Scapulothoracic Dissociation in a Patient with Polytrauma: a Case Report
CASE REPORT
Scapulothoracic Dissociation in a Patient with Polytrauma: a Case Report
HM Kwok, ES Lo, NY Pan, RLS Chan, SC Wong, LF Cheng, JKF Ma
Department of Diagnostic and Interventional Radiology, Princess Margaret Hospital, Hong Kong
Correspondence: Dr HM Kwok, Department of Diagnostic and Interventional Radiology, Princess Margaret Hospital, Hong Kong. Email: khm778@ha.org.hk
Submitted: 18 Feb 2021; Accepted: 4 Jun 2021.
Contributors: HMK and NYP designed the study. HMK acquired the data. HMK and ESL analysed the data and drafted the manuscript. HMK,
ESL, NYP, RLSC, LFC and JKFM critically revised the manuscript for important intellectual content. SCW did the final approval. All authors
had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and
integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: The patient was treated in accordance with the tenets of the Declaration of Helsinki. The requirement for patient consent was waived by the Kowloon West Cluster Research Ethics Committee [Ref: KW/EX-21-056(157-21)].
Acknowledgement: The authors would like to thank the Department of Orthopaedics and Traumatology, Princess Margaret Hospital, Hong Kong for acute management for the patient.
INTRODUCTION
Scapulothoracic dissociation (SD) is a rare but severe
injury to the shoulder girdle. It is characterised by
complete disruption of the scapulothoracic articulation
with lateral scapular displacement and intact skin.[1] [2] [3] It
is a spectrum of musculoskeletal and neurovascular
injuries,[3] involving high-energy trauma with lateral
tractional forces applied to the shoulder girdle.[2] [4]
Scapular Index (SI) is an indicator that is relevant to
SD. It is obtained by measuring the distance from the
spinous process to the medial border of the scapula, then
divide the value of the injured side by the value of the
non-injured side. Laterally displaced scapula with SI >1
has been commonly used as a diagnostic criterion for
SD in previous studies.[5] Nonetheless this requires a non-rotated
anteroposterior (AP) chest radiograph that may
be impractical in the urgent trauma setting. Moreover,
SD may be initially missed in the polytrauma setting
with multiple significant injuries.[2] Herein, we report
a case of SD in a young adult who was involved in a road traffic accident with polytrauma presenting with
absent brachial pulse and significant vascular injuries
on initial trauma computed tomography (CT) with CT
angiogram.
CASE REPORT
In December 2020, a 23-year-old man was admitted to
the Accident and Emergency Department of Princess
Margaret Hospital, Hong Kong having been found
lying on the ground after his motorcycle was involved
in a traffic accident. On admission, he had stable vital
signs with 98% oxygen saturation on 2 L/min oxygen.
Left-sided pneumothorax was detected and chest drain
insertion was required. There was left shoulder swelling
and left forearm deformity. The left brachial, radial,
and ulnar pulses were all non-palpable with absence of
Doppler signal on bedside ultrasound. Delayed capillary
refill time was also evident. Other limb pulses were
normal. The Glasgow Coma Scale score was 3/15 and
the patient was intubated for airway protection.
