Imaging Features of Gastrointestinal Stromal Tumour: Diagnosis and Evaluation of Treatment Response
PICTORIAL ESSAY
Imaging Features of Gastrointestinal Stromal Tumour: Diagnosis and Evaluation of Treatment Response
YT Wong, KY Kwok, OL Chan, SH Lee, ML Tsang
Department of Radiology, Tuen Mun Hospital, Hong Kong
Correspondence: Dr YT Wong, Department of Radiology, Tuen Mun Hospital, Hong Kong. Email: wyt521@ha.org.hk
Submitted: 28 Aug 2021; Accepted: 4 Feb 2022.
Contributors: YTW designed the study. YTW, OLC, SHL and MLT acquired the data. YTW and KYK analysed the data. YTW drafted the
manuscript. All authors critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: The study was approved by the New Territories West Cluster Research Ethics Committee of the Hospital Authority (Ref: NTWC/REC/21076). A waiver for written informed consent of patients was granted by the Committee as this manuscript is for pictorial review
only and does not involve patientʼs treatment/procedure.
INTRODUCTION
Gastrointestinal stromal tumours (GISTs) are the most
common mesenchymal neoplasms of the gastrointestinal
tract and more commonly found in middle-aged patients.
They arise from the interstitial cells of Cajal in the
myenteric plexus and are potential malignancies that
can occur anywhere along the gastrointestinal tract, most
commonly in the stomach (50-60%), followed by the
small intestine (30-35%), colon and rectum (5%), and
oesophagus (<1%).[1] GISTs can also be extraintestinal
and originate in the mesentery, omentum or
retroperitoneum. In the Chinese population, the incidence
among those aged ≥50 years is higher than in those under
50 years old with a mean age at diagnosis of 55.2 years.[2]
Most GISTs have a KIT or platelet-derived growth
factor receptor alpha (PDGFRA) mutation. Neoadjuvant
therapy with imatinib acts by blocking the signalling via
KIT and PDGFRA. Nonetheless, 10% to 15% of GISTs
do not have a detectabletable KIT or PDGFRA mutation and
have a poor response to imatinib. Some are associated
with neurofibromatosis type 1, Carney–Stratakis
syndrome and Carney triad.[3] Biopsy is preferred to
confirm the diagnosis for large resectable tumours or
metastatic GISTs. This article evaluates the radiological
images of pathologically proven GISTs.
RISK STRATIFICATION OF GASTROINTESTINAL STROMAL TUMOURS
There are several guidelines for assessing the malignant potential of GISTs; the most common are the modified
National Institutes of Health criteria and the Armed
Forces Institute of Pathology criteria. In both guidelines,
risk of recurrence varies with tumour size and mitotic
rate. The presence of tumour rupture is an additional
prognostic indicator. Intermediate tumours, i.e., large
tumours with a low mitotic rate or small tumours with
a high mitotic rate, that arise from the stomach have a
more favourable prognosis than those in other parts of
the gastrointestinal tract.[4]
IMAGING FEATURES OF GASTROINTESTINAL STROMAL TUMOURS AT THE TIME OF DIAGNOSIS
General Features
The radiological appearance of GISTs varies depending
on their anatomical location and size. Most GISTs are
submucosal and located in the muscularis propria so
have a propensity for exophytic growth and manifest
as masses outside the organ of origin.[5] GISTs have near-universal expression of CD117 antigen compared
with other submucosal gastrointestinal tract tumours
that are typically CD117 negative.[6] Small GISTs
usually show homogeneous enhancement; larger GISTs
can be heterogeneous with central necrosis or cystic
degeneration. The incidence of GIST rupture is about
7%.[7] Extensive calcification of GISTs is rare with only a
few cases reported in the literature.
Oesophagus
GISTs account for only about 25% of oesophageal
mesenchymal neoplasms, and the oesophagus is the
only site where leiomyomas predominate.[8] GISTs and
oesophageal leiomyomas have overlapping imaging
features although oesophageal GISTs tend to be more
distal in location, larger, and more heterogeneous with a
higher degree of enhancement on computed tomography
(Figures 1 and 2).[9]
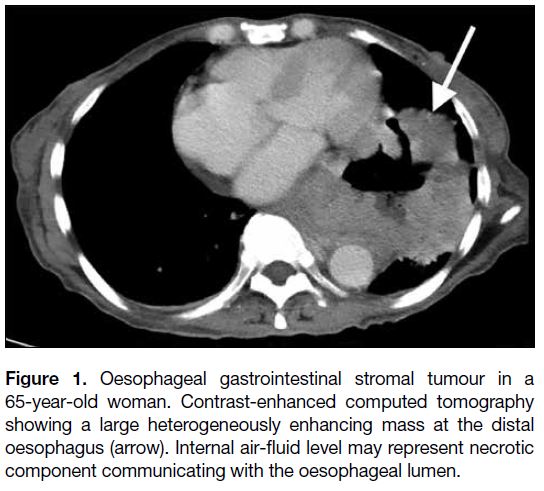
Figure 1. Oesophageal gastrointestinal stromal tumour in a
65-year-old woman. Contrast-enhanced computed tomography
showing a large heterogeneously enhancing mass at the distal
oesophagus (arrow). Internal air-fluid level may represent necrotic
component communicating with the oesophageal lumen.
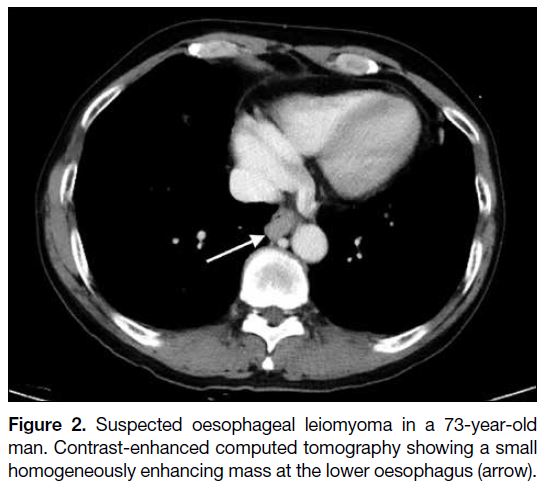
Figure 2. Suspected oesophageal leiomyoma in a 73-year-old
man. Contrast-enhanced computed tomography showing a small
homogeneously enhancing mass at the lower oesophagus (arrow).
