Absolute Lymphocyte Count in Cervical Cancer Patients Prior to Definitive Chemoradiotherapy: a Prognostic Indicator?
ORIGINAL ARTICLE CME
Absolute Lymphocyte Count in Cervical Cancer Patients Prior to Definitive Chemoradiotherapy: a Prognostic Indicator?
EYH Chuk, JCH Chow, KM Cheung, SSW Tse, RCY Ho, HY Wong, ANY Yeung, KH Wong
Department of Clinical Oncology, Queen Elizabeth Hospital, Hong Kong
Correspondence: Dr EYH Chuk, Department of Clinical Oncology, Queen Elizabeth Hospital, Hong Kong. Email: cyh087@ha.org.hk
Submitted: 6 Sep 2021; Accepted: 7 Jan 2022.
Contributors: All authors designed the study, acquired the data, analysed the data, drafted the manuscript, and critically revised the manuscript
for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for publication, and
take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: The research was approved by the ethics committee of Kowloon Central Cluster/Kowloon East Cluster of the Hospital
Authority (Ref: KC/KE-20-0246/ER-2) and conducted in compliance with the Declaration of Helsinki. Informed consent of patients was
waived because of the retrospective nature of the study.
Declaration: Part of this study was presented at the ESMO Asia Virtual Congress 2020 and is published online (https://doi.org/10.1016/j.annonc.2020.10.235).
Abstract
Objective
Baseline lymphopenia is associated with poor prognosis in various malignancies. This study aimed to
examine the prognostic value of pretreatment lymphocyte count in cervical cancer patients in Hong Kong.
Methods
A cohort of 198 cases of cervical cancer patients without evidence of metastatic disease (i.e., International
Federation of Gynecology and Obstetrics stage IB to IVA), who completed definitive chemoradiotherapy from
January 2009 to December 2014 was analysed. Baseline clinical and pretreatment blood test data were collected.
Definitive treatment had included external radiotherapy and brachytherapy with concurrent weekly cisplatin
40 mg/m2. Log-rank tests and multivariable Cox regression were used to evaluate the association between
haematological parameters and survival. Study endpoints were overall survival (OS), recurrence-free survival (RFS),
and late radiation-induced grade 3-4 toxicity.
Results
Median follow-up period was 6.52 years. A pretreatment absolute lymphocyte count ≤1.7 × 109/L was
associated with a significantly worse 5-year OS (68.7% vs. 84.4%, p = 0.005). Multivariate analysis confirmed
pretreatment lymphocyte count to be an independent predictor of RFS (adjusted hazard ratio = 0.58; 95% confidence
interval [CI] = 0.34-0.99, p = 0.046) and OS (adjusted hazard ratio = 0.47; 95% CI = 0.25-0.88, p = 0.018). Absolute
lymphocyte count was not associated with late grade 3-4 radiation toxicity.
Conclusion
Our data in a local cohort add evidence to findings in other studies that pretreatment absolute
lymphocyte count is an independent predictor of both OS and RFS in cervical cancer patients receiving definitive
chemoradiotherapy.
Key Words: Lymphopenia; Lymphocyte count; Chemoradiotherapy; Cervical cancer; Prognostic factor
中文摘要
子宮頸癌病人在接受根治性放化療前的淋巴細胞絕對值:預後指標?
祝菀馨、周重行、張嘉文、謝思華、賀俊義、黃馨誼、楊雅茵、黃錦洪
目的
基線淋巴細胞減少症與多種惡性腫瘤的預後不佳有關。本研究旨在找出香港子宮頸癌病人在接受治療前的淋巴細胞數的預後價值。
方法
本研究分析了198例子宮頸癌病人個案,沒有證據顯示他們有轉移性疾病(即國際婦產科協會分期IB期至IVA期),於2009年1月至2014年12月期間完成根治性放化療。研究收集了基線臨床及治療前的血液檢查數據。根治性治療包括了體外放射治療及腔內治療,同時每星期使用40 mg/m2順鉑。本研究採用了對數等級檢定及多變項Cox迴歸分析來找出血液學參數與存活之間的關係,並以總生存率、無復發生存率及晚期3至4級放射毒性為研究終點。
結果
中位隨訪期為6.52年。治療前的淋巴細胞絕對值≤1.7 × 109/L與明顯較差的五年存活率有關(68.7%比84.4%,p = 0.005)。多變項分析確認了治療前的淋巴細胞數能獨立預測無復發生存率(調整風險比 = 0.58;95% 置信區間 = 0.34-0.99,p = 0.046)及總生存率(調整風險比 = 0.47;95% 置信區間 = 0.25-0.88,p = 0.018)。淋巴細胞絕對值與晚期3至4級放射毒性不相關。
結論
本研究利用本港病人的數據,得出與先前研究結果一致的結論,即治療前的淋巴細胞絕對值能獨立預測接受根治性放化療的子宮頸癌病人之總生存率及無復發生存率。
INTRODUCTION
According to the World Health Organization’s statistics
in 2021, cervical cancer is the fourth most common
cancer in the female population. In 2018, it was
estimated that 570,000 women were diagnosed with
cervical cancer and approximately 311,000 patients died
of the disease.[1] Unprecedented progress in oncological
management has been seen in the last decade, with the
emergence of immunotherapy and adoptive cell transfer
therapy.[2] [3] However, management of cervical cancer
around the world is still performed with chemotherapy
and radiotherapy, usually in a combined way
(chemoradiotherapy). Given the toxicity of definitive
chemoradiotherapy, enhanced knowledge of prognostic
factors for better patient selection is warranted.