Supine AP chest radiograph on admission was
significantly rotated and measurement of the SI was not
feasible. Left acromioclavicular joint dislocation was the
only salient finding on frontal left shoulder radiograph
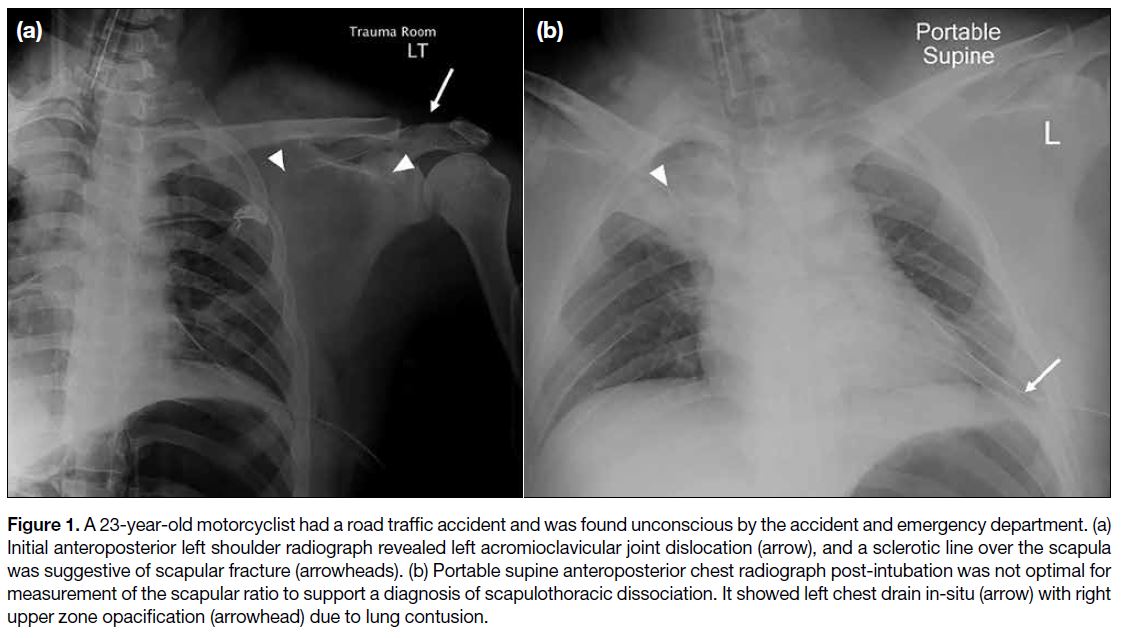
(Figure 1).
Figure 1. A 23-year-old motorcyclist had a road traffic accident and was found unconscious by the accident and emergency department. (a)
Initial anteroposterior left shoulder radiograph revealed left acromioclavicular joint dislocation (arrow), and a sclerotic line over the scapula
was suggestive of scapular fracture (arrowheads). (b) Portable supine anteroposterior chest radiograph post-intubation was not optimal for
measurement of the scapular ratio to support a diagnosis of scapulothoracic dissociation. It showed left chest drain in-situ (arrow) with right
upper zone opacification (arrowhead) due to lung contusion.
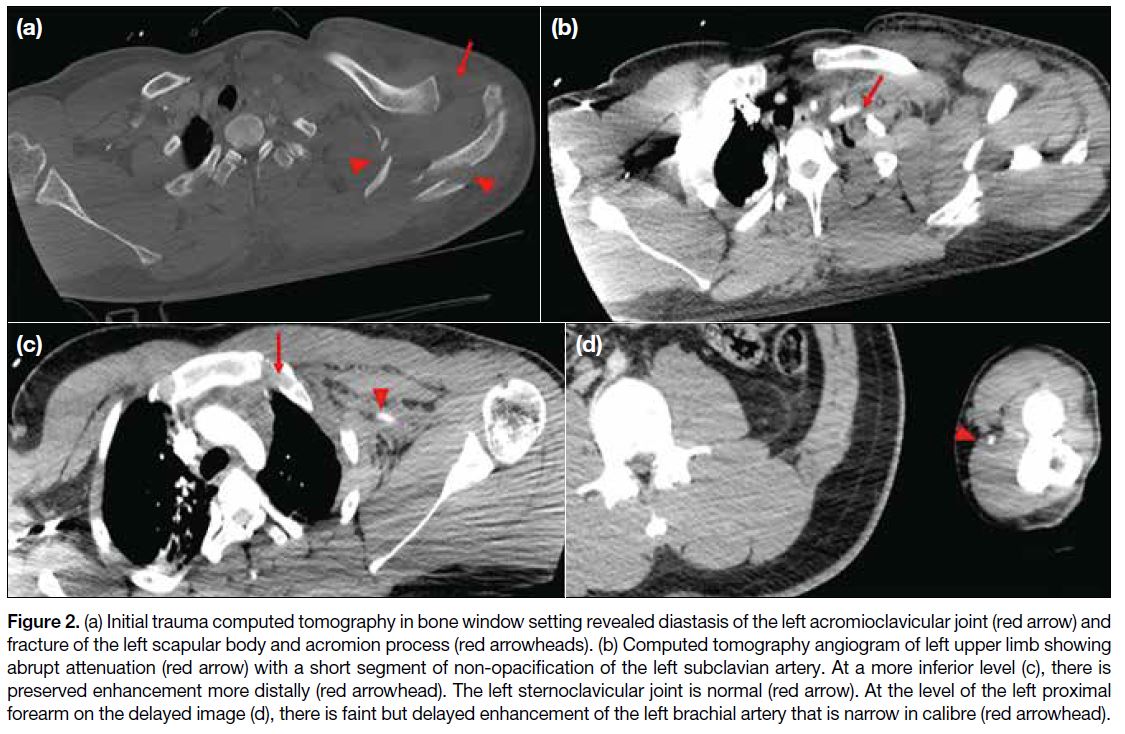
Urgent trauma CT series and CT angiogram (with
delayed images included) of the left upper limb
(Figure 2) revealed lateral displacement and
comminuted fracture of the left scapula, dislocated
left acromioclavicular joint and evidence of subclavian
artery dissection with high-grade thrombosis. There
was also evidence of left subclavian vein injury and left
supraclavicular fossa haematoma. No active contrast
extravasation was detected. The constellation of findings
was suggestive of left SD and associated neurological
injury of the brachial plexus was strongly suspected.
Faint, delayed opacification of the left axillary and
