Neoadjuvant Treatment of Locally Advanced Rectal Cancer in Elderly Patients: Real-World Experience at a Tertiary Institution
ORIGINAL ARTICLE CME
Neoadjuvant Treatment of Locally Advanced Rectal Cancer in Elderly Patients: Real-World Experience at a Tertiary Institution
GTC Cheung1, EYH Chuk1, KM Cheung1, JCH Chow1, L Fok2, HL Leung2, CC Law1
1 Department of Clinical Oncology, Queen Elizabeth Hospital, Hong Kong
2 Department of Oncology, United Christian Hospital, Hong Kong
Correspondence: Dr GTC Cheung, Department of Clinical Oncology, Queen Elizabeth Hospital, Hong Kong. Email: ctc069@ha.org.hk
Submitted: 14 Jun 2022; Accepted: 23 Oct 2022.
Contributors: GTCC designed the study and acquired the data. All authors analysed the data. GTCC, EYHC, KMC and JCHC drafted the
manuscript. All authors critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: As an editor of the journal, GTCC was not involved in the peer review process. Other authors have disclosed no conflicts of interest.
Funding/Support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: This research was approved by the Research Ethics Committee (Kowloon Central/Kowloon East Cluster) of Hospital Authority, Hong Kong (Ref: KC/KE-21-0264/ER-3) and was conducted according to the Declaration of Helsinki. Informed consent of patients
was waived because of the retrospective nature of the study.
Abstract
Introduction
The incidence of rectal cancer increases with age. Neoadjuvant radiotherapy, with or without concurrent
chemotherapy, has been shown to improve outcomes. Elderly patients are underrepresented in clinical trials. In
Hong Kong, there is a lack of consensus and local data to inform patient selection and formulate optimal treatment
strategies for them. We sought to examine the outcomes of elderly patients undergoing neoadjuvant treatment for
locally advanced rectal cancer.
Methods
Cases of patients with locally advanced rectal cancers who received neoadjuvant treatment in Department
of Clinical Oncology, Queen Elizabeth Hospital from 2015 to 2018 were reviewed. ‘Elderly patient’ was defined as
those ≥70 years at diagnosis. The key study endpoints were local relapse-free survival (RFS), regional RFS, distant
RFS, overall RFS, and overall survival. Other endpoints included rate of downstaging, rate of conversion from
threatened/involved margins to clear margins, and treatment-related toxicities.
Results
In all, 74 elderly patients and 142 non-elderly patients were identified. The proportion of patients receiving
concurrent chemotherapy during radiotherapy was lower in the elderly patients (p < 0.001). Chemoradiation
administered to patients of all ages did not result in statistically significant differences in any survival endpoint.
Elderly patients deemed unfit for concurrent chemotherapy had a higher incidence of treatment toxicities. Age was
not a significant prognostic factor in any categories of survival.
Conclusion
Age should not be a deterministic factor in treatment planning in locally advanced rectal cancer.
Satisfactory oncological outcomes can be achieved in selected elderly patients. Utilisation of geriatric assessment
and consideration of patients’ preference are required to optimise treatment outcomes.
Key Words: Aged; Chemoradiotherapy; Neoadjuvant therapy; Radiotherapy; Rectal cancers
中文摘要
局部晚期直腸癌年老病人的術前輔助治療:第三層醫療機構的實際經驗
張天俊、祝菀馨、張嘉文、周重行、霍善智、梁海量、羅志清
簡介
直腸癌發病率隨年齡增長而上升。已有研究顯示術前輔助放射治療(不論有否同步進行化學治療)能改善病情。年老病人在臨床測試中的代表性不足。在香港,在選取病人及為該類病人制訂最佳治療策略方面,醫學界尚未達成共識,本地數據亦不足。我們嘗試分析進行局部晚期直腸癌術前輔助治療的年老病人的病情。
方法
本研究回顧了於2015至2018年期間在伊利沙伯醫院臨床腫瘤科接受術前輔助治療的局部晚期直腸癌病人個案。「年老病人」的定義為於確診時年屆70歲或以上的病人。關鍵研究終點為無局部復發存活、無區域復發存活、無遠處轉移存活、整體無復發生存率及總生存率。其他研究終點包括癌症降期率、從受威脅/侵犯切緣轉為陰性切緣率及治療相關毒性。
結果
我們分析了74名年老病人及142名非年老病人。年老病人在接受放射治療期間同時進行化學治療的比例較低(p < 0.001)。為所有年齡的病人進行放化療在任何存活終點並沒有出現統計學上的顯著差異。不適合同時接受化學治療的年老病人,其治療毒性發生率較高。年齡並非任何類別存活的重要預後因素。
結論
在規劃局部晚期直腸癌的治療時,年齡不應被視為決定性因素。部分年老病人可以獲得令人滿意的腫瘤治療結果。要達至最佳治療結果,須使用老年評估及考慮病人的偏好。
INTRODUCTION
According to the latest Hong Kong Cancer Statistics
2019, colorectal cancer ranked second in annual
incidence of neoplasms in Hong Kong.[1] Within the 5556
new cases in 2019, 2072 were rectal/anal malignancies.