Lymphopenia has been suggested to reflect low host
immune reactivity.[4] The tumour microenvironment has
been of interest in tumour immunology. Although not
directly reflecting tumour microenvironment, peripheral
lymphocytes, especially cytotoxic T lymphocytes,[5] are
critical to anti-tumour immunity. The neutrophil-to-lymphocyte
ratio, platelet-to-lymphocyte ratio, and
lymphocyte-to–white blood cell count percentage[6] [7] [8] have
been shown to be of prognostic value in cancer outcomes. Pretreatment lymphopenia in cancer outcomes has been
found to be associated with poor prognosis in colorectal
cancer, breast cancer, renal carcinoma, bladder cancer,
sarcomas and gynaecological cancers.[9] [10] [11] [12] [13] This study
aimed to add evidence on the prognostic value of
pretreatment lymphopenia in cervical cancers in a local
cohort.
METHODS
This was a retrospective cohort study conducted in
a tertiary clinical oncology centre in Hong Kong. A
total of 218 consecutive patients with primary cervical
cancer who completed definitive chemoradiotherapy
from January 2009 to December 2014 were analysed.
Definitive chemoradiotherapy consisted of concurrent
weekly cisplatin 40 mg/m2, external beam radiotherapy
comprising 40 Gy in 20 fractions with high-dose-rate
intracavitary brachytherapy twice weekly for four
fractions up to 7 Gy per fraction at point A (2 cm
lateral to the central uterine canal and 2 cm from the
mucous membrane of the lateral fornix in the axis
of the uterus) and additional pelvic irradiation and
parametrial boost up to a total of 64 to 68 Gy at point
B (which was designated as 5 cm from midline at the
level of point A) according to the recommendations of the International Commission on Radiation Units and
Measurements[13] [14]; aiming for a total biologically EQD2
(equivalent dose delivered in 2 Gy fractions) of 80 Gy
to the tumour and limiting the dose to bladder and
rectum to EQD2 of 75 Gy. Pretreatment investigations
included physical examination, comprehensive baseline
blood investigations, magnetic resonance imaging of
the pelvis, computed tomography of thorax, abdomen
and pelvis or positron emission tomography/computed
tomography, and endoscopic examination including
sigmoidoscopy/cystoscopy in cases of suspected
mucosal invasion. Patients were staged according to the
2018 FIGO (International Federation of Gynecology and
Obstetrics) criteria. The study inclusion criteria consisted
of: (1) histologically confirmed cervical cancer; (2)
FIGO stage IB to IVA; (3) completion of treatment; and
(4) pretreatment blood counts available in our records.
Patients were excluded if they had (1) synchronous
malignancy at baseline; (2) pre-existing autoimmune
diseases; or (3) defaulted or failed to complete treatment.
Patients were followed up by physical examination
and interval imaging at 3- to 6-month intervals in the
first 2 years after treatment and every 6 to 12 months
subsequently.
Data Collection
Clinical variables, including patients’ baseline
demographics and clinicopathologic and treatment
details, were collected from the electronic patient
record system in our institution. Pretreatment blood
values, including absolute neutrophil counts, absolute
lymphocyte counts (ALCs), haemoglobin levels, and
absolute platelet counts were collected. A Charlson
Comorbidity Index[15] was calculated for each patient as a
reference for baseline comorbidity. Grade 3-4 toxicities
were classified according to The National Cancer
Institute’s Common Terminology Criteria for Adverse
Events (CTCAE), version 5.0 of the United States.