brachial arteries, with non-opacification of distal
branches was evident, suggestive of acute left upper limb
ischaemia.
Figure 2. (a) Initial trauma computed tomography in bone window setting revealed diastasis of the left acromioclavicular joint (red arrow) and
fracture of the left scapular body and acromion process (red arrowheads). (b) Computed tomography angiogram of left upper limb showing
abrupt attenuation (red arrow) with a short segment of non-opacification of the left subclavian artery. At a more inferior level (c), there is
preserved enhancement more distally (red arrowhead). The left sternoclavicular joint is normal (red arrow). At the level of the left proximal
forearm on the delayed image (d), there is faint but delayed enhancement of the left brachial artery that is narrow in calibre (red arrowhead).
Other significant CT findings included a treated small
left pneumothorax, and bilateral lung contusions and
lacerations. Fracture of the left humerus, left radius and
ulna, and left distal femur were also evident. Thin layers
of left perinephric haematoma, perisplenic haematoma, together with the American Association for the Surgery
of Trauma grade II splenic laceration were noted
intraabdominally. A thin layer of subdural haematoma
along the right tentorium cerebelli and posterior cerebral
falx were detected intracranially.
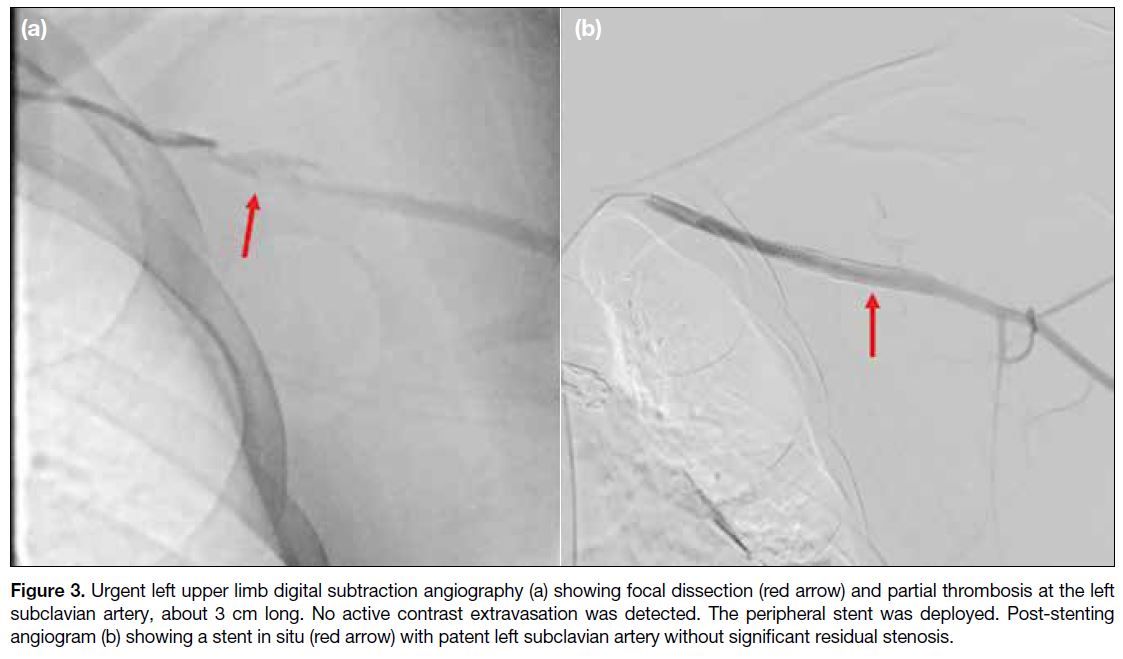
An urgent digital subtraction angiography (Figure 3)
performed on the same day confirmed a short segment
