90Yttrium Selective Internal Radiation Therapy in Unresectable or Otherwise High-Risk Hepatocellular Carcinoma: Single-Centre Experience
ORIGINAL ARTICLE
90Yttrium Selective Internal Radiation Therapy in Unresectable or Otherwise High-Risk Hepatocellular Carcinoma: Single-Centre Experience
KH Leung, MY Lim
Department of Oncology, Princess Margaret Hospital, Hong Kong
Correspondence: Dr KH Leung, Department of Oncology, Princess Margaret Hospital, Hong Kong. Email: lkh017@ha.org.hk
Submitted: 30 Jul 2022; Accepted: 20 Nov 2022.
Contributors: Both authors designed the study. KHL acquired the data. Both authors analysed the data, drafted the manuscript, and critically revised the manuscript for important intellectual content. Both authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: Both authors have disclosed no conflicts of interest.
Funding/Support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: The research was approved by the Kowloon West Cluster Research Ethics Committee of Hospital Authority, Hong Kong [Ref No.: KW/EX-22-027 (170-03)] and was conducted in compliance of Declaration of Helsinki. A waiver of patient consent was approved by the
Committee.
Abstract
Objectives
We reviewed prognostic factors and clinical outcomes of selective internal radiation therapy (SIRT)
with 90Yttrium (90Y) microsphere using transarterial embolisation in unresectable hepatocellular carcinoma (HCC).
Methods
All cases of hepatocellular carcinoma patients who underwent 90Y SIRT at Princess Margaret Hospital
between July 2017 and September 2021 were retrospectively reviewed. Overall survival (OS), progression-free
survival (PFS), and prognostic factors, as well as tumour response according to modified Response Evaluation
Criteria in Solid Tumors criteria and safety, were evaluated.
Results
Thirty HCC patients were treated with 90Y SIRT , of whom 26 (87%) were male. The median age of patients
was 66.5 years (range, 40-93). Fifty-seven percent were chronic hepatitis B carriers and the majority (93%) had Child–Pugh class A liver disease. Patients had portal vein thrombosis, or tumour size >8 cm. After a median follow-up of 14.6 months, the objective response rate was 26.9% and the local control rate was 76.9%, including three complete responses, four partial responses and 13 cases of stable disease. The median PFS was 6.3 months and the 1-year PFS was 40.2%. Median OS was not yet reached and the 1-year OS was 57.5%. In multivariable analysis, alpha-fetoprotein level was a significant prognostic factor for OS (p = 0.045) and PFS (p = 0.011). Most side-effects
were grades 1-2 only.
Conclusion
90Y SIRT via transarterial embolisation is an effective and safe treatment for intermediate- to advancedstage
HCC patients which provides satisfactory local control with minimal toxicity. Longer survival was observed
in patients with alpha-fetoprotein level <400 μg/L at baseline.
Key Words: Carcinoma, hepatocellular; Radiotherapy; Survival; Yttrium radioisotopes
中文摘要
不可切除或其他高危肝細胞癌的釔90選擇性內放射治療:單中心經驗
梁君豪、林美瑩
目的
本研究檢視在不可切除肝細胞癌使用經動脈栓塞術的釔90微粒選擇性內放射治療的預後因素及臨床結果。
方法
本研究回顧於2017年7月至2021年9月期間在瑪嘉烈醫院進行釔90選擇性內放射治療的所有肝細胞癌病人個案,評估了整體存活、疾病無惡化存活、預後因素及根據經修訂固體腫瘤反應評估標準的準則及安全程度評估的腫瘤反應。
結果
共30名病人接受了釔90選擇性內放射治療,當中26名(87%)為男性。病人年齡中位數為66.5歲(範圍,40-93)。57%病人為慢性乙型肝炎帶菌者,當中大部分(93%)為Child–Pugh分級A肝病病人。病人有肝門靜脈栓塞或腫瘤>8 cm。在覆診期中位數14.6個月後,客觀緩解率為26.9%,局部疾病控制率為76.9%,包括3個完全緩解、4個部分緩解及13個無變化個案。疾病無惡化存活中位數為6.3個月,一年疾病無惡化存活為40.2%。整體存活中位數尚未達到,一年整體存活為57.5%。在多變量分析中,甲型胎兒蛋白水平是整體存活(p = 0.045)及疾病無惡化存活(p = 0.011)的重要預後因素。大部分副作用只屬1-2級。
結論
對於中期至晚期肝細胞癌病人而言,使用經動脈栓塞術的釔90選擇性內放射治療是有效及安全的治療,能提供毒性最低而令人滿意的局部控制。本研究顯示,甲型胎兒蛋白基線水平<400 μg/L 的病人的存活期較長。
INTRODUCTION
In Hong Kong, hepatocellular carcinoma (HCC) ranks
fifth most common cancer and third among the most
common causes of cancer death since 2014.[1] Transarterial
embolisation or transarterial chemoembolisation (TACE)
has been shown to improve the survival of patients with
unresectable HCC.[2] [3]
Selective internal radiation therapy (SIRT) is a directed liver therapy making use of the tumour vascularity in
HCC in which the hepatic artery is usually the sole blood
supply. SIRT involves the injection of beta emitters
within resin or glass microspheres via the hepatic artery,
where the spheres form microemboli, thus giving a very
high radiation dose (100 to 1000+ Gy) to the tumour(s)
while at the same time minimising the radiation exposure
to normal liver tissue by not going through the hepatic
veins or the portal system.