Age is an important risk factor for rectal cancers; over
half of the newly diagnosed rectal/anal cancer patients in
2019 were >65 years old.[1]
Surgery is the mainstay of curative treatment for rectal
adenocarcinomas. Extensive research efforts were made
in search of the optimal neoadjuvant therapy modalities
to improve outcomes. The German Rectal Cancer Study
Group’s phase III study published in 2004 set the standard
of neoadjuvant chemoradiotherapy for clinical T3/4 or
lymph node positive diseases,[2] and the role of concurrent
chemotherapy during neoadjuvant radiotherapy (RT)
was confirmed in a 2013 Cochrane review in lowering
the incidence of local recurrence.[3] Mesorectal fascial
involvement threatening the circumferential resection
margin (CRM) and distal rectal tumours were included in
international treatment guidelines as relative indications
for neoadjuvant treatment.[4] [5]
Elderly patients were underrepresented in these clinical
trials. Ageing is associated with poor performance status, an increased incidence of comorbidities, and
suboptimal treatment tolerance. Retrospective studies
have investigated the outcomes of elderly patients
undergoing rectal cancer treatments,[6] [7] [8] [9] but conclusions
were divided regarding the adequate methodology of
patient selection, the optimal magnitude of neoadjuvant
treatment in patients with marginal performance status,
and the side-effect profile of this population. There is
also a lack of local data specifically for this controversial
topic. We therefore performed this study to examine the
outcomes of elderly patients undergoing neoadjuvant
treatment for locally advanced rectal cancers.
METHODS
We retrospectively reviewed the medical records
of consecutive patients that received neoadjuvant
treatment for rectal cancer in Department of Clinical
Oncology, Queen Elizabeth Hospital during the period
from 1 January 2015 to 31 December 2018. Patients
were considered for neoadjuvant treatment if they
satisfied the following inclusion criteria: (1) biopsy-proven
adenocarcinoma of rectum (located ≤12 cm
from anal verge); (2) staging by pelvic magnetic
resonance imaging, and either computed tomography
scan covering thorax, abdomen and pelvis, or positron
emitted tomography/computed tomography of the whole body, showing non-metastatic disease that was at
local stage T3 or above by American Joint Committee
on Cancer’s Cancer Staging Manual 7th edition, or
with involved or threatened mesorectal fascia; and (3)
deemed fit for neoadjuvant treatment and subsequent
surgery by the attending physician based on performance
status, age, and comorbidities. The diagnostic scans and
the decision to institute neoadjuvant treatment were
discussed in a multidisciplinary team meeting involving
clinical oncologists, colorectal surgeons, and diagnostic
radiologists.
Neoadjuvant treatment consisted of pelvic RT with or
without concurrent chemotherapy. For RT, gross tumour
volume was determined from clinical data (physical
examination, colonoscopy and imaging findings). Clinical
target volume included gross tumour volume plus a 2-cm
circumferential margin, the entire mesorectum, and high-risk
nodal areas including presacral, mesorectal, obturator,
and internal iliac nodes. A 1-cm circumferential margin
was added to clinical target volume to form the planning
target volume. Patients were treated with long-course
RT with conformal, intensity-modulated radiotherapy
or volumetric modulated arc therapy techniques. A
minimum dose of 45 Gy in 25 fractions was prescribed
to the 100% isodose line; an optional boost to the gross
disease was allowed up to a total equivalent dose of
54 Gy in 30 fractions, either with two-phase techniques
(in conformal RT) or simultaneous integrated boost (in
intensity-modulated radiotherapy/volumetric modulated
arc therapy).
Similar to reported local practice,[10] two regimens of
concurrent chemotherapy were adopted: intravenous
bolus 5-fluorouracil at 500 mg/m2 on Days 1-3 and Days
29-31, or oral capecitabine at 825 mg/m2 twice daily,
5 days per week. Omission of concurrent chemotherapy
due to advanced age, comorbidities, or patient preference
was allowed.
Cross-sectional imaging was repeated at around 4-6
weeks after completion of RT to evaluate treatment
response after neoadjuvant treatment and to assess
operability. If operable, total mesorectal excision was
performed ideally 6-10 weeks after RT completion.
Based on our institution protocol, further adjuvant
chemotherapy was not routinely offered due to the lack
of evidence supporting benefit of adjuvant chemotherapy
in randomised controlled trials and meta-analyses.
After treatment, surveillance was performed with regular history taking, physical examination, carcinoembryonic
antigen monitoring, and surveillance colonoscopy.
Cross-sectional imaging with computed tomography
or positron emitted tomography/computed tomography
scan was arranged when clinically indicated.
In this study, we defined elderly patients as those
≥70 years old at the time of histological diagnosis.
The key endpoints of this study were local relapse-free
survival (RFS), regional RFS, distant RFS, overall RFS,
and overall survival (OS). Other endpoints included
rate of downstaging (both T and N stages) and rate of
conversion from threatened/involved CRM to clear
margins. Treatment-related toxicities were assessed by
the National Cancer Institute Common Terminology
Criteria for Adverse Events version 4.03 and 30-day
postoperative mortality. Data were analysed using
commercial software SPSS (Windows version 24.0;
IBM Corp, Armonk [NY], United States). Baseline
characteristics between groups were tabulated and
compared using the Mann-Whitney U test for continuous
data and the Chi squared test for categorical data.
Survival endpoints were estimated by the Kaplan-Meier
method. Prognostic significance of clinical predictors
was analysed using the log-rank test in simple analysis
and the Cox proportional hazards model in multivariable
regression analysis.
RESULTS
A total of 238 cases of patients with rectal cancer who
received neoadjuvant therapy were identified. Twenty-two
cases were excluded for Stage IV disease at baseline.