Statistical Analysis
The abovementioned clinical variables were summarised
with descriptive statistics. A low pretreatment ALC was
defined as lower than the median of the pretreatment
ALC in the patient sample, i.e., ≤1.7 × 109/L. This
pretreatment lymphocyte cut-off was based on previous
pilot studies that attempted to find the optimal cut-off
in baseline haematological parameters.[11] The primary
outcomes of the study were recurrence-free survival
(RFS) and overall survival (OS). RFS was calculated
from the start of treatment to the date of first evidence of
recurrence or death. OS was defined as the time period from the date of start of treatment to the date of last
follow-up or death. RFS and OS were estimated using
the Kaplan–Meier method and were compared using
the log-rank test. Clinically known prognostic factors
were also evaluated using multivariate Cox regression.
The association between low and high pretreatment
lymphocyte counts with grade 3-4 toxicity was evaluated
with the Chi squared test. All statistical analyses were
performed with commercial software SPSS (Windows
version 24.0; IBM Corp, Armonk [NY], United States).
All p values were two-sided and a p value <0.05 was
considered statistically significant. The STROBE
checklist for observational studies was used.
RESULTS
A total of 198 patients undergoing definitive
chemoradiotherapy in our institution from January
2009 to December 2014 met our inclusion criteria. The
median follow-up period for our cohort was 6.52 years.
Demographics, clinicopathological and treatment details
for the study cohort are summarised in Table 1.
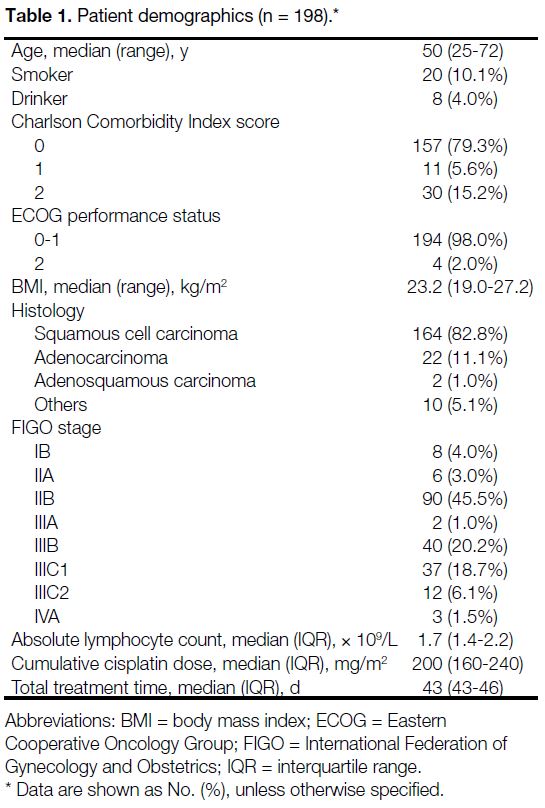
Table 1. Patient demographics (n = 198).
The median age of the population was 50 years (range,
25-72). The majority of our study patients were non-smokers,
non-drinkers, had good baseline comorbidities
with a Charlson Comorbidity Index of 0 and an Eastern
Cooperative Oncology Group performance score of 0
to 1. The histology of the cohort consisted of squamous
cell carcinoma (82.8%), adenocarcinoma (11.1%),
adenosquamous carcinoma (1.0%), and others (5.1%).
Out of the 198 patients, eight patients (4.0%) had stage
IB disease, 96 (48.5%) had stage II disease, 42 (21.2%)
had stage IIIA-B disease, 49 (24.7%) had stage IIIC
disease and three (1.5%) had stage IVA disease (i.e.,
invasion of adjacent organs). The cohort had a median
cumulative cisplatin dose of 200 mg/m2 (interquartile
range, 160-240) and a median total treatment time of
43 days (interquartile range, 43-46).
The median pretreatment ALC was 1.7 × 109/L. Patients with pretreatment ALC ≤1.7 × 109/L were classified as
the ‘low pretreatment ALC group’ whereas patients with
pretreatment ALC >1.7 × 109/L were classified as the
‘high pretreatment’ ALC group.