focal dissection at the left mid subclavian artery,
causing proximal flow stagnation. No active contrast
extravasation was seen. Post-stenting left upper limb
angiogram revealed a patent left subclavian artery
without significant residual stenosis. Clinically there was
normalisation of capillary refill time.
Figure 3. Urgent left upper limb digital subtraction angiography (a) showing focal dissection (red arrow) and partial thrombosis at the left
subclavian artery, about 3 cm long. No active contrast extravasation was detected. The peripheral stent was deployed. Post-stenting
angiogram (b) showing a stent in situ (red arrow) with patent left subclavian artery without significant residual stenosis.
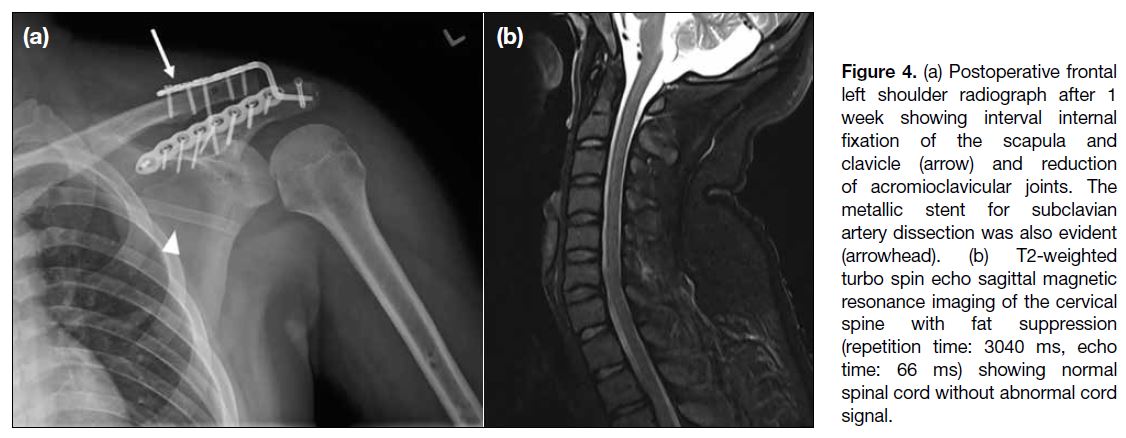
Open reduction and internal fixation of the scapula,
clavicle and left acromioclavicular joint were performed
1 week after the initial injury. With a gradual return of
Glasgow Coma Scale score to 15/15, the patient was
extubated. Magnetic resonance imaging (MRI) of the
cervical spine showed no evidence of spinal cord injury
(Figure 4).
Figure 4. (a) Postoperative frontal left shoulder radiograph after 1 week showing interval internal fixation of the scapula and clavicle (arrow) and reduction of acromioclavicular joints. The metallic stent for subclavian artery dissection was also evident (arrowhead). (b) T2-weighted turbo spin echo sagittal magnetic resonance imaging of the cervical spine with fat suppression (repetition time: 3040 ms, echo time: 66 ms) showing normal spinal cord without abnormal cord signal.