A total of 74 of the remaining 216 patients were elderly
patients. Median ages of elderly patients and non-elderly
patients were 76.0 and 61.2 years, respectively. In
total, 91.7% of the patients had an Eastern Cooperative
Oncology Group performance status (ECOG PS) score
of 0-1; a higher proportion of ECOG PS score of 2
was noted in the elderly population (18.9% vs. 2.8%,
p = 0.011). Elderly patients had a significantly higher
prevalence of comorbidities (hypertension: 66.2% vs.
26.8%, p < 0.001; ischaemic heart disease: 16.2% vs.
4.9%, p = 0.019). No statistically significant difference
was noted between elderly and non-elderly groups
in clinical stage or CRM status. The full baseline
demographics and comorbidities before treatment
initiation are shown in Table 1.
Table 1. Baseline demographics by age-group and treatment.
A total of 53 elderly patients and six non-elderly
patients had neoadjuvant RT alone; the proportion of
patients who received concurrent chemotherapy was lower in elderly patients than non-elderly patients
(28.4% vs. 95.8%, p < 0.001). The most common
reasons for chemotherapy omission in elderly
patients were advanced age (n = 44, 83%), poor
performance status (n = 4, 7.5%), and patient refusal
(n = 4, 7.5%). Technique of RT, boost dose frequency,
and time of RT completion were similar between elderly
and non-elderly groups.
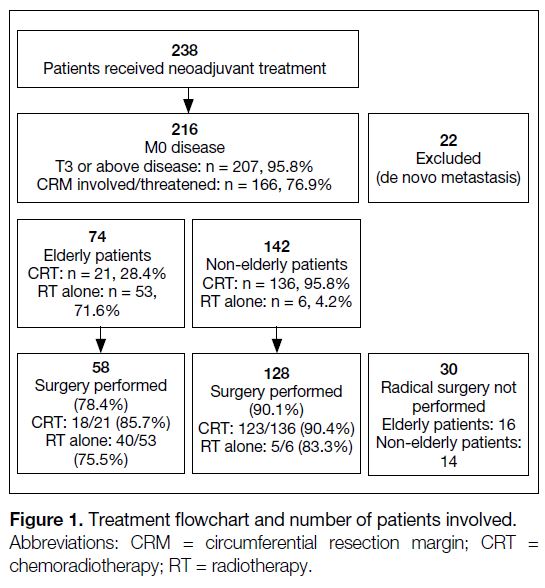
After neoadjuvant treatments, 58 elderly cases (78.4%) and 128 non-elderly (90.1%) cases went on to
undergo radical surgery as planned; 16 elderly patients
(21.6%) and 14 non-elderly patients (9.9%) did not
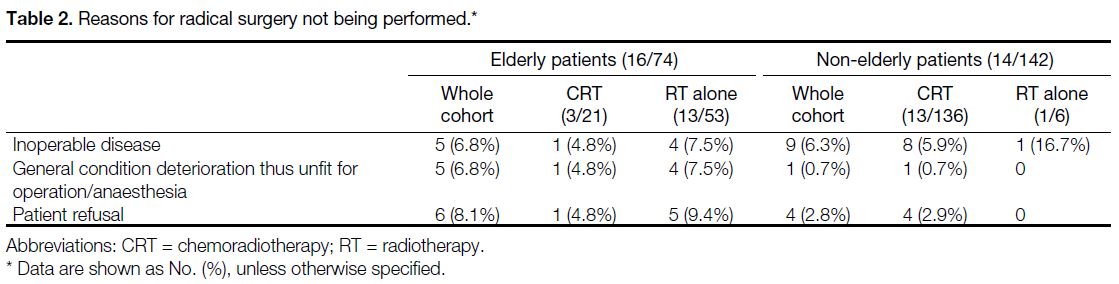
undergo radical surgery. The reasons are shown in
Table 2. These patients were excluded from survival
analyses but were included in toxicity and safety
analyses.
Table 2. Reasons for radical surgery not being performed.
Figure 1 illustrates the treatment scheme with number
of cases involved. The time to radical surgery and the rate of sphincter preservation were similar between
elderly and non-elderly patients. A total of 27 patients
subsequently received further adjuvant chemotherapy
after surgery. Treatment details are listed in the online supplementary Appendix.
Figure 1. Treatment flowchart and number of patients involved.
