Breast-Implant-Related Fibromatosis in a Patient with Free Silicone Injection: a Case Report
CASE REPORT
Breast-Implant-Related Fibromatosis in a Patient with Free Silicone Injection: a Case Report
YS Chan, C Tsoi, HY Hung, WCW Chu, HL Chau
Department of Imaging and Interventional Radiology, Prince of Wales Hospital, Hong Kong
Correspondence: Dr YS Chan, Department of Imaging and Interventional Radiology, Prince of Wales Hospital, Hong Kong. Email: juliannayschan@cuhk.edu.hk
Submitted: 20 Oct 2021; Accepted: 14 Jan 2022.
Contributors: YSC and CT designed the study. YSC and HLC acquired and analysed the data. YSC drafted the manuscript. YSC, HYH, WCWC and HLC critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: As an editor of the journal, WCWC was not involved in the peer review process. Other authors have disclosed no conflicts of interest.
Funding/Support: This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: This study was conducted in accordance with the Declaration of Helsinki. The patient provided consent for all tests and procedures.
CASE REPORT
A 36-year-old woman, gravida 4 and parity 1 with
three previous miscarriages, with good past health and
no family history of malignancy, was referred to our
institution. She had been prescribed oral contraceptives
for the last 13 years but had stopped taking them prior
to presentation. She had a history of bilateral breast
augmentation at age 23 years. The material injected was
unknown.
At the age of 36, she presented to an outside institution with a 6-month history of self-detected left breast
lump, increasing in size and associated with mastalgia.
A lesion at left 5 o’clock (L5H) position was detected
and subsequent biopsy revealed focal fat necrosis with
scarring.
Physical examination at our institution revealed an
immobile, hard left breast mass at L5H position with no
palpable lymphadenopathy. The overall clinical picture
warranted a repeated core biopsy due to suspicion of a
malignant disease process.
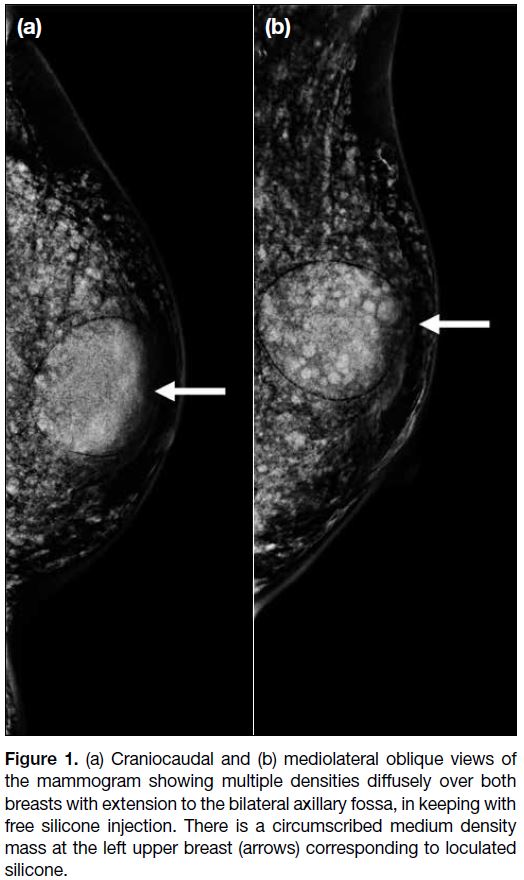
Review of her previous mammogram showed multiple
densities diffusely over both breasts suggestive of free
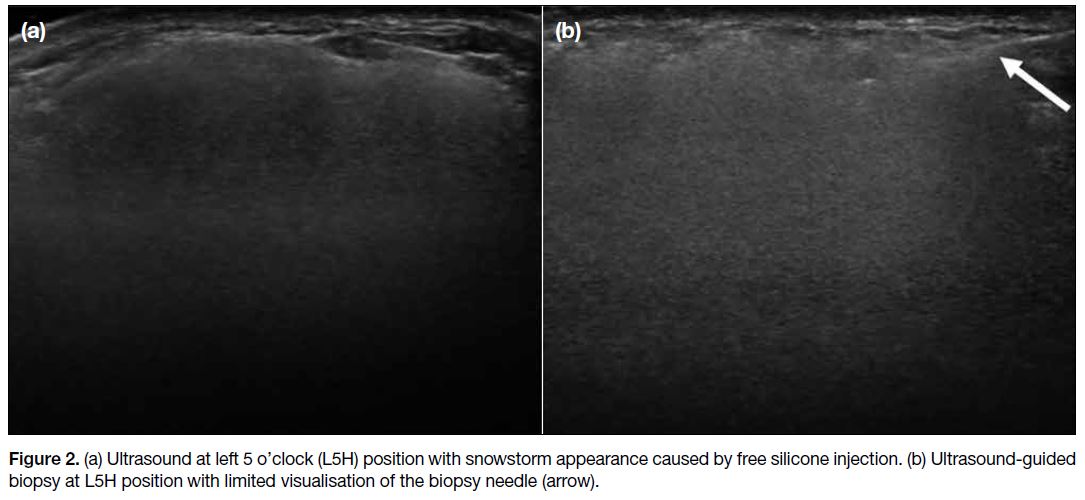
silicone injection (Figure 1). Ultrasound revealed a
snowstorm appearance in both breasts, also in keeping
with the presence of free silicone (Figure 2a). The
presenting lump was not well visualised, likely due to
the heavy shadowing of injected silicone. Ultrasound-guided
core biopsy was performed assisted by palpation
of the mass with an 18-gauge biopsy needle and two
cores of tissue obtained (Figure 2b). Histology showed benign breast tissue with fat necrosis and inflammation. She was offered a lumpectomy but was indecisive.