One month after the accident, there was persistent
complete loss of motor and sensory function of the left
upper limb. MRI of the left brachial plexus (Figures 5 and 6) revealed evidence of a high-grade brachial plexus injury with postganglionic injury of C5 but
preganglionic injury at C6 to T1 level. Electromyography
of the left upper limb showed absent motor response of
both ulnar and median nerves, and sensory response of
the ulnar nerve. There was a markedly decreased median
nerve sensory response with preserved conduction
velocity. Overall findings were compatible with left
brachial plexopathy with doubtful viability of the
nerves.
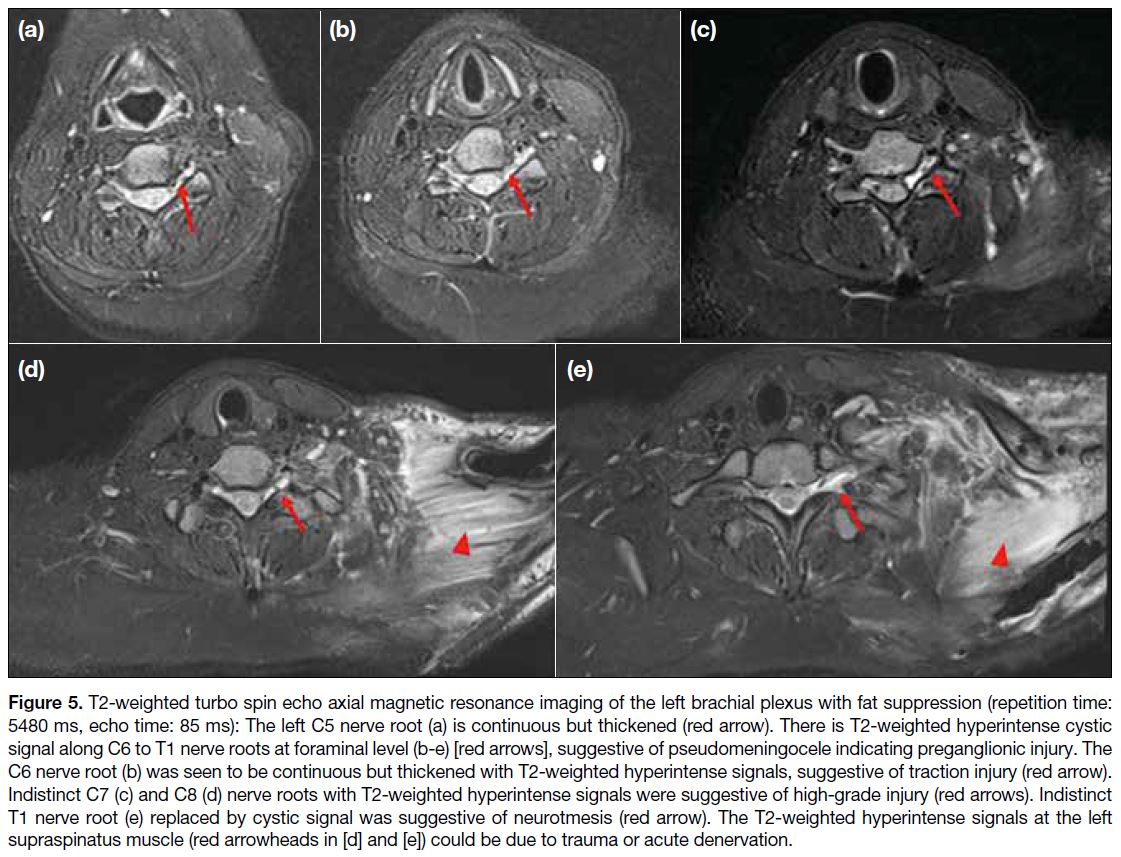
Figure 5. T2-weighted turbo spin echo axial magnetic resonance imaging of the left brachial plexus with fat suppression (repetition time:
5480 ms, echo time: 85 ms): The left C5 nerve root (a) is continuous but thickened (red arrow). There is T2-weighted hyperintense cystic
signal along C6 to T1 nerve roots at foraminal level (b-e) [red arrows], suggestive of pseudomeningocele indicating preganglionic injury. The
C6 nerve root (b) was seen to be continuous but thickened with T2-weighted hyperintense signals, suggestive of traction injury (red arrow).