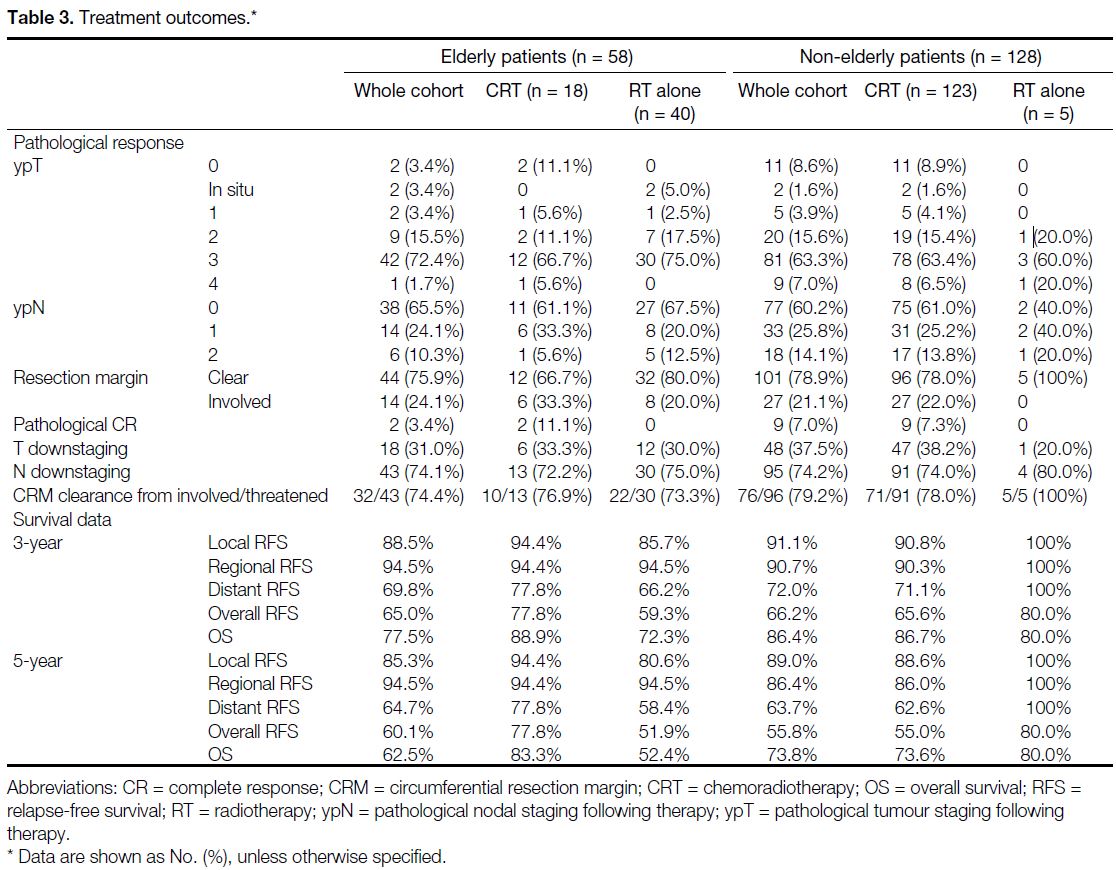
No statistically significant difference in treatment
outcomes was observed between elderly and non-elderly
cases, or between the CRT and RT-alone arms. Eleven
patients achieved a pathological complete response after
neoadjuvant treatment, with all of them had received
neoadjuvant CRT; the incidence was similar in the
elderly and non-elderly CRT cohorts. Detailed treatment
outcomes are listed in Table 3.
Table 3. Treatment outcomes.
The 5-year local RFS, regional RFS, distant RFS,
overall RFS and OS of the entire cohort were 88.0%,
88.7%, 63.9%, 57.1% and 70.2%, respectively. Elderly
and non-elderly patients who underwent CRT did not
demonstrate a statistically significant difference in any
survival endpoints. Five patients in the elderly RT-alone
subgroup died of non-cancer causes (12.5% of the
subgroup; 3 due to infection, 1 due to acute myocardial
infarction, and 1 due to cerebrovascular accident); no
non-cancer mortality was documented in the elderly
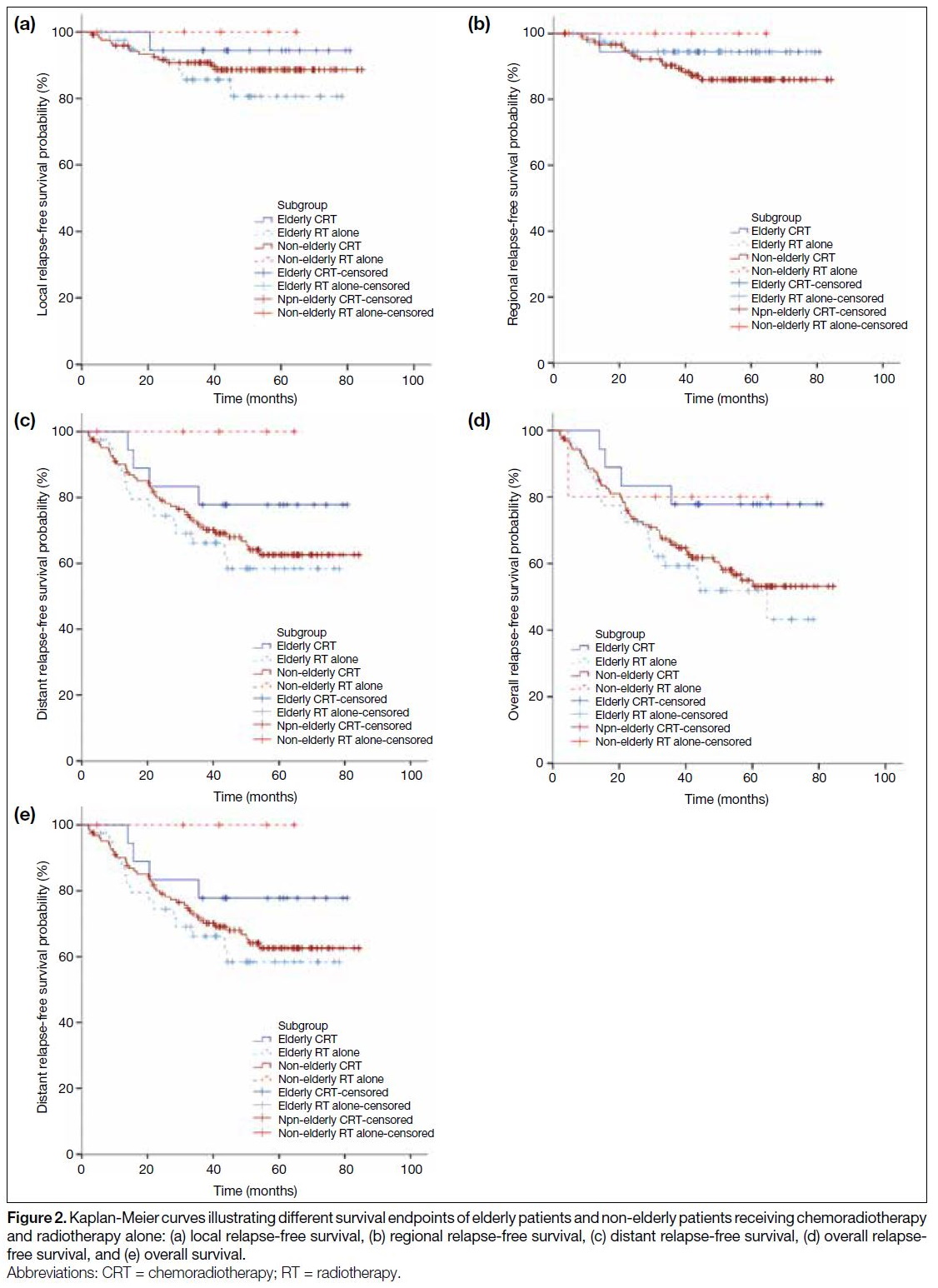
CRT subgroup. The Kaplan-Meier curves of the survival
endpoints are demonstrated in Figure 2.
