multi-modal ultrasound imaging between triple negative
and non-triple negative breast cancer. Ultrasound Med Biol.
2016;42:882-90. Crossref
13. Kim MY, Choi N. Mammographic and ultrasonographic features
of triple-negative breast cancer: a comparison with other breast
cancer subtypes. Acta Radiol. 2013;54:889-94. Crossref
14. Boisserie-Lacroix M, Macgrogan G, Debled M, Ferron S,
Asad-Syed M, McKelvie-Sebileau P, et al. Triple-negative breast
cancers: associations between imaging and pathological findings
for triple-negative tumors compared with hormone receptor–positive/human epidermal growth factor receptor-2-negative breast
cancers. Oncologist. 2013;18:802-11. Crossref
15. Pu H, Zhao LX, Yao MH, Xu G, Liu H, Xu HX, et al. Conventional
US combined with acoustic radiation force impulse (ARFI)
elastography for prediction of triple-negative breast cancer and
the risk of lymphatic metastasis. Clin Hemorheol Microcirc.
2017;65:335-47. Crossref
16. Li JW, Zhang K, Shi ZT, Zhang X, Xie J, Liu JY, et al. Triple-negative
invasive breast carcinoma: the association between the
sonographic appearances with clinicopathological feature. Sci Rep.
2018;8:9040. Crossref
17. Ko ES, Lee BH, Kim HA, Noh WC, Kim MS, Lee SA. Triple-negative
breast cancer: correlation between imaging and
pathological findings. Eur Radiol. 2010;20:1111-7. Crossref
18. Çelebi F, Pilancı KN, Ordu Ç, Ağacayak F, Alço G, İlgün S, et al.
The role of ultrasonographic findings to predict molecular subtype,
histologic grade, and hormone receptor status of breast cancer.
Diagn Interv Radiol. 2015;21:448-53. Crossref
19. Tian L, Wang L, Qin Y, Cai J. Systematic review and meta-analysis of the malignant ultrasound features of triple-negative breast cancer.
J Ultrasound Med. 2020;39:2013-25. Crossref
20. Krizmanich-Conniff KM, Paramagul C, Patterson SK, Helvie MA,
Roubidoux MA, Myles JD, et al. Triple receptor-negative breast
cancer: imaging and clinical characteristics. AJR Am J Roentgenol.
2012;199:458-64. Crossref
21. Costantini M, Belli P, Bufi E, Asunis AM, Ferra E, Bitti GT.
Association between sonographic appearances of breast cancers and
their histopathologic features and biomarkers. J Clin Ultrasound.
2016;44:26-33. Crossref