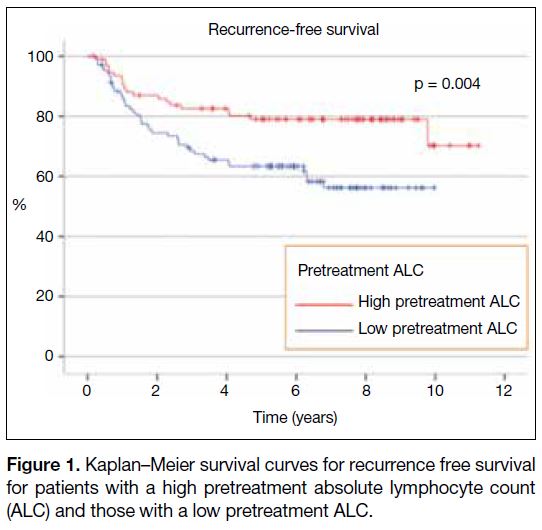
At the median follow-up period of 6.52 years, the 5-year RFS was 63.4% in the low pretreatment ALC group
compared to 79.0% for those in the high pretreatment
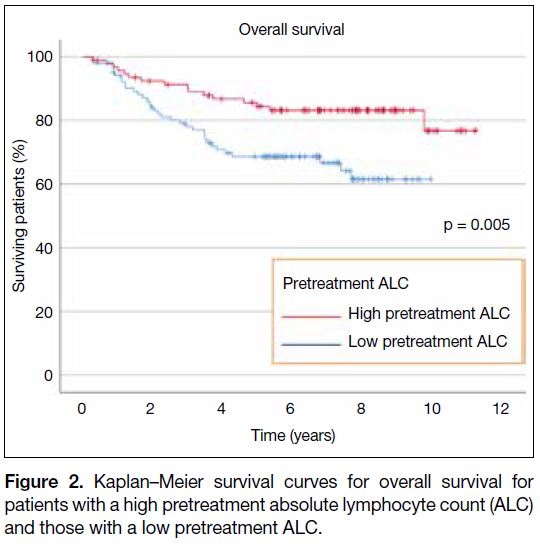
ALC group (p = 0.004; Figure 1). The 5-year OS was
68.7% in the low pretreatment ALC group compared to
84.4% in the high pretreatment ALC group (p = 0.005;
Figure 2).
Figure 1. Kaplan–Meier survival curves for recurrence free survival
for patients with a high pretreatment absolute lymphocyte count
(ALC) and those with a low pretreatment ALC.
Figure 2. Kaplan–Meier survival curves for overall survival for
patients with a high pretreatment absolute lymphocyte count (ALC)
and those with a low pretreatment ALC.
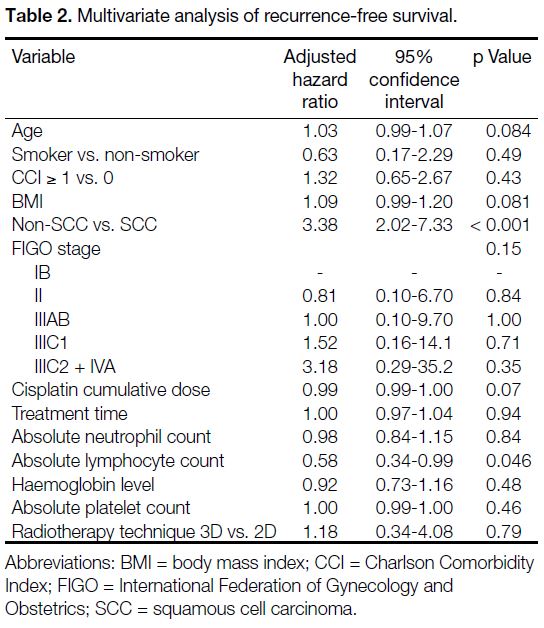
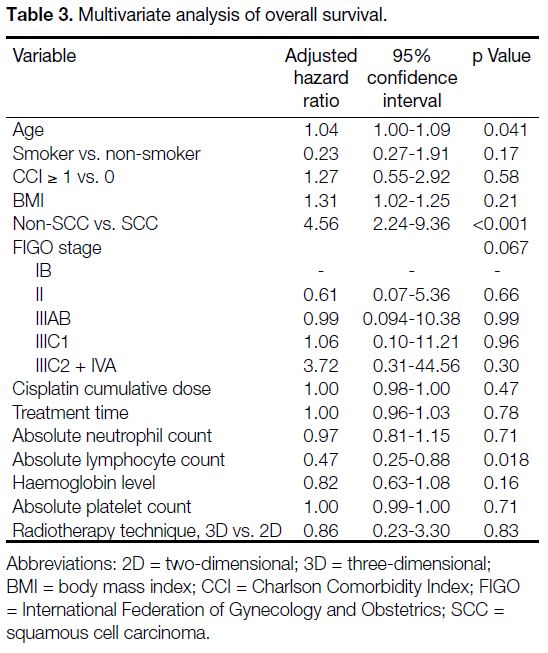
On multivariate analysis (Table 2), a high pretreatment
ALC remained an independent prognostic factor for
longer RFS with an adjusted hazard ratio (HR) of 0.58
(95% confidence interval [CI] = 0.34-0.99; p = 0.046).