Figure 2. Kaplan-Meier curves illustrating different survival endpoints of elderly patients and non-elderly patients receiving chemoradiotherapy
and radiotherapy alone: (a) local relapse-free survival, (b) regional relapse-free survival, (c) distant relapse-free survival, (d) overall relapse-free survival, and (e) overall survival.
Elderly patients who underwent neoadjuvant CRT
had noninferior toxicities and safety profiles in both
neoadjuvant treatment and surgery when compared to
their non-elderly counterparts. The incidence of grade ≥3
toxicities in neoadjuvant treatment in elderly and non-elderly
CRT arms were 4.8% and 14.0%, respectively.
Postoperative 30-day morbidities were 16.7% and
24.4%, respectively. Elderly patients who underwent
neoadjuvant RT alone had higher incidence of significant
treatment toxicities, with 7.5% having grade ≥3 RT
toxicities, and 32.5% having significant morbidities after
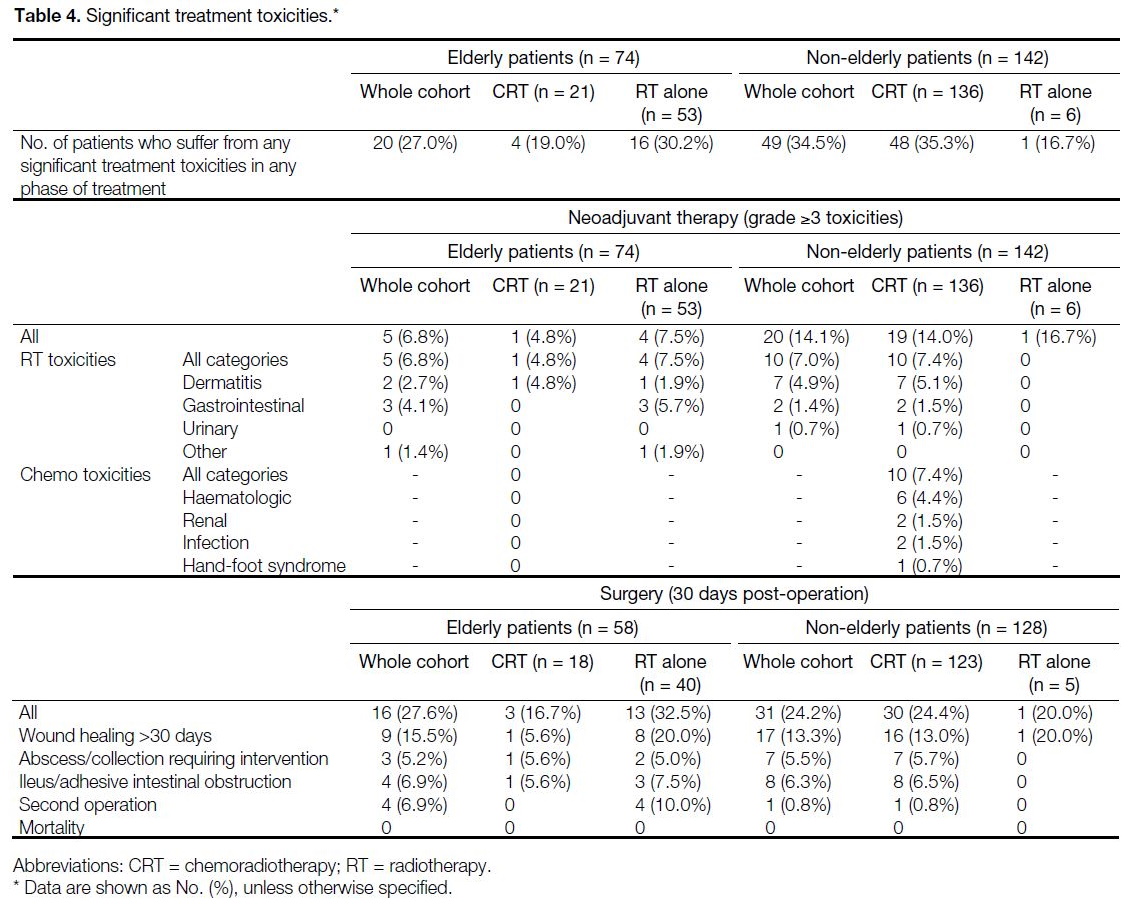
operation. No treatment-induced mortality was observed in this cohort. Table 4 shows the incidence of significant treatment toxicities.