Figure 1. (a) Craniocaudal and (b) mediolateral oblique views of
the mammogram showing multiple densities diffusely over both
breasts with extension to the bilateral axillary fossa, in keeping with
free silicone injection. There is a circumscribed medium density
mass at the left upper breast (arrows) corresponding to loculated
silicone.
Figure 2. (a) Ultrasound at left 5 o’clock (L5H) position with snowstorm appearance caused by free silicone injection. (b) Ultrasound-guided biopsy at L5H position with limited visualisation of the biopsy needle (arrow).
Unfortunately, 4 months later the patient presented
again with rapid increase in size and pain that was not
relieved by analgesics. She expressed her wish for
resection in view of the worsening symptoms. Due to the
rapid disease progression, the surgical team requested
magnetic resonance imaging (MRI) for further
evaluation and a core biopsy was repeated to exclude
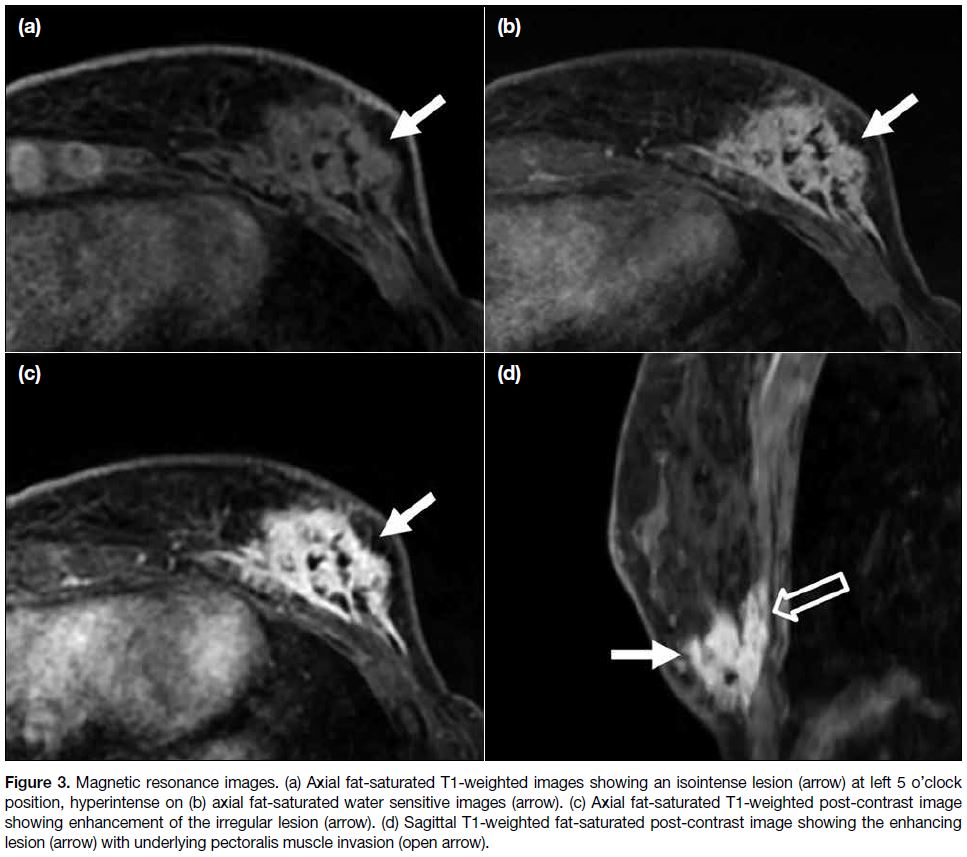
the possibility of malignancy. An enhancing mass at
L5H position was evident with chest wall invasion (Figure 3). Dynamic post-contrast images showed a type
I kinetic curve. Imaging features remained suspicious of
malignancy. Core biopsy was repeated with a 14-gauge
biopsy needle under ultrasound guidance and palpation,
with three cores of tissue obtained. Histology confirmed
fibromatosis. In view of this unusual diagnosis, the case
was taken to our multidisciplinary meeting for further
discussion of management.
Figure 3. Magnetic resonance images. (a) Axial fat-saturated T1-weighted images showing an isointense lesion (arrow) at left 5 o’clock
position, hyperintense on (b) axial fat-saturated water sensitive images (arrow). (c) Axial fat-saturated T1-weighted post-contrast image
showing enhancement of the irregular lesion (arrow). (d) Sagittal T1-weighted fat-saturated post-contrast image showing the enhancing
lesion (arrow) with underlying pectoralis muscle invasion (open arrow).