Squamous cell carcinoma was also associated with longer
RFS (p < 0.001). As shown in Table 3, high pretreatment
ALC remained an independent predictor of longer OS
in multivariate analysis (adjusted HR = 0.47; 95% CI =
0.25-0.88, p = 0.018). Negative prognostic factors for OS
included old age (adjusted HR = 1.04; 95% CI = 1.00-1.09; p = 0.041) and non-squamous histology (adjusted
HR = 4.56; 95% CI = 2.24-9.36; p < 0.001).
Table 2. Multivariate analysis of recurrence-free survival.
Table 3. Multivariate analysis of overall survival.
The distribution of cisplatin dose was similar across
the two groups (p = 0.139). Cumulative cisplatin dose
was not an independent prognostic factor for either RFS
or OS. In all, 52 out of 94 patients (55.3%) in the high
pretreatment ALC group needed chemotherapy dose
reduction, compared to 63 out of 104 patients (60.6%)
in the low pretreatment ALC group (p = 0.32). In total, 18 out of 94 patients (19.1%) in the high pretreatment
ALC group experienced grade 3-4 toxicity, compared to
13 out of 104 patients (12.5%) in the low pretreatment
ALC group (p = 0.199).
We explored the relationship of local RFS and distant
recurrence-free survival (DRFS) in the two groups. The
5-year DRFS was 72.4% in the low pretreatment ALC
group compared to 83.1% in the high pretreatment ALC group (p = 0.047). The 5-year local RFS was 85.0%
in the group with low pretreatment ALC, compared to
92.0% for those with high pretreatment ALC (p = 0.065).
DISCUSSION
In our study, we found that low pretreatment ALC was
associated with inferior RFS of borderline significance
in cervical cancer patients who underwent definitive
chemoradiotherapy. OS was significantly improved in
the high pretreatment ALC group. The prognostic value
of pretreatment ALC in cervical cancer was independent
of other major prognostic factors across cancer stages
and regardless of chemotherapy intensity. This finding
is consistent with multiple studies, which established the
relationship between pretreatment ALC with survival
outcome of multiple solid tumours, including tumours of
the cervix[16] [17] [18] and haematological malignancies.[19]
The cut-off for the pretreatment ALC was defined as
a median of 1.7 × 109/L in our patient population. To
our knowledge, there is no consensus as to the cut-off
between high or low pretreatment ALC.[20] Previous
studies on prognostic impact of ALC had found the
median ALC of the study population to be an independent
prognostic factor of survival.[18] According to a cohort
study by den Ouden et al[21] comparing haematological
abnormalities of metastatic and benign ovarian tumours,
the authors found that the median lymphocyte counts of
the malignant group (1.2 g/L) were significantly lower
than those in the benign tumour (1.8 g/L) and age-matched
control groups (2 g/L) with p values of 0.02
and 0.00005, respectively. Although the study was not
done in cervical cancer patients, it reflects that cancer
patients often have intrinsically lower pretreatment
ALC. In 2016, Cho et al[17] studied the prognostic
value of lymphopenia according to the CTCAE grade
during chemoradiotherapy in cervical cancer. Grade
4 lymphopenia during chemoradiotherapy predicted
a significantly shorter disease-specific survival and
progression-free survival (PFS). The authors also found
that patients with Grade 4 lymphopenia had relatively
lower baseline ALC despite not statistically significant
(p = 0.07) and a more rapid decrease during treatment.[17]
It is known that concurrent chemoradiotherapy might
have both a positive effect on sustaining peripheral
lymphocytes by tumour control and a deleterious effect
from lymphocyte depletion in the radiation portal.[22] This
suggests that ALC before, during, and after treatment is
probably reflective of baseline disease extent, treatment
toxicity, and treatment response.
A systematic review and meta-analysis based on 42
studies by Zhao et al[16] evaluated the pretreatment ALC
cut-off; the largest effect size was observed with a
cut-off of ≤1.0 × 109/L, followed by a cut-off between >1.0 and 2.0 × 109/L. Their high ALC cut-off (>2.0 ×
109/L) subgroup was not associated with poorer OS.[16]
They found similar results on subgroup analysis of
PFS.[16] This shows a trend for a lower pretreatment ALC
cut-off for a larger effect on the prognostic value of
pretreatment ALC. The pretreatment ALC cut-off used
in our patient population falls into the range reported.
Some authors had arbitrarily chosen to use CTCAE as
cut-off for pretreatment ALC but this was less applicable
to this study, as pretreatment ALC was the study interest
instead of treatment-related lymphopenia. This reflects
the complexity of finding a definitive cut-off for clinical
utility; however, this does not diminish the importance
of pretreatment ALC as a prognostic factor of survival
outcomes.