90Yttrium (90Y) is a pure beta-emitting isotope (maximum
energy 2.28 MeV; mean energy 0.934 MeV), with a
mean and maximum penetration range of 2.5 mm and 11
mm, respectively. It is commonly used to treat HCC.[4] 90Y
SIRT is effective, with one study showing an objective response rate up to 40.0% and a median overall survival
(OS) of 16.4 months.[5] It has shown effectiveness in
terms of survival, response rates, and safety profile
similar to that with TACE in unresectable HCC in
several studies and meta-analyses.[6] [7] [8] [9] It was also shown
to be an effective treatment to accomplish downstaging
as a bridge to transplantation, surgical resection, or
radiofrequency ablation.[10] Survival in patients receiving
90Y SIRT for intermediate-advanced HCC can vary from
12-24 months (1-year pooled OS = 63%) to 6-12 months
(1-year pooled OS = 37%), should portal vein
thrombosis be present.[11] [12]
Careful selection of suitable candidates for 90Y SIRT is necessary. Several prognostic factors, including a
low Child–Pugh score, percentage of liver replaced
by tumour (≤50%) and alpha-fetoprotein (AFP) level
(<400 μg/L) are associated with better survival.[13]
Unilobar disease before SIRT and tumour response
(complete response/partial response) have also been
found to be significant predictors of survival.[14] It is
believed that survival can be prolonged in unresectable
HCC to a similar extent using TACE[15] with careful
selection of candidates.
In our hospital (Princess Margaret Hospital), HCC
patients are under the care of a multidisciplinary
hepatoma team with oncologists, surgeons, and
radiologists. Since 2012, 90Y SIRT has been offered as
a funded treatment by Hospital Authority, a statutory
body managing the public healthcare services in Hong
Kong, to high-risk HCC patients whose tumours are not
resectable or ablatable, with portal vein thrombosis, or
with tumour size >8 cm. Patients with infiltrative HCC,
Child–Pugh class C disease, ascites, or inadequate liver
reserve (with bilirubin level >34 μmol/L) are generally
considered ineligible for SIRT. In this study, we report
the outcome together with prognostic factors in the use
of 90Y in the treatment of these advanced cases of HCC
in our centre.
METHODS
We retrospectively enrolled HCC patients who received
90Y SIRT, either resin microspheres containing 90Y
(Sirtex, Australia) or 90Y-impregnated glass microspheres
(TheraSphere; MDS Nordion, Canada), at our hospital
between July 2017 and September 2021. Before SIRT,
patients underwent hepatic angiography, 90mtechnetium-macroaggregated
albumin scintigraphy, and computed
tomography (CT) scans to estimate the potential doses to
tumour, liver, and lung. A catheter was guided through
the femoral artery and into the hepatic artery by an
interventional radiologist. Blood vessels feeding the
gastrointestinal tract and extrahepatic sites such as the
pancreas were identified and prophylactically embolised
if necessary. Patients were deemed ineligible when lung
shunting was >20%. The dose activity calculation was
based on a partition model.[16] The aim of the treatment
was to administer a minimum dose of 120 Gy to the
tumour while keeping the dose to normal liver at <40 Gy
and to <50 Gy in patients with poor liver reserve. The
lung dose was planned to be <20 Gy.
Treatment
Intrahepatic administration of radioactive 90Y microspheres using either resin microspheres containing
90Y or 90Y-impregnated glass microspheres was
performed. Understanding of radiation exposure of
patients implanted with pure beta emitters is very limited.
Patients were kept in a radiation isolation room to wait
for assessment by physicists and considered safe if
radiation activity was <1.5 GBq according to Radiation
Ordinance of Hong Kong and Hospital Authority Code of
Practice on Radiation Safety and Protection. They were
discharged with medications including pantoprazole,
ursodeoxycholic acid, prednisolone, and entecavir if they were viral hepatitis B carriers. A bremsstrahlung
scan was performed on day 2 or 3 to document any
extrahepatic reflux of 90Y microspheres.
Outcome Assessment
Patients were followed up by surgeons after 90Y treatment with liver function tests and AFP tumour
marker test. The first follow-up was within 4 weeks
after discharge to assess for any treatment-related
toxicities. All patients had reassessment with triphasic
CT at approximately 3 months after 90Y treatment for an
objective evaluation of treatment outcome according to
mRECIST (modified Response Evaluation Criteria in
Solid Tumors) based on combined assessment of target
lesions, non-target lesions, and new lesions. Appearance
of one or more new lesions was counted as progression
regardless of the response of treated target and non-target
lesions classified according to mRECIST. Subsequent
follow-up was performed at approximately 1- to 3-month
intervals with laboratory tests and/or CT at the discretion
of surgeons. Any toxicity or adverse events noted during
the first 6 months after completion of 90Y treatment were
reviewed and graded according to CTCAE (Common
Terminology Criteria for Adverse Events) version 5.0 criteria.
Statistical Measures
Treatment responses were assessed radiologically
according to mRECIST. Local control rate was defined
as the proportion of patients with at least stable disease
of an irradiated target lesion. Objective response rate
was defined as the proportion of patients with partial or
complete response in target lesions and at least stable
disease in non-target lesions to 90Y SIRT. Progression-free
survival (PFS) was defined from the date of SIRT
to the date of a radiological sign of progression or death
from any cause. OS was calculated from the date of SIRT
to the date of death from any cause. Survival curves
were determined by the Kaplan-Meier method and
comparison between different Barcelona Clinic Liver
Cancer (BCLC) stages[17] and AFP levels was done by the
log-rank test. Statistical significance was defined at p < 0.05. Univariate and multivariable analyses of different
prognostic factors of survival outcomes, including
patient and tumour factors, were performed using the
Cox proportional hazards analysis. Only factors with p
values < 0.05 were considered significant and included
in the multivariable analysis. Commercial software
SPSS (Windows version 28.0; IBM Corp, Armonk
[NY], United States) was used to perform the statistical
analyses.