The radiological differential diagnoses of oesophageal
GISTs depend on the size and origin of the lesion. For
small mucosal lesions, papilloma and fibrovascular polyp
should be considered. For small submucosal lesions,
leiomyoma and granular cell tumour are the differential
diagnoses. If a tumour is large and aggressive-looking,
carcinoma and leiomyosarcoma need to be considered.
Stomach
The stomach is the most common location of a GIST. In
contrast to small gastric GISTs that are confined to the
organ of origin (Figures 3 and 4a), large gastric GISTs
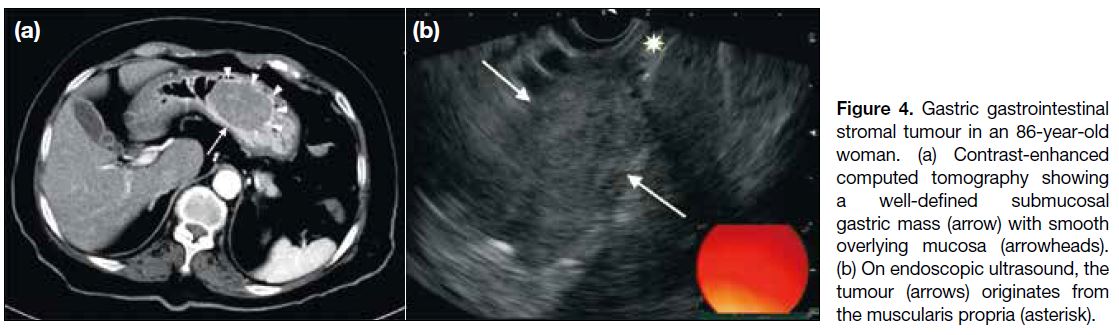
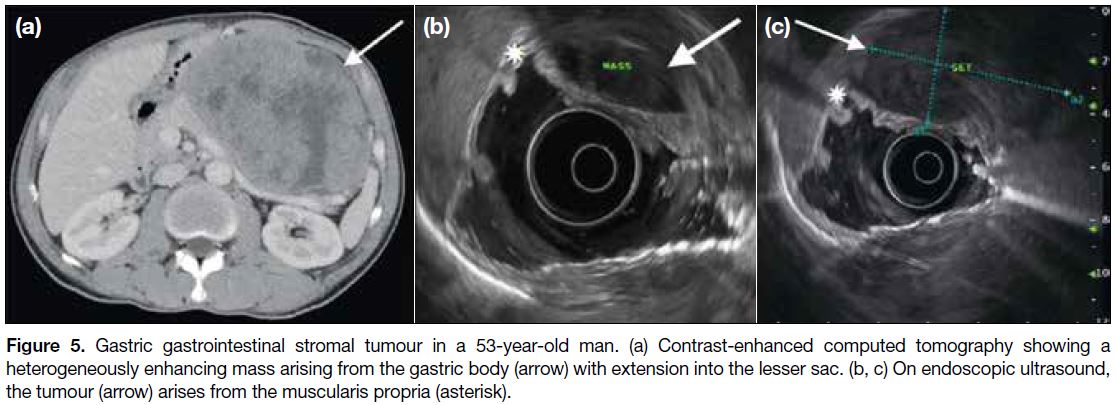
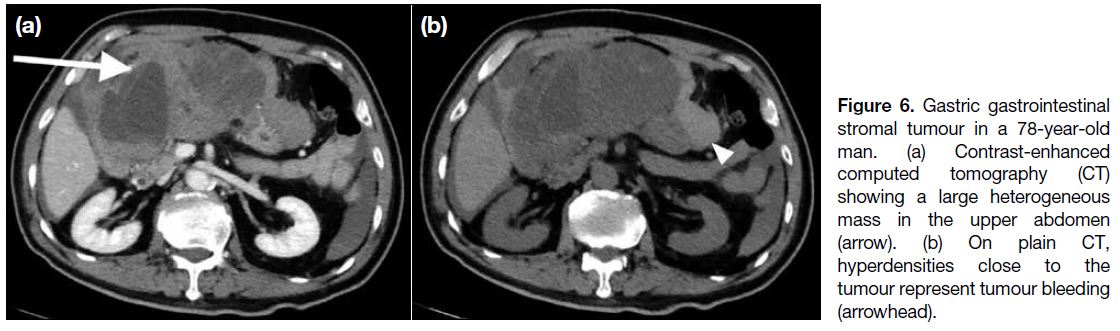
may extend into the gastrohepatic ligament, gastrosplenic ligament or lesser sac (Figure 5a). Endoscopic ultrasonography is useful to identify the layer of origin of the mass (Figures 4b, 5b and 5c). Gastric GISTs may also be complicated by perforation or bleeding
(Figure 6).
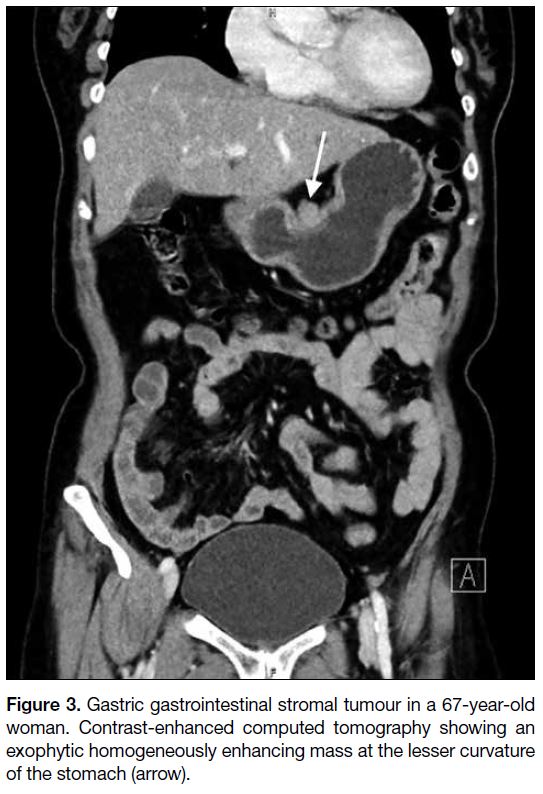
Figure 3. Gastric gastrointestinal stromal tumour in a 67-year-old
woman. Contrast-enhanced computed tomography showing an
exophytic homogeneously enhancing mass at the lesser curvature
of the stomach (arrow).