In 2016, Wu et al[18] conducted a cohort study of
lymphopenia in 71 patients and its association with
locally advanced cervical cancer. They found that
subjects with low pretreatment ALC <1 × 103/L and
persistent lymphopenia <500 cells/mm3 2 months after
initiating treatment tended towards shorter OS, though
not to a statistically significant degree on multivariate
analysis, contrary to our finding of pretreatment ALC
being an independent prognostic factor of OS. This
might be explained by their smaller study population of
71 patients and of which only 47 had ALC documented
2 months after initiating treatment.[18] Another cohort
study by Jeong et al[23] found pretreatment lymphocyte
percentage (calculated as the proportion of the ALCs
in the total white blood cell count) predictive of PFS
and OS; however it also did not remain significant in
multivariate analysis for OS.
The mechanisms that govern the relationship between
pretreatment ALC and poorer treatment outcomes are
likely multifactorial. In our study, we found no significant
association among pretreatment ALC, chemotherapy
tolerance, and treatment-related toxicities. Therefore,
the negative survival outcomes in patients with low
pretreatment ALC were not mediated by suboptimal
therapy nor by treatment complications. Lower
pretreatment ALC was associated with poorer DRFS
in solid cancers,[8] [10] although it was only of borderline
significance in our cohort.
Lymphopenia and decrease in T and B lymphocyte
subpopulations leads to a lower ability to activate an
effective antitumour cellular immune response.[24] T
lymphocytes drive cancer cell apoptosis,[25] and induce
cancer cell death in response to chemotherapy by presenting tumour-associated antigens to immune cells.[26]
Current concepts of tumour immunoediting explain
the interplay between tumour growth and the cellular
immune system.[27] Tumour growth is suppressed by a host
of immune cells including natural killer T cells and CD8+
T cells. It is followed by an equilibrium phase, where
tumour cells withstand the selection pressure of immune
cells. Tumour cells then escape from the immune system
by inhibition of immune cells or inducing tolerance.[28]
The authors postulated that a low pretreatment
absolute lymphocyte cell count would reflect a lower
ability of the host’s immune response to react to the
tumour. Another possible mechanism is a reduction in
cytokine production. Circulating lymphocytes produce
cytokines to inhibit tumour growth.[29] The balance
between immunostimulatory cytokines and blocking of
immunosuppressive cytokines facilitates antitumoural
immune responses.[30] Failure to maintain the cytokine
response tips the equilibrium towards tumour
proliferation. A low pretreatment ALC could represent a
depleted host immune state that leads to a poorer ability to
respond to the subsequent treatment. Pre-clinical studies
have shown that a depletion of CD8+ T cells significantly
reduced treatment efficacy of radiotherapy.[31] Failure to
stimulate the innate and adaptive immunity negatively
impacts treatment responses to radiotherapy.[32] Given
that the exact levels of CD8+ T cells and CD4+ T cells
are not easily measurable in clinical practice, peripheral
pretreatment ALC may act as a surrogate to reflect the
robustness of the host immune system.
Peripheral pretreatment ALC may act as a surrogate to
reflect the robustness of the host immune system. This
study contributes to the evidence of utilising pretreatment
ALC as a prognostic factor clinically.
There are several limitations of our study. First, this was a retrospective cohort study with limited cohort size which
made it an exploratory analysis. Second, the choice of
median as the cut-off for high and low pretreatment ALC
was based on observation and further external validation
is warranted to confirm our findings. Third, the choice
of brachytherapy practised in our institution during
the study period adopted the conventional Manchester
system instead of image-guided brachytherapy, which
is now the standard of care that improves pelvic control
and reduces treatment toxicities.[33] However, this
should not diminish the importance of this study as the
prognostic relationship of pretreatment ALC focuses
on the immune mechanism towards tumourigenesis
and antitumour responses. The choice of brachytherapy would be unlikely to affect the prognostic implication of
pretreatment ALC.
Cytokine boost has been a subject of interest given
the plethora of evidence supporting better treatment
responses with a strong innate and adaptive immune
system. Cervical cancer is one of the best-known
cancers related to chronic viral infection, specifically
with the human papillomavirus (HPV) types 16 and 18,
making them an attractive target for immunotherapy.[34] [35] [36]
Studies have shown that enhanced CD4+ and CD8+ T
cell expression in response to HPV type 16 E7 peptides
was associated with better treatment prognosis in HPV-positive
oropharyngeal cancer.[36]
CONCLUSION
In conclusion, our study shows the independent
prognostic value of pretreatment ALC in OS and RFS
in cervical cancer patients. Further tumour immunology
investigations are warranted to explore the mechanism
underlying pretreatment ALC and treatment outcomes.