RESULTS
Baseline Characteristics
From July 2017 to September 2021, 30 HCC patients
were treated with 90Y, of whom 26 (86.7%) were male.
The median age was 66.5 years (range, 40-93). The
majority of them (n = 17, 56.7%) were chronic hepatitis
B carriers. Liver tumour sizes ranged from 4 cm to
19.7 cm, with a median size of 11.7 cm. Most of them
had Child–Pugh class A liver disease (n = 28, 93.3%)
and about one-third (n = 11, 36.7%) had AFP level
≥400 μg/L. Eleven (36.7%) patients had portal vein
thrombosis with four (13.3%) having thrombosis
involving the main portal vein. Half of the patients
received TACE with cisplatin and one-third of them
received targeted therapy (either sorafenib or lenvatinib)
as subsequent treatment. Other characteristics and
laboratory investigations are listed in Table 1.
Table 1. Baseline characteristics of the patients treated with 90Yttrium (n = 29).
Outcomes
Median follow-up time was 14.6 months. One patient
with ECOG performance status score of 3 and BCLC
stage D was excluded from the analysis. Of the 29
patients, 26 had assessable responses on CT (median
time = 2.76 months after 90Y treatment). Three patients
had complete responses (11.5%), four with partial
responses (15.4%), 13 with stable disease (50%), and
six with progressive disease (23.1%). The objective
response rate (defined as the sum of complete and
partial responses) was 26.9% while the local control rate (defined as the sum of complete and partial responses
and stable disease) was 76.9%. Three patients were
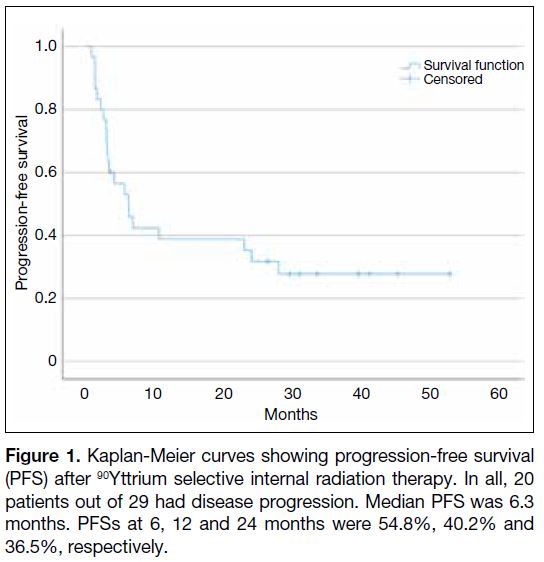
lost to follow-up. The median PFS was 6.3 months and
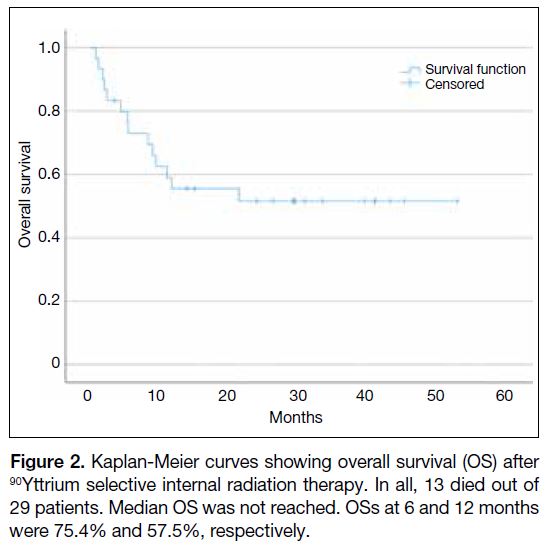
1-year PFS was 40.2% (Figure 1). Median OS was not
yet reached and 1-year OS was 57.5% (Figure 2).
Figure 1. Kaplan-Meier curves showing progression-free survival
(PFS) after 90Yttrium selective internal radiation therapy. In all, 20
patients out of 29 had disease progression. Median PFS was 6.3
months. PFSs at 6, 12 and 24 months were 54.8%, 40.2% and
36.5%, respectively.
Figure 2. Kaplan-Meier curves showing overall survival (OS) after
90Yttrium selective internal radiation therapy. In all, 13 died out of
29 patients. Median OS was not reached. OSs at 6 and 12 months
were 75.4% and 57.5%, respectively.
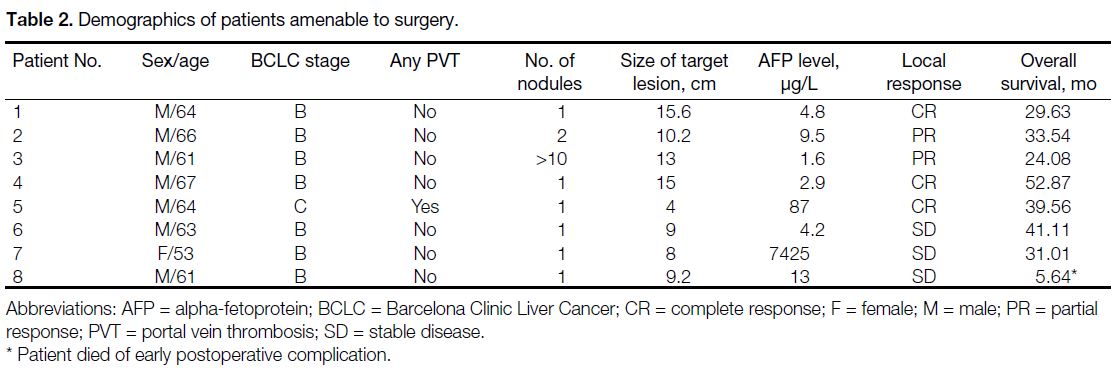
Eight out of 29 patients (27.6%) had surgery done after
downstaging of disease (Table 2). Median time from 90Y
treatment to surgery was 6.1 months. One achieved a
pathological complete response (Figure 3). Six of them
had residual HCC completely resected and one resected
with focally involved margin. Length of hospital stay
was 5-24 days. One had significant intra-operative
blood loss requiring massive blood transfusions. One
had postoperative ileus and pulmonary embolism which resolved with anticoagulation. One died early
postoperatively due to aspiration pneumonia.