Figure 4. Gastric gastrointestinal stromal tumour in an 86-year-old woman. (a) Contrast-enhanced computed tomography showing a well-defined submucosal gastric mass (arrow) with smooth overlying mucosa (arrowheads). (b) On endoscopic ultrasound, the tumour (arrows) originates from the muscularis propria (asterisk).
Figure 5. Gastric gastrointestinal stromal tumour in a 53-year-old man. (a) Contrast-enhanced computed tomography showing a
heterogeneously enhancing mass arising from the gastric body (arrow) with extension into the lesser sac. (b, c) On endoscopic ultrasound,
the tumour (arrow) arises from the muscularis propria (asterisk).
Figure 6. Gastric gastrointestinal stromal tumour in a 78-year-old man. (a) Contrast-enhanced computed tomography (CT) showing a large heterogeneous mass in the upper abdomen (arrow). (b) On plain CT, hyperdensities close to the tumour represent tumour bleeding (arrowhead).
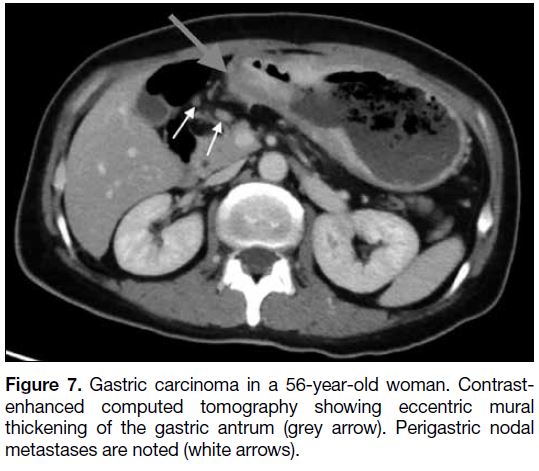
Common differential diagnoses of gastric masses include
carcinoma and lymphoma. Advanced gastric carcinoma is
commonly associated with perigastric lymphadenopathy
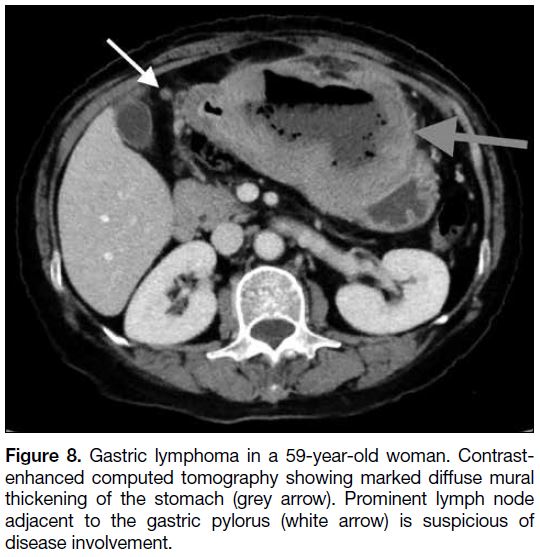
(Figure 7). Lymphoma causes significant circumferential
mural thickening of the stomach with lymphadenopathy
(Figure 8). Absence of lymphadenopathy is a radiological
feature favouring a diagnosis of GIST.
Figure 7. Gastric carcinoma in a 56-year-old woman. Contrast-enhanced
computed tomography showing eccentric mural
thickening of the gastric antrum (grey arrow). Perigastric nodal
metastases are noted (white arrows).
Figure 8. Gastric lymphoma in a 59-year-old woman. Contrastenhanced
computed tomography showing marked diffuse mural
thickening of the stomach (grey arrow). Prominent lymph node
adjacent to the gastric pylorus (white arrow) is suspicious of
disease involvement.
Small Intestine
The small intestine is the second most common site of
GISTs. There can be extraintestinal extension of the
neoplasm into the pelvic cavity mimicking a pelvic
mass (Figure 9), rendering it radiologically difficult to
assess the origin of the tumour. Tumour bleeding and
perforation are also complications of small bowel GISTs (Figure 10). Intestinal obstruction is an uncommon
complication due to the exophytic nature of GISTs.
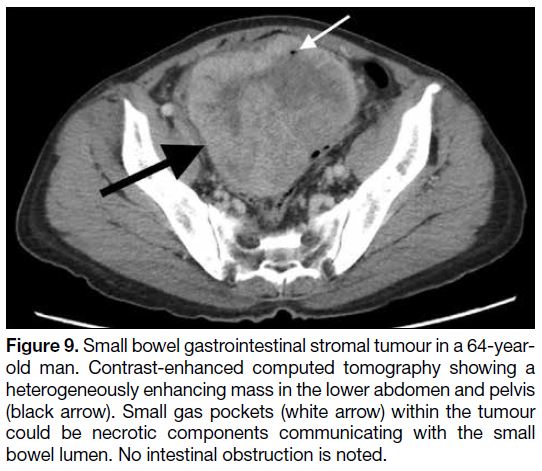
Figure 9. Small bowel gastrointestinal stromal tumour in a 64-year-old
man. Contrast-enhanced computed tomography showing a
heterogeneously enhancing mass in the lower abdomen and pelvis
(black arrow). Small gas pockets (white arrow) within the tumour
could be necrotic components communicating with the small
bowel lumen. No intestinal obstruction is noted.