Indistinct C7 (c) and C8 (d) nerve roots with T2-weighted hyperintense signals were suggestive of high-grade injury (red arrows). Indistinct
T1 nerve root (e) replaced by cystic signal was suggestive of neurotmesis (red arrow). The T2-weighted hyperintense signals at the left
supraspinatus muscle (red arrowheads in [d] and [e]) could be due to trauma or acute denervation.
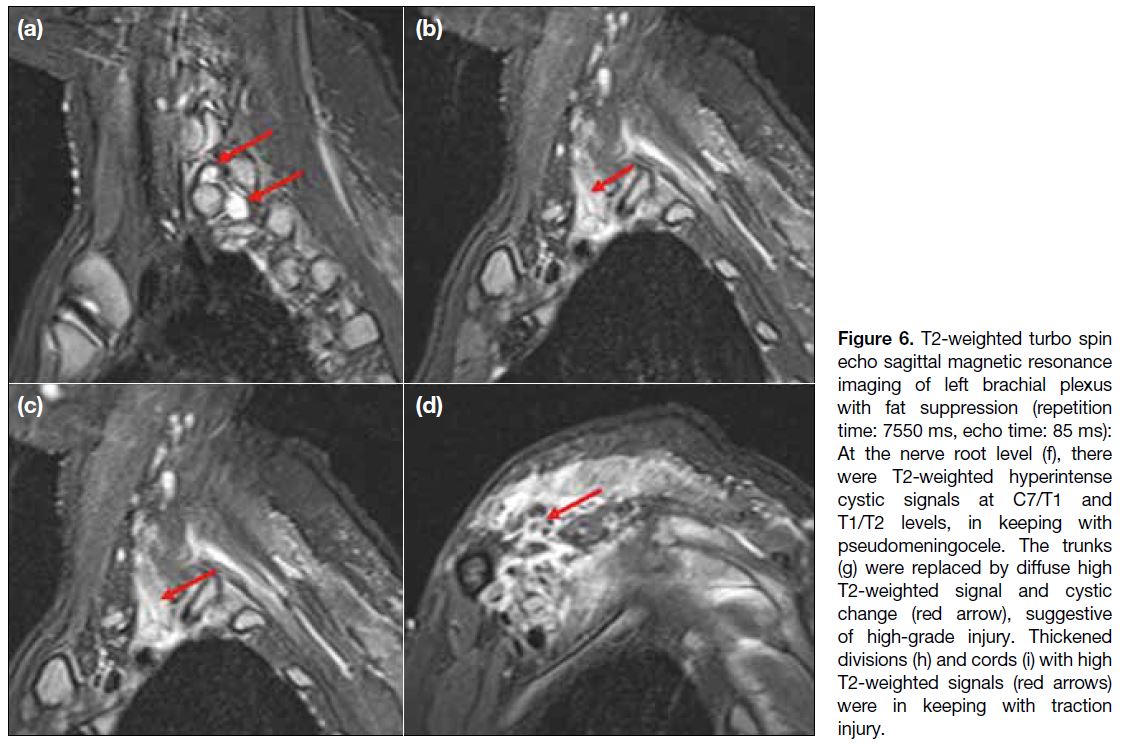
Figure 6. T2-weighted turbo spin echo sagittal magnetic resonance imaging of left brachial plexus with fat suppression (repetition time: 7550 ms, echo time: 85 ms): At the nerve root level (f), there were T2-weighted hyperintense cystic signals at C7/T1 and T1/T2 levels, in keeping with pseudomeningocele. The trunks (g) were replaced by diffuse high T2-weighted signal and cystic change (red arrow), suggestive of high-grade injury. Thickened divisions (h) and cords (i) with high T2-weighted signals (red arrows) were in keeping with traction injury.