Table 4. Significant treatment toxicities.
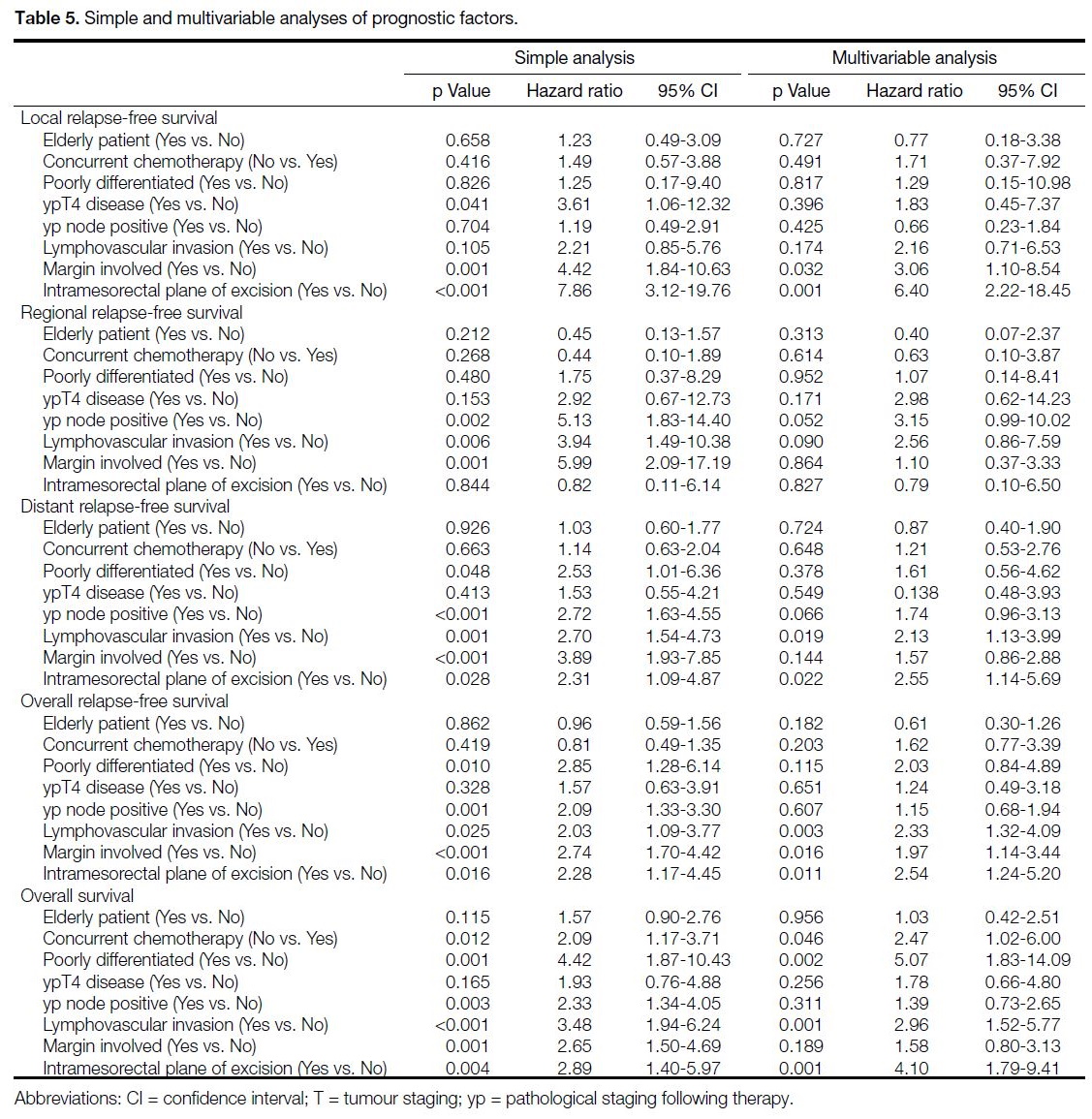
Analyses of prognostic factors indicated that older
age was not a significant prognostic factor in any of
the categories of survival on simple and multivariable
analyses. After multivariable regression analysis,
resection margin involvement and intramesorectal plane
of excision were significantly associated with shorter
local RFS. Presence of lymphovascular invasion and
intramesorectal plane of excision were significantly
associated with shorter distant RFS. Lymphovascular
invasion, margin involved, and intramesorectal plane
of excision were significantly associated with shorter
overall RFS. Absence of concurrent chemotherapy,
poorly differentiated histology, lymphovascular
invasion, and intramesorectal plane of excision were
significantly associated with shorter OS. The simple and
multivariable analyses results are shown in Table 5.
Table 5. Simple and multivariable analyses of prognostic factors.