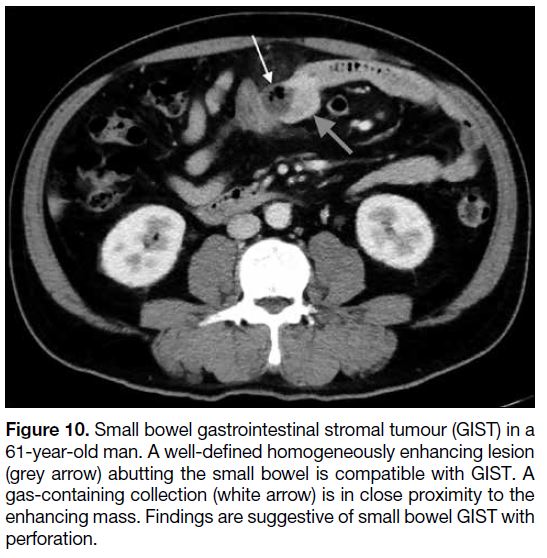
Figure 10. Small bowel gastrointestinal stromal tumour (GIST) in a
61-year-old man. A well-defined homogeneously enhancing lesion
(grey arrow) abutting the small bowel is compatible with GIST. A
gas-containing collection (white arrow) is in close proximity to the
enhancing mass. Findings are suggestive of small bowel GIST with
perforation.
Apart from GISTs, other common primary small bowel
tumours include adenocarcinoma, lymphoma and
carcinoid tumour. Adenocarcinoma usually presents
with a circumferential mass with shouldered border
(Figure 11). Both lymphoma and GISTs may show
aneurysmal dilatation of the bowel but the absence
of lymphadenopathy favours the diagnosis of GIST.
Carcinoid tumour usually shows avid homogeneous
enhancement with desmoplastic reaction that can be a
distinguishing imaging feature.
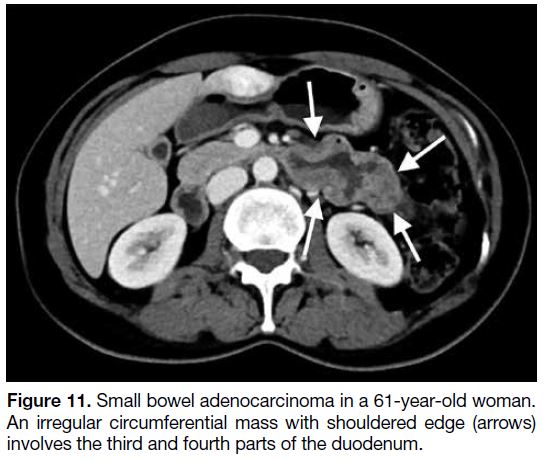
Figure 11. Small bowel adenocarcinoma in a 61-year-old woman.
An irregular circumferential mass with shouldered edge (arrows)
involves the third and fourth parts of the duodenum.
Colon and Rectum
Colonic GISTs are rarer than rectal GISTs and were not
found in our case series. Colonic GISTs are typically
transmural tumours with frequent intraluminal and
extraserosal components.[10] Circumferential growth with
aneurysmal dilatation of the affected colonic segment is
also common.[10]
Rectal GIST is usually seen as a well-defined eccentric
mural mass with extraserosal extension that may involve
the ischiorectal fossa, prostate or vagina (Figure 12).
On magnetic resonance imaging, GISTs are usually T1
hypointense to isointense and T2 hyperintense relative to
muscle (Figure 12).[11]
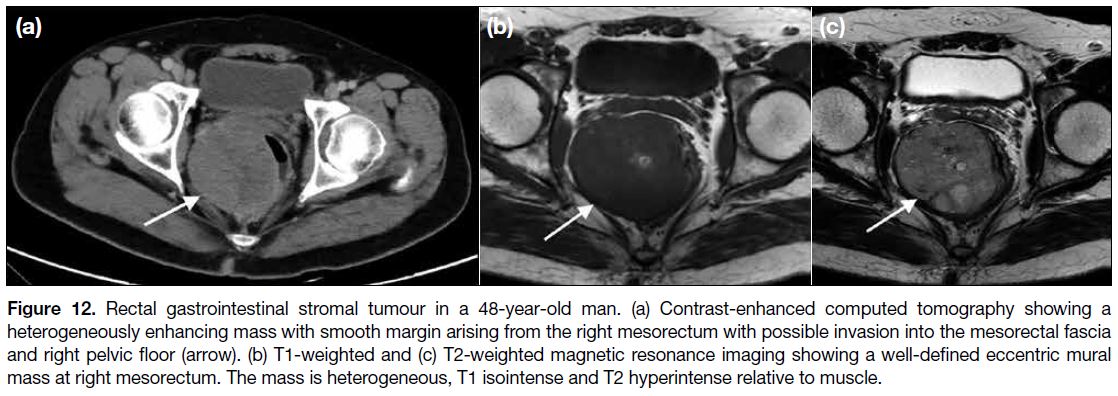
Figure 12. Rectal gastrointestinal stromal tumour in a 48-year-old man. (a) Contrast-enhanced computed tomography showing a
heterogeneously enhancing mass with smooth margin arising from the right mesorectum with possible invasion into the mesorectal fascia
and right pelvic floor (arrow). (b) T1-weighted and (c) T2-weighted magnetic resonance imaging showing a well-defined eccentric mural
mass at right mesorectum. The mass is heterogeneous, T1 isointense and T2 hyperintense relative to muscle.