The patient commenced rehabilitation of his left
upper limb to preserve elbow, wrist, and distal hand
joint mobility. Nortriptyline 10 mg night time and
pregabalin 300 mg three times daily and at night were
prescribed for neuropathic pain. He was referred to a
subspecialist orthopaedic hand team for brachial plexus
reconstruction.
DISCUSSION
Oreck et al[4] coined the term ‘scapulothoracic
dissociation’ in 1984 to describe an injury involving
complete closed separation of the scapula and upper
extremity from the thoracic attachments. SD is defined as violent lateral or rotational displacement of the shoulder
girdle from its thoracic attachments and causes severe
neurovascular injury.[1] [2] [3]
First, SD is an easily overlooked injury in the polytrauma
setting.[2] [6] It has been suggested that the diagnosis of
SD requires a combination of clinical findings and
radiographic findings that rely heavily on the SI.[3] The SI
is calculated by measuring the distance from the medial
border of the scapula to the thoracic spinous process of
both the injured and uninjured sides on a non-rotated
posteroanterior or AP chest radiograph.[5] The normal
value is 1.07 ± 0.04.[5] A SI >1.29 is consistent with SD
until proven otherwise.[6] The limitation of the need for
a well-centred radiograph for measurement has been
addressed, and no similar technique for CT measurement
has been described.[3] It is impractical to obtain a non-rotated
radiograph due to use of multiple immobilisation
and monitoring devices in the polytrauma setting.
Also, urgent trauma series CT would be performed to
facilitate clinical management in the urgent setting. The
above case nicely illustrates how a diagnosis of SD is
established with typical clinical findings of an absent brachial pulse with CT angiography showing subclavian
artery dissection with thrombosis, together with the
scapular fracture and dislocated acromioclavicular
joint.
Second, SD is a spectrum of injuries that comprises
muscular, osseous, ligamentous, nerve, and vascular
injuries of varying degrees and combination. The high-energy
lateral distraction force disrupts muscular tissues and acromioclavicular ligaments and/or sternoclavicular
ligaments.[4] What radiologists can ‘see’ on initial trauma
CT or radiographs is the ‘tip of the iceberg’, and the
associated neurological injury in particular should alert
the clinical team to a need for subsequent investigation
after initial resuscitation. It is not surprising that SD is
associated with other life-threatening injuries[7] that should
also be reported and prioritised in order to facilitate acute
management.
There is no current universally agreed treatment
algorithm for SD due to its rarity and variation of injury
pattern and presence of systemic injuries.[3] General
principles of polytrauma care with cardiopulmonary
stabilisation and resuscitation should be the top priority.[2]
For SD, the urgency of surgical intervention is determined
by vascular injury and the need to prevent ischaemic
complications, while neurological injury is managed in
a delayed manner.[2] [3] There is little evidence for the best
timing of osseous stabilisation.[3] In haemodynamically
stable patients, angiography is widely recommended
prior to surgery. Nonetheless in haemodynamically
unstable cases, urgent surgical intervention is required
to control arterial bleeding.[2] [8] Prior studies with analysis
of angiographic findings suggested that subclavian
or axillary artery active haemorrhage is exceedingly
rare, and angiography of the injured extremity is
recommended for all haemodynamically stable patients
to determine the presence and location of a vascular lesion.[3] As endovascular repair becomes more popular,[3]
our case illustrates that successful stenting can help
blunt subclavian arterial injury to re-establish upper limb
perfusion in the urgent setting. Sampson et al[9] suggested
a conservative approach to revascularisation for the
arterial injury in SD in view of the dismal functional
outcome of brachial plexus injury. Nonetheless in
the urgent setting with acute upper limb ischaemia
with unknown neurological status of the upper limb,
revascularisation is our preferred approach.