Table 2. Demographics of patients amenable to surgery.
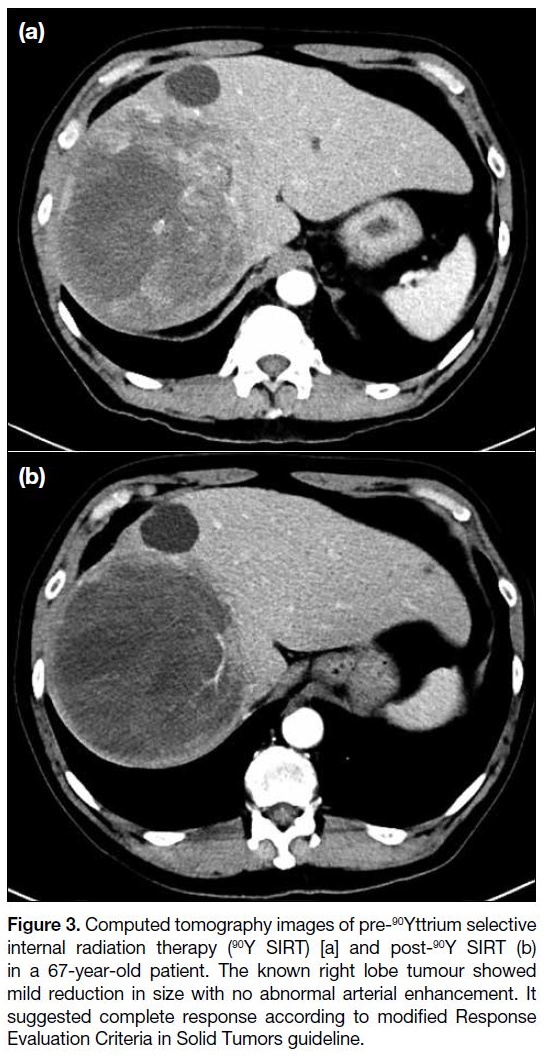
Figure 3. Computed tomography images of pre-90Yttrium selective
internal radiation therapy (90Y SIRT) [a] and post-90Y SIRT (b)
in a 67-year-old patient. The known right lobe tumour showed
mild reduction in size with no abnormal arterial enhancement. It
suggested complete response according to modified Response
Evaluation Criteria in Solid Tumors guideline.
Univariate and Multivariable Analyses
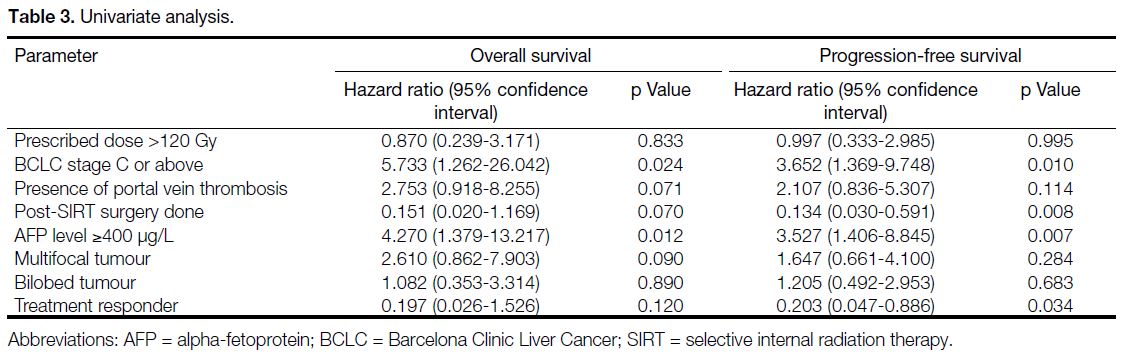
In univariate analysis (Table 3), BCLC stage C or above
(hazard ratio [HR] = 5.733, p = 0.024), and AFP level
≥400 μg/L (HR = 4.270, p = 0.012) were significant
prognostic factors for OS whereas BCLC stage C or
above (HR = 3.652, p = 0.010), post-SIRT surgery
(HR = 0.134, p = 0.008), AFP level ≥400 μg/L
(HR = 3.527, p = 0.007), and treatment responder (defined
as those with complete response or partial response)
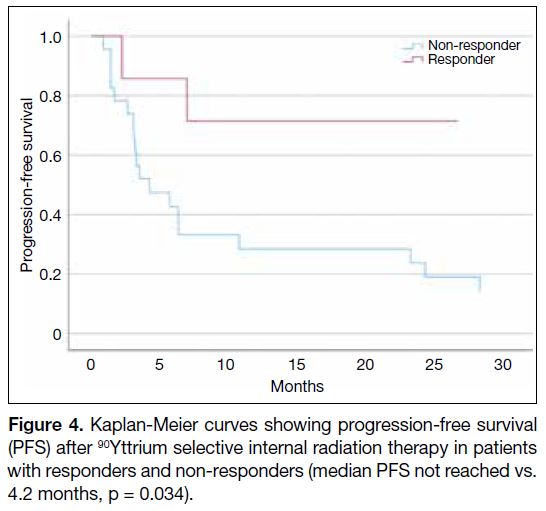
[HR = 0.203, p = 0.034; Figure 4] were significant
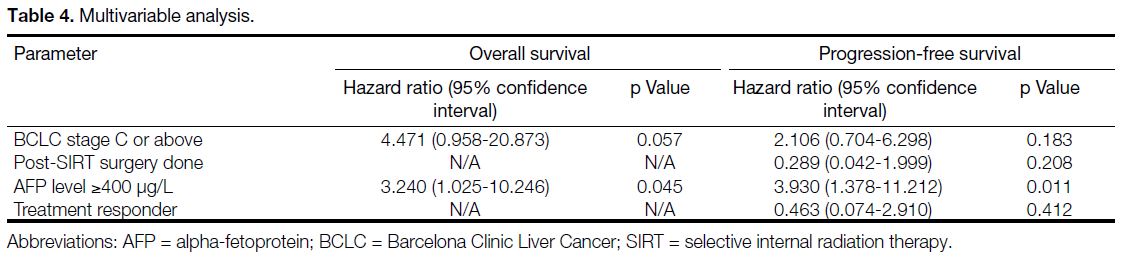
prognostic factors for PFS. In multivariable analysis
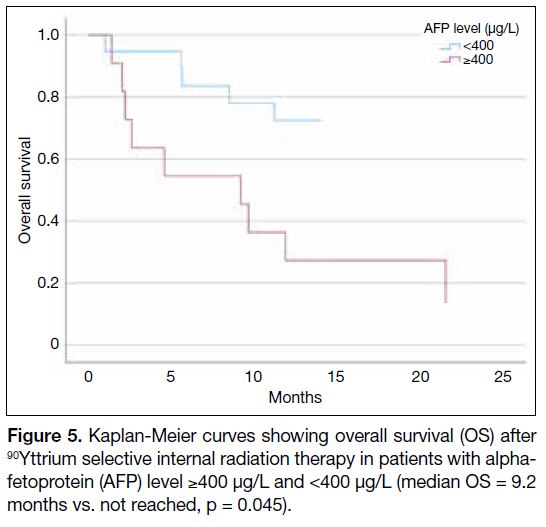
(Table 4), AFP level ≥400 μg/L remained as a significant prognostic factor for OS (HR = 3.240, p = 0.045;
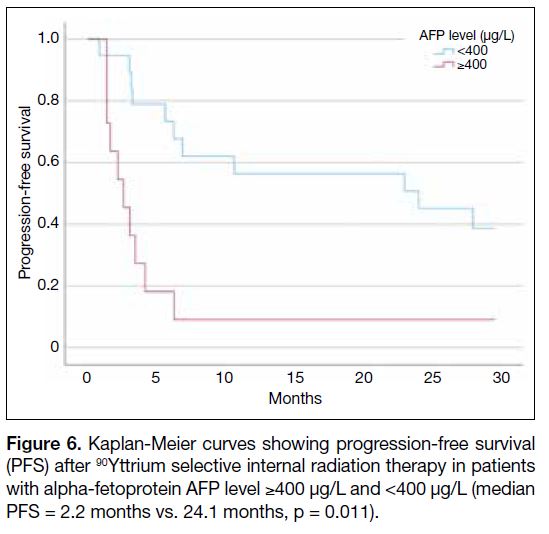
Figure 5) as well as for PFS (HR = 3.930, p = 0.011; Figure 6).
Table 3. Univariate analysis.
Figure 4. Kaplan-Meier curves showing progression-free survival
(PFS) after 90Yttrium selective internal radiation therapy in patients with responders and non-responders (median PFS not reached vs.