Adenocarcinoma is the most common colorectal
neoplasm. Compared with rectal GISTs that usually
have a smooth margin, rectal adenocarcinoma tends to
have an irregular margin (Figure 13) and may have soft
tissue stranding extending into the ischiorectal fossa or supralevator space.[11] Perirectal lymphadenopathy is
common in rectal adenocarcinomas (Figure 13) but not
in GISTs.[11] In addition, the presence of haemorrhage on
magnetic resonance imaging is a feature that favours
GISTs.[11]
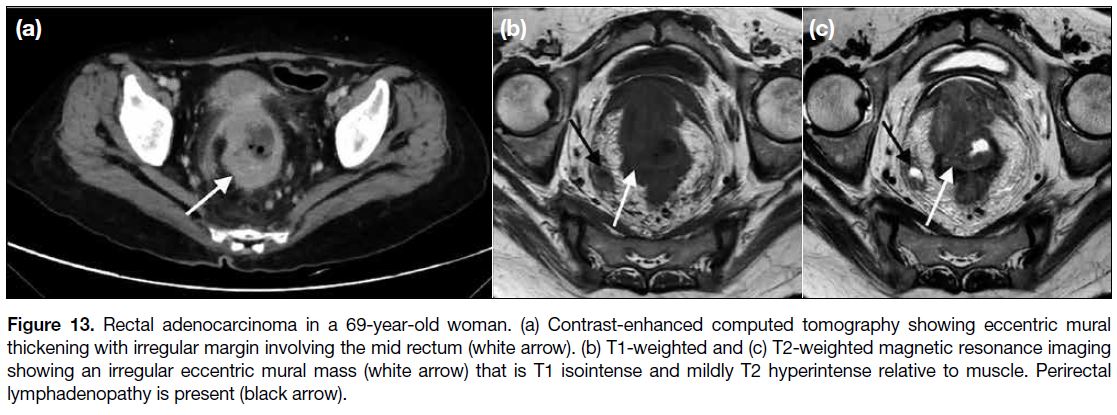
Figure 13. Rectal adenocarcinoma in a 69-year-old woman. (a) Contrast-enhanced computed tomography showing eccentric mural
thickening with irregular margin involving the mid rectum (white arrow). (b) T1-weighted and (c) T2-weighted magnetic resonance imaging
showing an irregular eccentric mural mass (white arrow) that is T1 isointense and mildly T2 hyperintense relative to muscle. Perirectal
lymphadenopathy is present (black arrow).
Mesentery and Omentum
Primary GISTs can occur in the mesentery and omentum.
Similar to GISTs in the gastrointestinal tract, mesenteric
and omental GISTs are usually heterogeneous with
central necrosis or cystic degeneration (Figure 14).
Nonetheless, they are commonly larger in size and most
exceed 10 cm.[12]
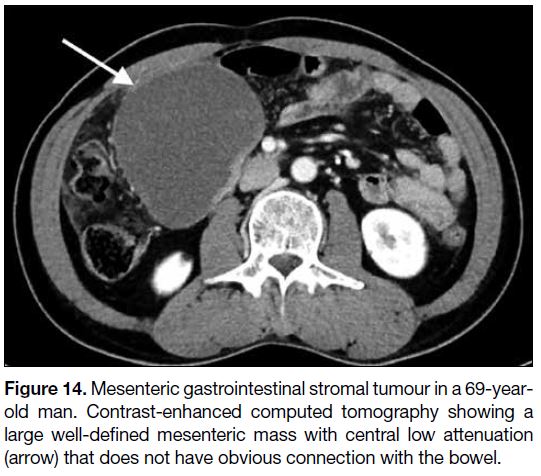
Figure 14. Mesenteric gastrointestinal stromal tumour in a 69-year-old
man. Contrast-enhanced computed tomography showing a
large well-defined mesenteric mass with central low attenuation
(arrow) that does not have obvious connection with the bowel.
GISTs in the gastrointestinal tract may metastasise to the
mesentery and omentum, usually manifesting as multiple
masses, whereas primary mesenteric or omental GISTs
are more often solitary. The imaging appearance of
mesenteric and omental GISTs can be indistinguishable
from that of other primary peritoneal tumours.
Metastasis
The most common sites of metastases are the liver and
peritoneum; less commonly, GISTs may metastasise to
lung and bone. They rarely metastasise to lymph nodes;
in the Surveillance, Epidemiology, and End Results
Program database study of the United States, nodal
involvement was identified in only 5% of cases and was
associated with decreased cancer-specific and overall
survival.[13]
EVALUATION OF TREATMENT RESPONSE
In the early post-treatment period with imatinib, tumour size reduction may not be significant and there can even
be a paradoxical increase in tumour size due to tumoural
haemorrhage, necrosis or myxoid degeneration. The first
radiographic response to imatinib is usually reduction