Raising pretreatment ALC by cytokine boost may be a
valuable direction in improving cervical cancer treatment
outcomes.
REFERENCES
1. World Health Organization. Cervical cancer. Available from:
https://www.who.int/health-topics/cervical-cancer#tab=tab_1.
Accessed 20 Jun 2021.
2. Caldwell KJ, Gottschalk S, Talleur AC. Allogeneic CAR cell therapy—more than a pipe dream. Front Immunol. 2021;11:618427. Crossref
3. June CH, O’Connor RS, Kawalekar OU, Ghassemi S, Milone MC.
CAR T cell immunotherapy for human cancer. Science.
2018;359:1361-5. Crossref
4. Patel S, Chiplunkar S. Host immune responses to cervical cancer.
Curr Opin Obstet Gynecol. 2009;21:54-9. Crossref
5. Tindle RW. Immune evasion in human papillomavirus-associated
cervical cancer. Nat Rev Cancer. 2002;2:59-65. Crossref
6. Howard R, Kanetsky PA, Egan KM. Exploring the prognostic
value of the neutrophil-to-lymphocyte ratio in cancer. Sci Rep.
2019;9:19673. Crossref
7. Bardash Y, Olson C, Herman W, Khaymovich J, Costantino P,
Tham T. Platelet-lymphocyte ratio as a predictor of prognosis in
head and neck cancer: a systematic review and meta-analysis. Oncol
Res Treat. 2019;42:665-77. Crossref
8. Kim GM, Koh HD, Kim JH, Park BW, Cho YU, Kim SI, et al.
Baseline lymphocyte counts predict distant recurrence in early
breast cancer. Ann Oncol. 2018;29(Suppl 8):viii62-3. Crossref
9. Joseph N, Dovedi SJ, Thompson C, Lyons J, Kennedy J, Elliott T,
et al. Pre-treatment lymphocytopaenia is an adverse prognostic
biomarker in muscle-invasive and advanced bladder cancer. Ann
Oncol. 2016;27:294-9. Crossref
10. Vicente Conesa MA, Garcia-Martinez E, Gonzalez Billalabeitia E,
Chaves Benito A, Garcia Garcia T, Vicente Garcia V, et al.
Predictive value of peripheral blood lymphocyte count in breast
cancer patients treated with primary chemotherapy. Breast.
2012;21:468-74. Crossref
11. Cho O, Noh OK, Oh YT, Chang SJ, Ryu HS, Lee EJ, et al.
Hematological parameters during concurrent chemoradiotherapy as
potential prognosticators in patients with stage IIB cervical cancer.
Tumour Biol. 2017;39:1010428317694306. Crossref
12. Ray-Coquard I, Cropet C, Van Glabbeke M, Sebban C, Le Cesne A,
Judson I, et al. Lymphopenia as a prognostic factor for overall
survival in advanced carcinomas, sarcomas, and lymphomas.
Cancer Res. 2009;69:5383-91. Crossref
13. Lee LJ, Sadow CA, Russell A, Viswanathan AN. Correlation of
point B and lymph node dose in 3D-planned high-dose-rate cervical
cancer brachytherapy. Int J Radiat Oncol Biol Phys. 2009;75:803-9. Crossref
14. Nag S, Erickson B, Thomadsen B, Orton C, Demanes JD,
Petereit D. The American Brachytherapy Society recommendations
for high-dose-rate brachytherapy for carcinoma of the cervix. Int J
of Radiation Oncol Biol Phys. 2000;48:201-11. Crossref
15. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method
of classifying prognostic comorbidity in longitudinal studies:
development and validation. J Chronic Dis. 1987;40:373-83. Crossref
16. Zhao J, Huang W, Wu Y, Luo Y, Wu B, Cheng J, et al. Prognostic
role of pretreatment blood lymphocyte count in patients with solid
tumors: a systematic review and meta-analysis. Cancer Cell Int.
2020;20:15. Crossref
17. Cho O, Chun M, Chang SJ, Oh YT, Noh OK. Prognostic value of
severe lymphopenia during pelvic concurrent chemoradiotherapy
in cervical cancer. Anticancer Res. 2016;36:3541-7.