4.2 months, p = 0.034).
Table 4. Multivariable analysis.
Figure 5. Kaplan-Meier curves showing overall survival (OS) after
90Yttrium selective internal radiation therapy in patients with alpha-fetoprotein (AFP) level ≥400 μg/L and <400 μg/L (median OS = 9.2
months vs. not reached, p = 0.045).
Figure 6. Kaplan-Meier curves showing progression-free survival
(PFS) after 90Yttrium selective internal radiation therapy in patients with alpha-fetoprotein AFP level ≥400 μg/L and <400 μg/L (median PFS = 2.2 months vs. 24.1 months, p = 0.011).
In this series, there were four long-term survivors
and three complete responders. Those with complete
response achieved long survivals ranging from 29.6
to 52.9 months compared to a median of 11.2 months
in non-responders. All of them had post-SIRT surgery
done with clear resection margins. The median dose of
90Y SIRT was higher in responders (200 Gy) than in
non-responders (170 Gy). However, OS did not differ
significantly with dose (lower dose: p = 0.268, 95%
confidence interval = 0.983-1.005; higher dose: p = 0.456, 95% confidence interval = 0.201-2.056). Patient demographics and liver tumour baseline characteristics
were investigated in treatment responders and non-responders
together with those amenable to post-SIRT
surgery and were compared to those that were not.
The treatment responder group had better Eastern
Cooperative Oncology Group (ECOG) performance
status score (p = 0.039) and the group amenable to
surgery had significantly more patients with BCLC stage
B (p = 0.002) and better ECOG performance status score
(p = 0.011).