in tumour attenuation, followed by a gradual decrease
in size (Figures 15 and 16). This response pattern is not well suited to the standard RECIST (Response
Evaluation Criteria in Solid Tumours) that is based on
tumour size. An alternative way to evaluate treatment
response is therefore proposed — the Choi response
criteria (Table).[14]
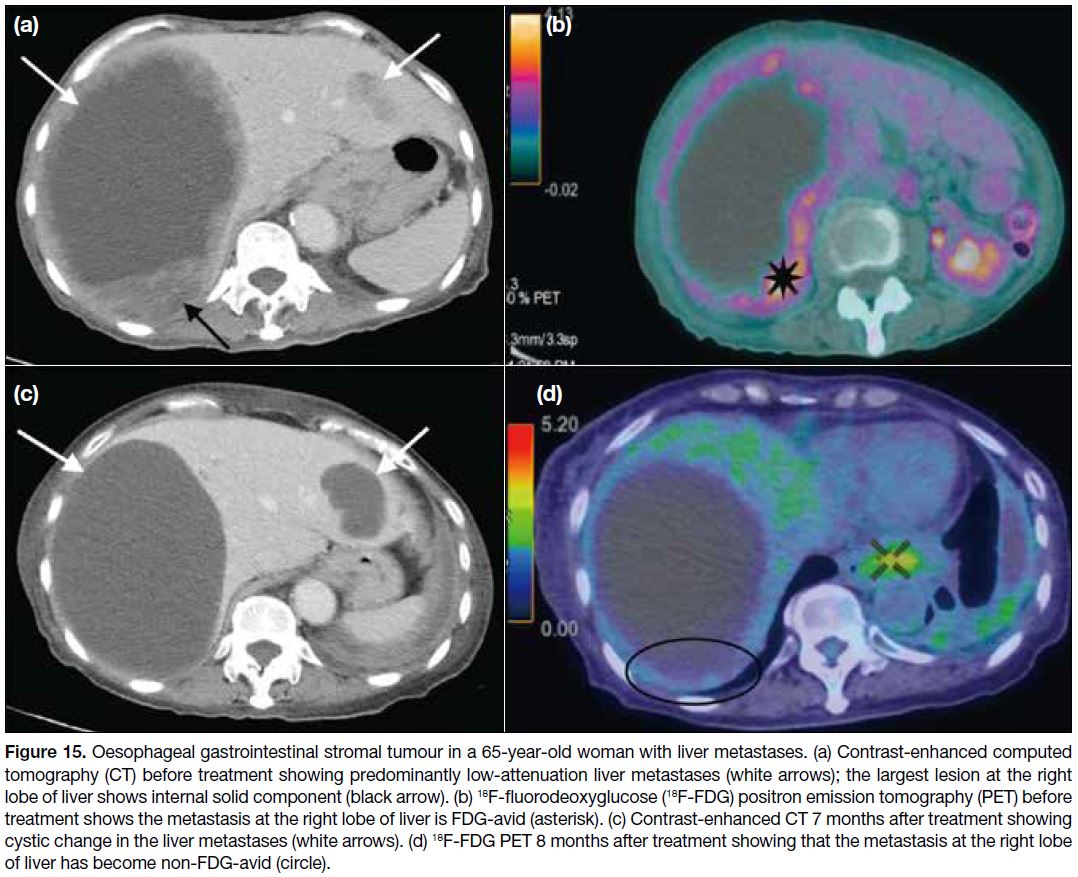
Figure 15. Oesophageal gastrointestinal stromal tumour in a 65-year-old woman with liver metastases. (a) Contrast-enhanced computed
tomography (CT) before treatment showing predominantly low-attenuation liver metastases (white arrows); the largest lesion at the right
lobe of liver shows internal solid component (black arrow). (b) 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET) before
treatment shows the metastasis at the right lobe of liver is FDG-avid (asterisk). (c) Contrast-enhanced CT 7 months after treatment showing
cystic change in the liver metastases (white arrows). (d) 18F-FDG PET 8 months after treatment showing that the metastasis at the right lobe
of liver has become non-FDG-avid (circle).
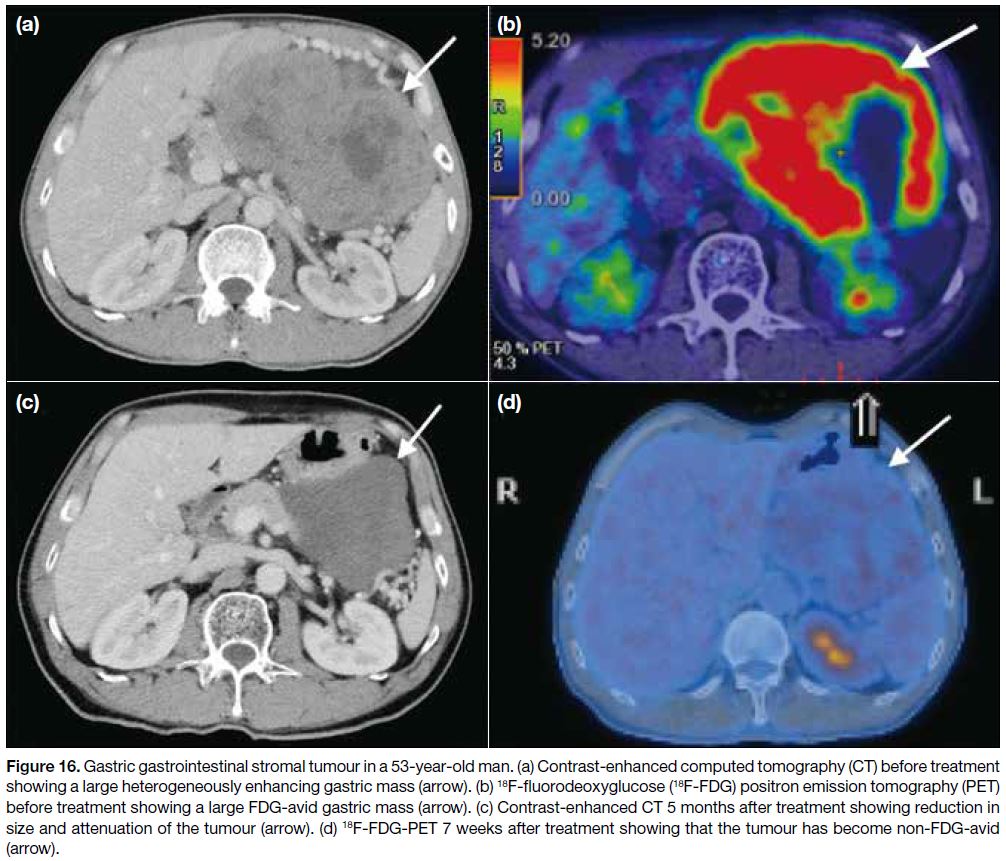
Figure 16. Gastric gastrointestinal stromal tumour in a 53-year-old man. (a) Contrast-enhanced computed tomography (CT) before treatment
showing a large heterogeneously enhancing gastric mass (arrow). (b) 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET)
before treatment showing a large FDG-avid gastric mass (arrow). (c) Contrast-enhanced CT 5 months after treatment showing reduction in
size and attenuation of the tumour (arrow). (d) 18F-FDG-PET 7 weeks after treatment showing that the tumour has become non-FDG-avid
(arrow).
Table. The Choi response criteria for GISTs.
18F-fluorodeoxyglucose positron emission tomography
is more sensitive for the assessment of early therapy
response than morphological imaging modalities.[14]
Studies show that a ≥50% reduction in maximum
standardised uptake value and/or a maximum standardised uptake value <2.5 in the follow-up scan can
be used to assess a sustained response.[14] Nonetheless,
18F-fluorodeoxyglucose positron emission tomography
cannot be used to assess treatment response in GISTs
that are initially non-FDG-avid.
The role of other advanced imaging for treatment
response assessment of GISTs remains under
investigation. Dual-energy computed tomography scan
is reported to enable visualisation and quantification
of iodine-related attenuation and has the potential for
accurate response assessment in GISTs.[15] Nonetheless,
further studies are required to prove the efficacy of new
imaging techniques.