Finally, SD contributes about 10% of the overall
mortality rate and the clinical outcome of patients is
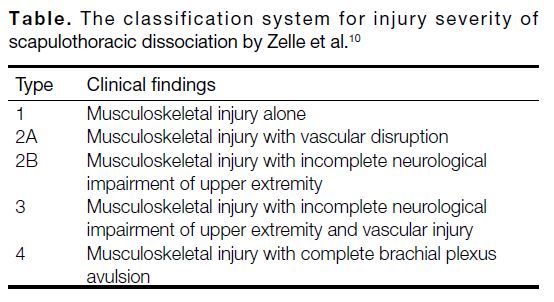
largely determined by neurological recovery.[3] [6] Zelle et al[10]
established a classification system for SD (Table) and
regarded the presence of a complete brachial plexus
avulsion as a predictor of poor functional outcome.
Differentiating a partial from a complete and a
postganglionic from a preganglionic brachial plexus injury is of utmost importance because the injury
type determines the expected chance of spontaneous
neurological recovery and responsiveness to surgical
intervention.[3] The extent of neurological injury is
characterised by clinical findings, CT myelography,
MRI, and electromyography.[3] The presence of a
pseudomeningocele on magnetic resonance images
has been strongly correlated with nerve root avulsion.
Therefore, it is important for radiologists to identify
nerve injuries in the brachial plexus and differentiate
between incomplete and complete avulsion. This will
guide subsequent management and overall prognosis.
Concomitant spinal cord injury, due to direct contusion
or as an indirect sign of nerve root avulsion, should also
be sought.[1] Historically, complete preganglionic injury
has been managed with early above-elbow amputation
with or without shoulder arthrodesis,[1] [3] with consequent
superior functional outcomes.[2] In recent decades, there
has been increasing interest in nerve transfer with
adjacent uninjured donor nerves or neurotisation as a
way of reconstruction, and a means to restore elbow
function, shoulder stability, hand grasp, and sensation.[1]
Table. The classification system for injury severity of
scapulothoracic dissociation by Zelle et al.[10]
CONCLUSION
SD is a limb-threatening and life-threatening injury that
physicians should take care not to overlook in patients
with polytrauma. The highly complex injury spectrum
requires case-to-case multidisciplinary management in
which radiologists play a pivotal role.
REFERENCES
1. Lee GK, Suh KJ, Choi JA, Oh HY. A case of scapulothoracic dissociation with brachial plexus injury: magnetic resonance imaging findings. Acta Radiol. 2007;48:1020-3. Crossref
2. Brucker PU, Gruen GS, Kaufmann RA. Scapulothoracic
dissociation: evaluation and management. Injury. 2005;36:1147-55. Crossref
3. Choo AM, Schottel PC, Burgess AR. Scapulothoracic dissociation:
evaluation and management. J Am Acad Orthop Surg. 2017;25:339-
47. Crossref
4. Oreck SL, Burgess A, Levine AM. Traumatic lateral displacement
of the scapula: a radiographic sign of neurovascular disruption. J
Bone Joint Surg Am. 1984;66:758-63. Crossref
5. Kelbel JM, Jardon OM, Huurman WW. Scapulothoracic dissociation. A case report. Clin Orthop Relat Res. 1986;209:210-4. Crossref
6. Kani KK, Chew FS. Scapulothoracic dissociation. Br J Radiol. 2019;92:20190090. Crossref
7. Jangir R, Misra D. Scapulothoracic dissociation: a rare variant: a
case report. Malays Orthop J. 2014;8:46-8. Crossref
8. Kim JH, Cancelada D, Meghoo CA. Scapulothoracic dissociation:
case report and review of current management. J Surg Educ.
2007;64:174-7. Crossref
9. Sampson LN, Britton JC, Eldrup-Jorgensen J, Clark DE,
Rosenberg JM, Bredenberg CE. The neurovascular outcome of
scapulothoracic dissociation. J Vasc Surg. 1993;17:1083-8. Crossref
10. Zelle BA, Pape HC, Gerich TG, Garapati R, Ceylan B, Krettek C.
Functional outcome following scapulothoracic dissociation. J Bone
Joint Surg Am. 2004;86:2-8. Crossref