18. Wu ES, Oduyebo T, Cobb LP, Cholakian D, Kong X, Fader AN,
et al. Lymphopenia and its association with survival in patients with
locally advanced cervical cancer. Gynecol Oncol. 2016;140:76-82. Crossref
19. Kusano Y, Yokoyama M, Terui Y, Nishimura N, Mishima Y, Ueda
K, et al. Low absolute peripheral blood CD4+ T-cell count predicts
poor prognosis in R-CHOP-treated patients with diffuse large B-cell
lymphoma. Blood Cancer J. 2017;7:e558. Crossref
20. Prochazka V, Trneny M, Salek D, Belada D, Kozak T, Papajik T,
et al. Median absolute lymphocyte count independently predicts
survival of elderly patients with diffuse large B-cell lymphoma
treated with R-chemotherapy: analysis of 651 patients included in
the Czech lymphoma project. Blood. 2010;116:2882. Crossref
21. den Ouden M, Ubachs JM, Stoot JE, van Wersch JW. Whole blood
cell counts and leucocyte differentials in patients with benign or
malignant ovarian tumours. Eur J Obstet Gynecol Reprod Biol.
1997;72:73-7. Crossref
22. Yovino S, Grossman SA. Severity, etiology and possible
consequences of treatment-related lymphopenia in patients with
newly diagnosed high-grade gliomas. CNS Oncol. 2012;1:149-54. Crossref
23. Jeong MH, Kim H, Kim TH, Kim MH, Kim BJ, Ryu SY. Prognostic
significance of pretreatment lymphocyte percentage and age at
diagnosis in patients with locally advanced cervical cancer treated
with definite radiotherapy. Obstet Gynecol Sci. 2019;62:35-45. Crossref
24. Ménétrier-Caux C, Ray-Coquard I, Blay JY, Caux C. Lymphopenia
in cancer patients and its effects on response to immunotherapy: an
opportunity for combination with cytokines? J Immunother Cancer.
2019;7:85. Crossref
25. Dworacki G, Meidenbauer N, Kuss I, Hoffmann TK, Gooding W,
Lotze M, et al. Decreased zeta chain expression and apoptosis in
CD3+ peripheral blood T lymphocytes of patients with melanoma.
Clin Cancer Res. 2001;7(3 Suppl):947s-57s.
26. Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A,
et al. Toll-like receptor 4-dependent contribution of the immune
system to anticancer chemotherapy and radiotherapy. Nat Med.
2007;13:1050-9. Crossref
27. Vesely MD, Schreiber RD. Cancer immunoediting: antigens,
mechanisms, and implications to cancer immunotherapy. Ann N
Y Acad Sci. 2013;1284:1-5. Crossref
28. Galon J, Bruni D. Tumor immunology and tumor evolution: intertwined histories. Immunity. 2020;52:55-81. Crossref
29. Berraondo P, Sanmamed MF, Ochoa MC, Etxeberria I, Aznar MA,
Pérez-Gracia JL, et al. Cytokines in clinical cancer immunotherapy.
Br J Cancer. 2019;120:6-15. Crossref
30. Shimizu K, Iyoda T, Okada M, Yamasaki S, Fujii SI. Immune
suppression and reversal of the suppressive tumor microenvironment.
Int Immunol. 2018;30:445-54. Crossref
31. Chen HY, Xu L, Li LF, Liu XX, Gao JX, Bai YR. Inhibiting
the CD8+ T cell infiltration in the tumor microenvironment after
radiotherapy is an important mechanism of radioresistance. Sci
Rep. 2018;8:11934. Crossref
32. Burnette BC, Liang H, Lee Y, Chlewicki L, Khodarev NN,
Weichselbaum RR, et al. The efficacy of radiotherapy relies upon
induction of type I interferon-dependent innate and adaptive
immunity. Cancer Res. 2011;71:2488-96. Crossref
33. Sturdza A, Pötter R, Fokdal LU, Haie-Meder C, Tan LT,
Mazeron R, et al. Image guided brachytherapy in locally
advanced cervical cancer: improved pelvic control and survival in
RetroEMBRACE, a multicenter cohort study. Radiother Oncol.
2016;120:428-33. Crossref
34. Atherton MJ, Stephenson KB, Pol J, Wang F, Lefebvre C,
Stojdl DF, et al. Customized viral immunotherapy for HPV-associated
cancer. Cancer Immunol Res. 2017;5:847-59. Crossref
35. Stevanović S, Draper LM, Langhan MM, Campbell TE, Kwong ML,
Wunderlich JR, et al. Complete regression of metastatic cervical
cancer after treatment with human papillomavirus-targeted tumor-infiltrating
T cells. J Clin Oncol. 2015;33:1543-50. Crossref
36. Masterson L, Lechner M, Loewenbein S, Mohammed H, Davies-Husband C, Fenton T, et al. CD8+ T cell response to
human papillomavirus 16 E7 is able to predict survival outcome in
oropharyngeal cancer. Eur J Cancer. 2016;67:141-51. Crossref