SURVEILLANCE
Recurrence of disease is common and usually occurs first
in the liver or peritoneum (Figures 17 and 18). Disease
progression and recurrence may fail to be detected by
RECIST since there may not be significant increase in
tumour size initially. Instead, recurrence commonly first
manifests as a new enhancing intratumoural solid lesion
within the previous hypodense lesion (Figure 19), and
some may show a hyperdense ‘nodule-within-a-mass’
pattern (Figure 17).
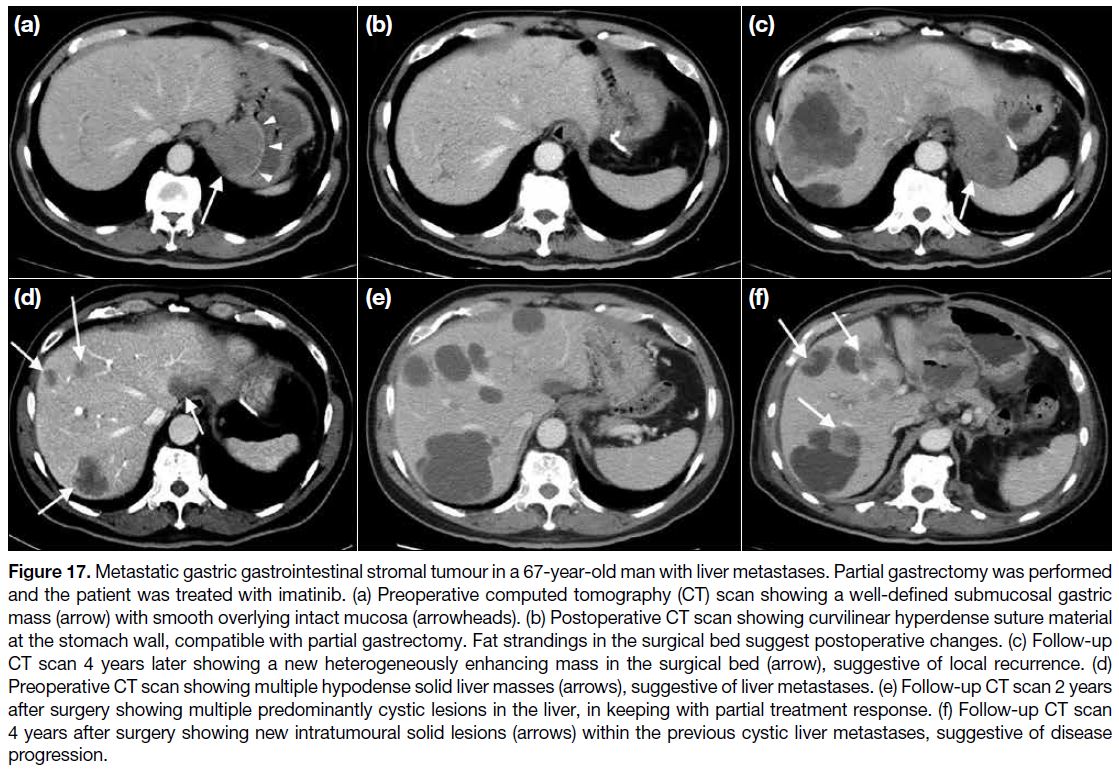
Figure 17. Metastatic gastric gastrointestinal stromal tumour in a 67-year-old man with liver metastases. Partial gastrectomy was performed
and the patient was treated with imatinib. (a) Preoperative computed tomography (CT) scan showing a well-defined submucosal gastric
mass (arrow) with smooth overlying intact mucosa (arrowheads). (b) Postoperative CT scan showing curvilinear hyperdense suture material
at the stomach wall, compatible with partial gastrectomy. Fat strandings in the surgical bed suggest postoperative changes. (c) Follow-up
CT scan 4 years later showing a new heterogeneously enhancing mass in the surgical bed (arrow), suggestive of local recurrence. (d)
Preoperative CT scan showing multiple hypodense solid liver masses (arrows), suggestive of liver metastases. (e) Follow-up CT scan 2 years
after surgery showing multiple predominantly cystic lesions in the liver, in keeping with partial treatment response. (f) Follow-up CT scan
4 years after surgery showing new intratumoural solid lesions (arrows) within the previous cystic liver metastases, suggestive of disease
progression.
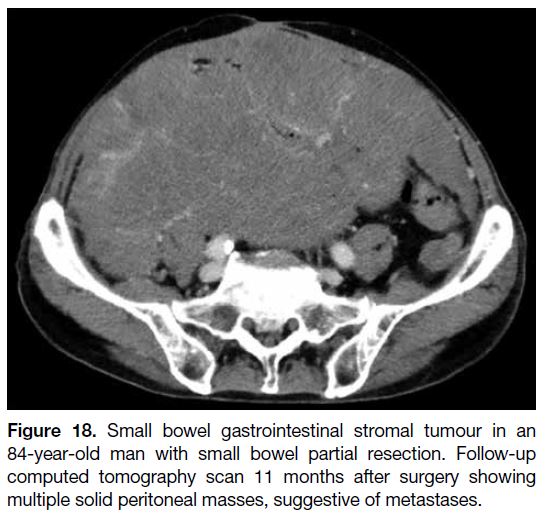
Figure 18. Small bowel gastrointestinal stromal tumour in an
84-year-old man with small bowel partial resection. Follow-up
computed tomography scan 11 months after surgery showing
multiple solid peritoneal masses, suggestive of metastases.