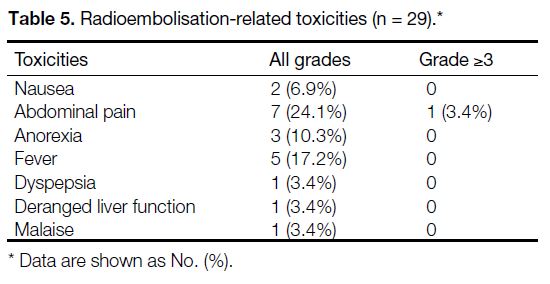
Toxicity
The median postoperative hospital stay was 6.5 days
(range, 2-16). Twelve patients (41.4%) had some forms
of post-90Y treatment complications, in total 29 different
kinds of toxicities experienced by them (Table 5). Most
side-effects were mild with abdominal pain and fever
being the most common. Only one patient had grade 3 abdominal pain requiring hospital admission 6 weeks
after 90Y SIRT. There was no identifiable cause found
in work-up and the patient was discharged the next day
after symptom subsided with analgesics. Abnormal liver
function with grade 1 hyperbilirubinemia occurred in
one patient and was self-limited.
Table 5. Radioembolisation-related toxicities (n = 29).
There was one case of suspected radiation pneumonitis
occurring approximately 7 weeks post-SIRT. The patient
was a non-smoker and presented with fever and shortness
of breath. The lung dose by the 90Y SIRT was 24.4 Gy.
Chest CT showed extensive ground-glass opacities
and patchy consolidation, which may have represented
oedema or infection. Multiple antibiotics and systemic
steroids were administered but patient succumbed due to
respiratory failure. Since the diagnosis was doubtful, it
was not regarded as post-SIRT toxicity.
DISCUSSION
In this study, it was demonstrated that 90Y SIRT was a feasible and effective treatment option in our local
population who had intermediate- and advanced-stage
HCC without serious adverse events. The response rate
to 90Y SIRT was high and the results were comparable to
other Asian series that reported OS and PFS of patients
receiving 90Y SIRT ranging from 11 to 16.4 months
and 2.4 to 11 months, respectively.[5] [18] [19] [20] The wide
range of survivals represents heterogeneity of patients’
demographics and disease status, thus making direct
comparison of survivals across different studies difficult.
In our study, 59% and 34% of patients belong to BCLC
stage C and had AFP level ≥400 μg/L, respectively.
Both were found to be poor prognostic factors, which
is consistent with the findings in a European multicentre
analysis.[21] Despite this, the results of our cohort were
impressive with encouraging results of local control,
PFS, and OS.
TACE is commonly used in intermediate-stage HCC but
is contraindicated in presence of portal vein thrombosis
due to the potential risk of precipitating liver necrosis
and failure by its effects on the already compromised
hepatic vascular supply. This limitation can be overcome
by 90Y SIRT due to the small size of the 90Y particles
which exert microembolic effects on hepatic vascular
dynamics.[22] Also, easy application in the left or right
hepatic arteries of 90Y SIRT makes it attractive for
patients compared to superselective TACE with longer
intervention times and repeated hospital admissions.[23]
Yet, presence of portal vein thrombosis was shown to
be associated with worse survival in treatment of 90Y
SIRT.[24] This was reconfirmed in our study in which
the OS after SIRT was shorter in those with portal vein
thrombosis compared to those without (median OS = 9.2
months vs. 26.4 months, p = 0.045).
In advanced HCC, targeted therapies are the mainstay
of treatment with a median survival of approximately 13.6 months with lenvatinib and 12.3 months with
sorafenib.[25] Although 90Y SIRT failed to demonstrate a
statistically significant difference in OS compared with
sorafenib in two recent phase III trials, SARAH[26] and
SIRveNIB[27], it had significantly fewer severe adverse
events and better health-related quality of life. Most of
the patients were classified as BCLC stage C in SARAH
study, 68% in the 90Y group and 67% in the sorafenib
group, whereas respective rates were 48.4% and 44.9%
in SIRveNIB study. Targeted therapy is associated with
numerous side-effects, namely hypertension, diarrhoea,
and hand-foot syndrome, and are known to lead to
treatment discontinuation permanently in approximately
11% of patients.[28] On the other hand, 90Y SIRT has better
toxicity profiles with most side-effects being only mild as
grades 1 to 2. This is also consistent with the observation
in our cohort with the most common side-effects being
grades 1 to 2 abdominal pain (23.3%) followed by fever
(16.7%). There was only one grade 3 abdominal pain in
our study with no identifiable causes. Symptom subsided
quickly with analgesics and the patient was discharged
the next day with no further complaints noted.
90Y SIRT is also effective in bridging to liver surgery
through tumour shrinkage and inducing future liver
remnant hypertrophy in initially unresectable HCC.[29] In
our study, 90% of the subjects underwent SIRT as the
initial treatment and eight (30%) of them had surgery
afterwards. They enjoyed a significantly longer survival
(ranging from 24 to 52 months, excluded one died of
postoperative complications). One of them demonstrated
radiological and pathological complete response in his
initial 4-cm tumour in subsequent hepatectomy after
SIRT. He remains well without any disease recurrence for
over 3 years by now. One of the long survivors received
90Y SIRT twice to the right lobe of liver. Radiological
complete response was achieved. Right hepatectomy
was performed 2 months after the second 90Y treatment.
Pathology showed residual pT1 grade III HCC with
clear resection margins. He has enjoyed >4 years of
survival by now without disease recurrence. The above
finding illustrated the potential role of downstaging and
facilitating curative resection. 90Y SIRT outperforms
TACE in the role of downstaging from T3 to T2 HCC[30]
and patients enjoyed better quality of life with 90Y
treatment.[31] Our series also showed 90Y SIRT is safe
with very low rates of grade ≥3 adverse events. Based on
our study results, those amenable to surgery mostly were
with ECOG performance status score of 0, classified as
BCLC stage B with low AFP level, and without portal
vein thrombosis, which could further guide our selection of 90Y SIRT candidates aiming for surgical resection.