DISCUSSION
Elderly rectal cancer patients were a heterogenous group
with diverse outcomes after neoadjuvant treatment, as
demonstrated by our study, which is to date the largest
reported Hong Kong cohort, and with neoadjuvant
CRT outcomes comparable to local and international
data.[2] [10] [11] [12]
Elderly patients deemed fit for neoadjuvant CRT
had comparable outcomes compared to their non-elderly
counterparts, including pathological complete
response rate, rate of tumour downstaging, probability
of conversion from involved/threatened CRM,
survival endpoints, and adverse events. On simple
and multivariable analyses, it was demonstrated that
old age was not an independent prognostic factor in
any categories of survival. This echoes the findings of
Kang et al,[7] demonstrating an elderly subgroup treated
with trimodality therapy with survival outcomes similar
to those of younger patients.
Whereas less fit patients in this study underwent
neoadjuvant RT alone, they tolerated the whole treatment
course less well, despite treatment de-escalation, with
30.2% experiencing significant treatment toxicities, and
17.0% not completing treatment due to frailty and non-compliance.
A total of five out of 40 patients (12.5% of
the subgroup) that completed treatment died due to non-cancer
causes. Our RT-alone cohort — mostly elderly
patients — had poorer OS, which was in contrast to
published data that suggested concurrent chemotherapy
omission was not detrimental to RFS or OS.[3] [13] The
reason behind poorer OS in our RT-alone cohort was
likely due to selection bias, as the patients were of more
advanced age, with more comorbidities and a higher
incidence of non-cancer mortalities, with limited choices
for palliative systemic treatment of disease recurrence.
In our institution, patients’ fitness for treatment
was assessed based on ECOG PS score, age, and
comorbidities. Inter-observer variability is inevitable, and discrepancies between physiological and
chronological ages exist. Despite multidisciplinary team
meeting endorsement, the treatments for a portion of less
fit patients were futile on retrospective review, ranging
from incapability to tolerate the whole treatment course
to short lifespan after treatment completion. There is
therefore a need for more accurate and objective tools
to stratify patients’ fitness for different intensities of
neoadjuvant treatments, or even radical treatment at all.
Multidisciplinary participation in comprehensive
geriatric assessment is recommended by the International
Society of Geriatric Oncology when assessing frailty
for tailoring the treatment plan in colorectal cancers.[14]
However, it is a time- and resource-consuming process
which hinders its routine use. Specific to rectal cancer,
an international expert panel recommends patients aged
≥70 years to receive mandatory office-based frailty
screening tests before being considered for usual care[15]; any presence of a frailty predictor requires a formal
geriatric assessment and a geriatrician’s presence in
multidisciplinary decision making.
After careful assessment of fitness and frailty, treatment
intent and its intensity should be adjusted accordingly. A
review by Wang et al[16] proposed a treatment algorithm
for locally advanced rectal cancer in elderly/comorbid
patients based on degree of frailty. Elderly patients
that are fully fit should undergo neoadjuvant treatment,
either long-course CRT or short-course RT alone, before
radical surgery. For less fit patients, different emerging
nonsurgical treatment approaches, e.g., watch and wait
approach or brachytherapy boost after CRT, should be
considered. Palliative RT, or even symptomatic care,
should be considered if patients are deemed frail. The
adaptation of the abovementioned approaches will likely
screen out unfit patients, avoid futile treatments in patients
with limited life expectancies due to comorbidities, and minimise unbearable treatment toxicities.
Besides fitness for treatment, elderly patients’ goals
and preferences in treatment outcomes should be
carefully respected during treatment planning. In our
study, 9.4% of the elderly patients subsequently refused
radical surgery after receiving neoadjuvant treatment.
The specific reasons of treatment refusal were not
documented in clinical notes, yet potential reasons
may be due to fear of surgical/anaesthetic risk, or the
subsequent inconvenience with a temporary/permanent
stoma. Thorough counselling, before and during treatment, would be helpful to formulate a tailor-made
treatment plan in respect of patients’ preferences, and
ensure compliance with treatment.
The concept of total neoadjuvant therapy for rectal
cancers was introduced in recent years, with two phase
III trials showing that such approach provided a superior
pathological complete response rate and outcomes at 3
years (3-year disease-free survival in the UNICANCER-PRODIGE
23 trial, disease-related treatment failure at
3 years in the RAPIDO trial).[17] [18] Although OS data were
immature to demonstrate superiority of total neoadjuvant therapy,[19] adding neoadjuvant chemotherapy is being
increasingly adopted as a treatment option worldwide.
This approach, however, will lead to more systemic
chemotherapy exposure and may pose extra toxicities
to patients. He et al[20] reported that the addition of
neoadjuvant chemotherapy to neoadjuvant CRT in the
elderly patients gave rise to similar disease-related survival
rates and oncological outcomes with that of younger
patients, but geriatric assessments and complications
data were lacking in the study. It is expected that as we
intensify the magnitude of our neoadjuvant treatment,
there will be a growing importance in proper patient
selection to balance the risk and benefits of treatment.
There were limitations to this study. The study data was
collected retrospectively, limiting the completeness of
data and introducing potential bias during data collection.
There was selection bias during referral and initiation of
neoadjuvant treatment, thus epidemiological data were
limited. There was also selection bias when patients
were assigned to different neoadjuvant treatment arms
(i.e., with or without concurrent chemotherapy), thus
confounding factors that were not identified in this
study may be present. A short-course RT scheme was
not adopted in this cohort, limiting our scope of analysis
and subsequent recommendations. Absence of detailed
parameters of elderly patients’ conditions and the small
sample size of this study limit the ability to identify
prognostic factors to predict tolerance to treatment, and
to derive further recommendations.
CONCLUSION
Age alone should not be a deterministic factor in treatment intensity consideration in locally advanced rectal cancer.