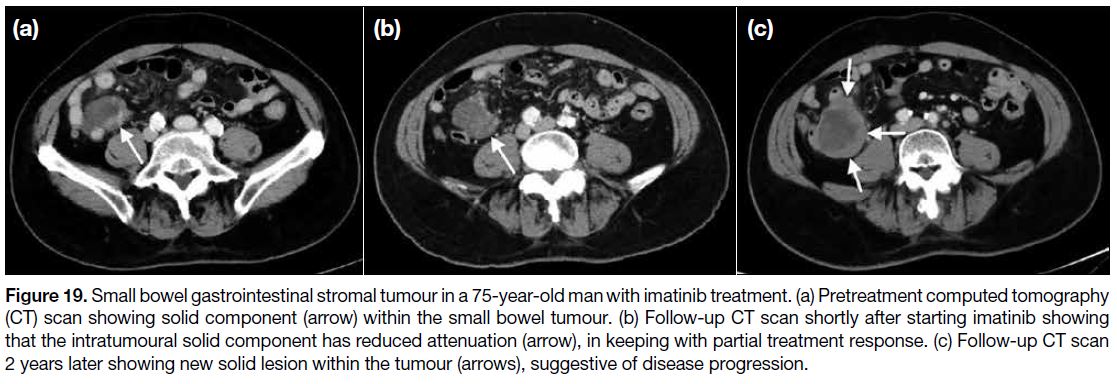
Figure 19. Small bowel gastrointestinal stromal tumour in a 75-year-old man with imatinib treatment. (a) Pretreatment computed tomography
(CT) scan showing solid component (arrow) within the small bowel tumour. (b) Follow-up CT scan shortly after starting imatinib showing
that the intratumoural solid component has reduced attenuation (arrow), in keeping with partial treatment response. (c) Follow-up CT scan
2 years later showing new solid lesion within the tumour (arrows), suggestive of disease progression.
The side-effects of imatinib include fluid retention, muscle
cramps and vomiting. Fluid retention with peripheral
oedema, pleural effusion and ascites are common,
especially in elderly patients. New onset of ascites on follow-up computed tomography should not be mistaken
for peritoneal metastasis or disease progression.
CONCLUSION
GISTs are the most common mesenchymal neoplasms
of the gastrointestinal tract. Although there is no
pathognomonic imaging feature of GIST, it is useful to
narrow the differential diagnoses of a gastrointestinal
tract neoplasm based on imaging findings. The Choi
criteria can effectively assess response in patients treated
with targeted therapies.
REFERENCES
1. Joensuu H, Vehtari A, Riihimäki J, Nishida T, Steigen SE,
Brabec P, et al. Risk of recurrence of gastrointestinal stromal tumour
after surgery: an analysis of pooled population-based cohorts.
Lancet Oncol. 2012;13:265-74. Crossref
2. Xu L, Ma Y, Wang S, Feng J, Liu L, Wang J, et al. Incidence
of gastrointestinal stromal tumor in Chinese urban population: a
national population-based study. Cancer Med. 2021;10:737-44. Crossref
3. Tirumani SH, Baheti AD, Tirumani H, O’Neill A, Jagannathan JP.
Update on gastrointestinal stromal tumors for radiologists. Korean
J Radiol. 2017;18:84-93. Crossref
4. Khoo CY, Chai X, Quek R, Teo MC, Goh BK. Systematic review
of current prognostication systems for primary gastrointestinal
stromal tumors. Eur J Surg Oncol. 2018;44:388-94. Crossref
5. Levy AD, Remotti HE, Thompson WM, Sobin LH, Miettinen M.
Gastrointestinal stromal tumours: radiologic features with
pathologic correlation. Radiographics. 2003;23:283-304. Crossref
6. Sarlomo-Rikala M, Kovatich AJ, Barusevicius A, Miettinen M.
CD117: a sensitive marker for gastrointestinal stromal tumors that
is more specific than CD34. Mod Pathol. 1998;11:728-34.
7. Nishida T, Cho H, Hirota S, Masuzawa T, Chiguchi G, Tsujinaka T, et al. Clinicopathological features and prognosis of primary
GISTs with tumor rupture in the real world. Ann Surg Oncol.
2018;25:1961-9. Crossref
8. Miettinen M, Sarlomo-Rikala M, Sobin LH, Lasota J. Esophageal
stromal tumors: a clinicopathologic, immunohistochemical,
and molecular genetic study of 17 cases and comparison with
esophageal leiomyomas and leiomyosarcomas. Am J Surg Pathol.
2000;24:211-22. Crossref
9. Winant AJ, Gollub MJ, Shia J, Antonescu C, Bains MS,
Levine MS. Imaging and clinicopathologic features of esophageal
gastrointestinal stromal tumors. AJR Am J Roentgenol.
2014;203:306-14. Crossref
10. Miettinen M, Sarlomo-Rikala M, Sobin LH, Lasota J.
Gastrointestinal stromal tumors and leiomyosarcomas in the colon:
a clinicopathologic, immunohistochemical, and molecular genetic
study of 44 cases. Am J Surg Pathol. 2000;24:1339-52. Crossref
11. Levy AD, Remotti HE, Thompson WM, Sobin LH, Miettinen M.
Anorectal gastrointestinal stromal tumors: CT and MR imaging
features with clinical and pathologic correlation. AJR Am J
Roentgenol. 2003;180:1607-12. Crossref
12. Feng F, Feng B, Liu S, Liu Z, Xu G, Guo M, et al. Clinicopathological
features and prognosis of mesenteric gastrointestinal stromal tumor:
evaluation of a pooled case series. Oncotarget. 2017;8:46514-22. Crossref
13. Güller U, Tarantino I, Cerny T, Schmied BM, Warschkow R.
Population-based SEER trend analysis of overall and cancer-specific
survival in 5138 patients with gastrointestinal stromal
tumor. BMC Cancer. 2015;15:557. Crossref
14. Dimitrakopoulou-Strauss A, Ronellenfitsch U, Cheng C, Pan L,
Sachpekidis C, Hohenberger P, et al. Imaging therapy response
of gastrointestinal stromal tumors (GIST) with FDG PET, CT and
MRI: a systematic review. Clin Transl Imaging. 2017;5:183-97. Crossref
15. Meyer M, Hohenberger P, Apfaltrer P, Henzler T, Dinter DJ,
Schoenberg SO, et al. CT-based response assessment of advanced
gastrointestinal stromal tumor: dual energy CT provides a more
predictive imaging biomarker of clinical benefit than RECIST or
Choi criteria. Eur J Radiol. 2013;82:923-8. Crossref