The retrospective nature and the small sample size in this cohort might affect survival analysis and determination
of the significance of different prognostic factors. Also,
the survival is not mature yet where longer follow-up of
patients is necessary. Another limitation was the time of
the reassessment of CT scans. The mean time to the first
response assessment CT was 2.35 months after SIRT
and not all patients had regular scans afterwards. Later
response might then be underreported. Yet, the first CT
was chosen to assess the treatment response to 90Y SIRT
as most of the patients had subsequent treatment which
might confound the response solely due to 90Y SIRT. In
fact, most of the subjects only had one CT scan done
within 6 months of 90Y treatment. Furthermore, 23 out of
26 patients had 90Y SIRT alone whereas only three cases
had planned combination treatment with SIRT and TACE
or systemic therapy. Two patients had concurrent TACE
and one was taking sorafenib during radioembolisation.
Hence, it is believed the CT could reflect the treatment
response of 90Y SIRT.
Our study confirmed the role of 90Y SIRT in intermediate- to advance-stage HCC patients. However, careful
patient selection is of utmost importance to optimise
treatment benefits. There has been evidence suggesting
that AFP level ≥400 μg/L predicts a higher rate of dual-tracer
positron emission tomography/CT–detected
metastasis.[32] Our study further confirms it as a negative
prognostic factor, probably due to occult extrahepatic
metastases. With discreet use of incorporating staging
dual tracer positron emission tomography/CT scan in
high-risk cases with AFP level ≥400 μg/L, we might
screen out those with extensive distant metastases whom
90Y SIRT is not advised as the initial therapy.
The result of the series further consolidated the role
of 90Y SIRT in our local practice. In locally advanced
HCC, selection of appropriate treatment modalities has
been challenging. The study reflects the importance
of careful selection of candidates for 90Y SIRT. Good
ECOG performance status score and the classification as
BCLC stage B HCC are shown to be favourable factors
for this expensive radioisotope treatment and should
be prioritised when it comes to selection of suitable
candidates in multidisciplinary meetings. Other local
ablative treatments such as radiofrequency ablation and
stereotactic radiotherapy should be reserved for solitary
or lower volume disease, whereas systemic therapy is for
clearly disseminated disease.
CONCLUSION
90Y SIRT is an effective and safe treatment for
intermediate-to advanced-stage HCC which provides
satisfactory local control with minimal toxicity. Longer
survival was observed in patients with AFP level
<400 μg/L.
REFERENCES
1. Hong Kong Cancer Registry. Top ten cancers. Available from: https://www3.ha.org.hk/cancereg/topten.html. Accessed 1 May 2022.
2. Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003;37:429-42. Crossref
3. D’Avola D, Iñarrairaegui M, Pardo F, Rotellar F, Marti P, Bilbao JI, et al. Prognosis of hepatocellular carcinoma in relation to treatment across BCLC stages. Ann Surg Oncol. 2011;18:1964-71. Crossref
4. Levillain H, Bagni O, Deroose CM, Dieudonné A, Gnesin S,
Grosser OS, et al. International recommendations for personalised
selective internal radiation therapy of primary and metastatic liver
diseases with yttrium-90 resin microspheres. Eur J Nucl Med Mol
Imaging. 2021;48:1570-84. Crossref
5. Hilgard P, Hamami M, Fouly AE, Scherag A, Müller S, Ertle J,
et al. Radioembolization with yttrium-90 glass microspheres in
hepatocellular carcinoma: European experience on safety and
long-term survival. Hepatology. 2010;52:1741-9. Crossref
6. Kooby DA, Egnatashvili V, Srinivasan S, Chamsuddin A, Delman KA, Kauh J, et al. Comparison of yttrium-90
radioembolization and transcatheter arterial chemoembolization
for the treatment of unresectable hepatocellular carcinoma. J Vasc
Interv Radiol. 2010;21:224-30. Crossref
7. Carr BI, Kondragunta V, Buch SC, Branch RA. Therapeutic equivalence in survival for hepatic arterial chemoembolization and yttrium 90 microsphere treatments in unresectable hepatocellular carcinoma: a two-cohort study. Cancer. 2010;116:1305-14. Crossref
8. Pitton MB, Kloeckner R, Ruckes C, Wirth GM, Eichhorn W, Wörns MA, et al. Randomized comparison of selective internal radiotherapy (SIRT) versus drug-eluting bead transarterial
chemoembolization (DEB-TACE) for the treatment of hepatocellular
carcinoma. Cardiovasc Intervent Radiol. 2015;38:352-60. Crossref
9. Facciorusso A, Serviddio G, Muscatiello N. Transarterial radioembolization vs chemoembolization for hepatocarcinoma patients: a systematic review and meta-analysis. World J Hepatol. 2016;8:770-8. Crossref
10. Kulik LM, Atassi B, van Holsbeeck L, Souman T, Lewandowski RJ, Mulcahy MF, et al. Yttrium-90 microspheres (TheraSphere) treatment of unresectable hepatocellular carcinoma: downstaging to resection, RFA and bridge to transplantation. J Surg Oncol.
2006;94:572-86. Crossref
11. Rognoni C, Ciani O, Sommariva S, Facciorusso A, Tarricone R,
Bhoori S, et al. Trans-arterial radioembolization in intermediate-advanced
hepatocellular carcinoma: systematic review and meta-analyses.