Satisfactory oncological outcomes can be achieved
in selected elderly patients with locally advanced
rectal cancers who undergo standard neoadjuvant
treatment and radical surgery, while the risk of shorter
survival and toxicities is higher in less fit candidates.
Utilisation of geriatric screening and assessment tools,
and consideration of patients’ preference and treatment
objectives are required to achieve tailor-made treatment
schemes and optimise treatment outcomes.
REFERENCES
1. Hong Kong Cancer Registry. Overview of Hong Kong Cancer
Statistics of 2019. October 2021. Available from: https://www3.ha.org.hk/cancereg/pdf/overview/Overview%20of%20HK%20Cancer%20Stat%202019.pdf
. Accessed 17 Apr 2022.
2. Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731-40. Crossref
3. De Caluwé L, Van Nieuwenhove Y, Ceelen WP. Preoperative
chemoradiation versus radiation alone for stage II and III resectable
rectal cancer. Cochrane Database Syst Rev. 2013;(2):CD006041. Crossref
4. Glynne-Jones R, Wyrwicz L, Tiret E, Brown G, Rödel C, Cervantes A, et al. Rectal cancer: ESMO Clinical Practice
Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;29(Suppl 4):iv22-40. Crossref
5. Benson AB, Venook AP, Al-Hawary MM, Azad N, Chen YJ, Ciombor KK, et al. Rectal cancer, version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw.
2022;20:1139-67. Crossref
6. Choi Y, Kim JH, Kim JW, Kim JW, Lee KW, Oh HK, et al. Preoperative chemoradiotherapy for elderly patients with locally advanced rectal cancer — a real-world outcome study. Jpn J Clin
Oncol. 2016;46:1108-17. Crossref
7. Kang S, Wilkinson KJ, Brungs D, Chua W, Ng W, Chen J, et al. Rectal cancer treatment and outcomes in elderly patients treated with curative intent. Mol Clin Oncol. 2021;15:256. Crossref
8. Mourad AP, De Robles MS, Putnis S, Winn RD. Current treatment approaches and outcomes in the management of rectal cancer above the age of 80. Curr Oncol. 2021;28:1388-401. Crossref
9. Tominaga T, Nagasaki T, Akiyoshi T, Fukunaga Y, Fujimoto Y, Yamaguchi T, et al. Feasibility of neoadjuvant therapy for elderly patients with locally advanced rectal cancer. Surg Today. 2019;49:694-703. Crossref
10. Lee SF, Chiang CL, Lee FA, Wong YW, Poon CM, Wong FC, et al.
Outcome of neoadjuvant chemoradiation in MRI staged locally
advanced rectal cancer: retrospective analysis of 123 Chinese
patients. J Formos Med Assoc. 2018;117:825-32. Crossref
11. Yeung WW, Ma BB, Lee JF, Ng SS, Cheung MH, Ho WM, et al.
Clinical outcome of neoadjuvant chemoradiation in locally
advanced rectal cancer at a tertiary hospital. Hong Kong Med J.
2016;22:546-55. Crossref
12. Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, Hess C,
et al. Preoperative versus postoperative chemoradiotherapy for
locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11
years. J Clin Oncol. 2012;30:1926-33. Crossref
13. Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355:1114-23. Crossref
14. Papamichael D, Audisio RA, Glimelius B, de Gramont A, Glynne-Jones R, Haller D, et al. Treatment of colorectal cancer in older patients: International Society of Geriatric Oncology (SIOG) consensus recommendations 2013. Ann Oncol. 2015;26:463-76. Crossref
15. Montroni I, Ugolini G, Saur NM, Spinelli A, Rostoft S, Millan M,
et al. Personalized management of elderly patients with rectal
cancer: Expert recommendations of the European Society
of Surgical Oncology, European Society of Coloproctology,
International Society of Geriatric Oncology, and American
College of Surgeons Commission on Cancer. Eur J Surg Oncol.
2018;44:1685-702 Crossref
16. Wang SJ, Hathout L, Malhotra U, Maloney-Patel N, Kilic S, Poplin E, et al. Decision-making strategy for rectal cancer management using radiation therapy for elderly or comorbid
patients. Int J Radiat Oncol Biol Phys. 2018;100:926-44. Crossref
17. Conroy T, Bosset JF, Etienne PL, Rio E, François É, Mesgouez-Nebout N, et al. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally
advanced rectal cancer (UNICANCER-PRODIGE 23): a
multicentre, randomised, open-label, phase 3 trial. Lancet Oncol.
2021;22:702-15. Crossref
18. Bahadoer RR, Dijkstra EA, van Etten B, Marijnen CA, Putter H, Kranenbarg EM, et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus
preoperative chemoradiotherapy, TME, and optional adjuvant
chemotherapy in locally advanced rectal cancer (RAPIDO): a
randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:29-42. Crossref
19. Kasi A, Abbasi S, Handa S, Al-Rajabi R, Saeed A, Baranda J, et al. Total neoadjuvant therapy vs standard therapy in locally advanced rectal cancer: a systematic review and meta-analysis. JAMA Netw Open. 2020;3:e2030097. Crossref
20. He F, Chen M, Xiao WW, Zhang Q, Liu Y, Zheng J, et al. Oncologic
and survival outcomes in elderly patients with locally advanced
rectal cancer receiving neoadjuvant chemoradiotherapy and total
mesorectal excision. Jpn J Clin Oncol. 2021;51:1391-9. Crossref