Oncotarget. 2016;7:72343-55. Crossref
12. Salem R, Lewandowski RJ, Mulcahy MF, Riaz A, Ryu RK,
Ibrahim S, et al. Radioembolization for hepatocellular carcinoma
using yttrium-90 microspheres: a comprehensive report of long-term
outcomes. Gastroenterology. 2010;138:52-64. Crossref
13. Geschwind JF, Salem R, Carr BI, Soulen MC, Thurston KG,
Goin KA, et al. Yttrium-90 microspheres for the treatment of
hepatocellular carcinoma. Gastroenterology. 2004;127(5 Suppl
1):S194-205. Crossref
14. Van Thai N, Thinh NT, Ky TD, Bang MH, Giang DT, Ha LN,
et al. Efficacy and safety of selective internal radiation therapy
with yttrium-90 for the treatment of unresectable hepatocellular
carcinoma. BMC Gastroenterol. 2021;21:216. Crossref
15. Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, et al.
Arterial embolisation or chemoembolisation versus symptomatic
treatment in patients with unresectable hepatocellular carcinoma:
a randomised controlled trial. Lancet. 2002;359:1734-9. Crossref
16. Ho S, Lau WY, Leung TW, Chan M, Ngar YK, Johnson PJ, et al.
Partition model for estimating radiation doses from yttrium-90
microspheres in treating hepatic tumours. Eur J Nucl Med.
1996;23:947-52. Crossref
17. Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-38. Crossref
18. Lee VH, Leung DK, Luk MY, Tong CC, Law MW, Ng SC, et al. Yttrium-90 radioembolization for advanced inoperable
hepatocellular carcinoma. Onco Targets Ther. 2015;8:3457-64. Crossref
19. Chaikajornwat J, Tanasoontrarat W, Phathong C, Pinjaroen N, Chaiteerakij R. Clinical outcome of yttrium-90 selective internal radiation therapy (Y-90 SIRT) in unresectable hepatocellular carcinoma: experience from a tertiary care center. Liver Res. 2022;6:30-8. Crossref
20. Mazzaferro V, Sposito C, Bhoori S, Romito R, Chiesa C, Morosi C, et al. Yttrium-90 radioembolization for intermediate-advanced hepatocellular carcinoma: a phase 2 study. Hepatology. 2013;57:1826-37. Crossref
21. Sangro B, Carpanese L, Cianni R, Golfieri R, Gasparini D,
Ezziddin S, et al. Survival after yttrium-90 resin microsphere
radioembolization of hepatocellular carcinoma across Barcelona
clinic liver cancer stages: a European evaluation. Hepatology.
2011;54:868-78. Crossref
22. Quirk M, Kim YH, Saab S, Lee EW. Management of hepatocellular
carcinoma with portal vein thrombosis. World J Gastroenterol.
2015;21:3462-71. Crossref
23. Kloeckner R, Ruckes C, Kronfeld K, Wörns MA, Weinmann A, Galle PR, et al. Selective internal radiotherapy (SIRT) versus transarterial chemoembolization (TACE) for the treatment of
intrahepatic cholangiocellular carcinoma (CCC): study protocol
for a randomized controlled trial. Trials. 2014;15:311. Crossref
24. Floridi C, Pesapane F, Angileri SA, De Palma D, Fontana F, Caspani F, et al. Yttrium-90 radioembolization treatment for unresectable hepatocellular carcinoma: a single-centre prognostic factors analysis. Med Oncol. 2017;34:174. Crossref
25. Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al.
Lenvatinib versus sorafenib in first-line treatment of patients with
unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163-73. Crossref
26. Vilgrain V, Pereira H, Assenat E, Guiu B, Ilonca AD, Pageaux GP,
et al. Efficacy and safety of selective internal radiotherapy with
yttrium-90 resin microspheres compared with sorafenib in locally
advanced and inoperable hepatocellular carcinoma (SARAH):
an open-label randomised controlled phase 3 trial. Lancet Oncol.
2017;18:1624-36. Crossref
27. Chow PK, Gandhi M, Tan SB, Khin MW, Khasbazar A, Ong J, et al.
SIRveNIB: selective internal radiation therapy versus sorafenib in
Asia-Pacific patients with hepatocellular carcinoma. J Clin Oncol.
2018;36:1913-21. Crossref
28. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-90. Crossref
29. Bekki Y, Marti J, Toshima T, Lewis S, Kamath A, Argiriadi P, et al.
A comparative study of portal vein embolization versus radiation
lobectomy with yttrium-90 microspheres in preparation for liver
resection for initially unresectable hepatocellular carcinoma.
Surgery. 2021;169:1044-51. Crossref
30. Lewandowski RJ, Kulik LM, Riaz A, Senthilnathan S, Mulcahy MF,
Ryu RK, et al. A comparative analysis of transarterial downstaging
for hepatocellular carcinoma: chemoembolization versus
radioembolization. Am J Transplant. 2009;9:1920-8. Crossref
31. Salem R, Gilbertsen M, Butt Z, Memon K, Vouche M, Hickey R,
et al. Increased quality of life among hepatocellular carcinoma
patients treated with radioembolization, compared with
chemoembolization. Clin Gastroenterol Hepatol. 2013;11:1358-65.e1. Crossref
32. Chu KK, Chan AC, Ma KW, She WH, Dai WC, Chok KS, et al. Role of C11-FDG dual-tracer PET-CT scan in metastatic screening of hepatocellular carcinoma — a cost-effectiveness analysis.
Hepatobiliary Surg Nutr. 2021;10:301-7. Crossref