The multidisciplinary meeting consensus was a trial
of systemic treatment before consideration of surgery
since the chest wall invasion of the fibromatosis
would necessitate radical surgery rather than a simple
lumpectomy, and the extent of surgical resection may be
scaled down if there was a good response to systemic
treatment. Due to the significant length of time between
the last MRI and the meeting, a repeated MRI was
performed to review the progress of the disease and
provide a new baseline prior to starting treatment, which
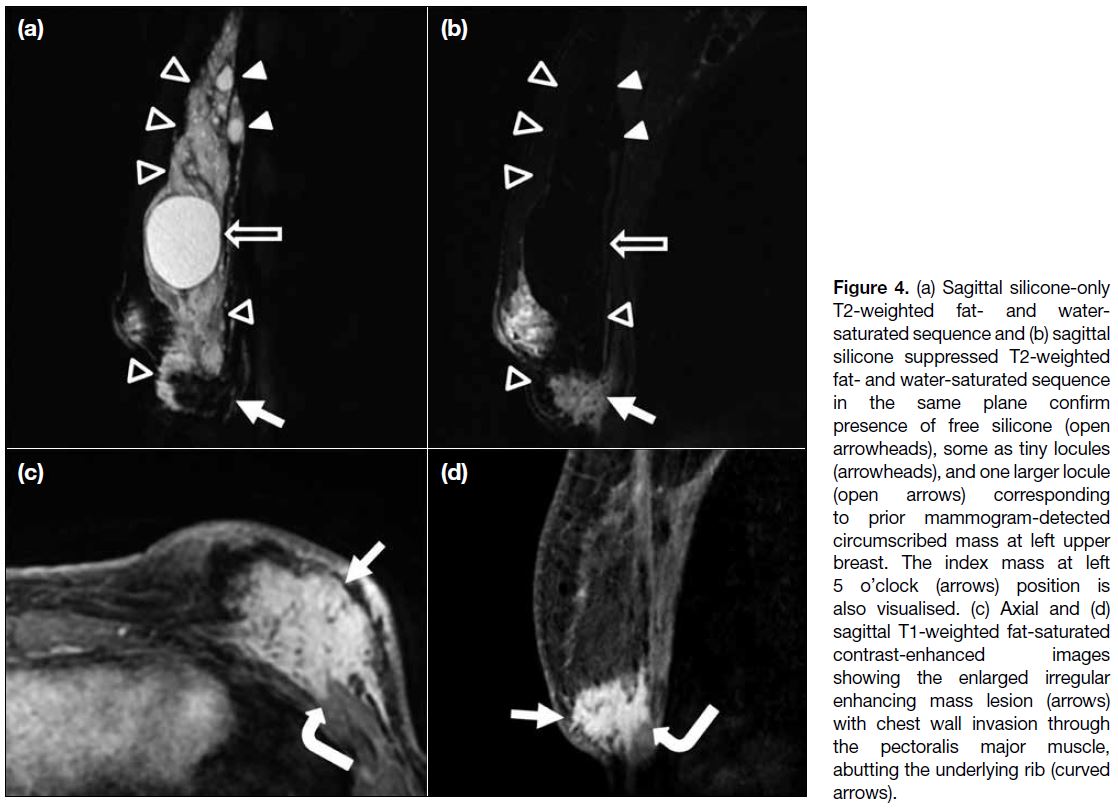
showed an increased size of the ill-defined enhancing
mass (Figure 4). The lesion invaded the pectoralis muscle
and directly abutted the underlying rib. It again showed
a type I kinetic curve on dynamic contrast images. The
patient was prescribed sulindac and tamoxifen and
reported static pain and size of lesion after 3 months. A
follow-up MRI has been arranged.
Figure 4. (a) Sagittal silicone-only T2-weighted fat- and water-saturated sequence and (b) sagittal silicone suppressed T2-weighted fat- and water-saturated sequence in the same plane confirm presence of free silicone (open arrowheads), some as tiny locules (arrowheads), and one larger locule (open arrows) corresponding to prior mammogram-detected circumscribed mass at left upper breast. The index mass at left 5 o’clock (arrows) position is also visualised. (c) Axial and (d) sagittal T1-weighted fat-saturated contrast-enhanced images showing the enlarged irregular enhancing mass lesion (arrows) with chest wall invasion through the pectoralis major muscle, abutting the underlying rib (curved arrows).
DISCUSSION
Fibromatosis is a rare soft tissue tumour that is considered of ‘intermediate nature’ due to its local aggressiveness.[1] It is not metastasising but has a high risk of recurrence.[1] [2] [3] [4]
It accounts for up to 4% of extra-abdominal fibromatosis
cases, and constitutes only 0.2% of breast tumours.[1] [4] [5]
It has been reported to be associated with trauma, prior
surgery, pregnancy, increased oestrogen level, implant,
and familial adenomatous polyposis (particularly
Gardner syndrome).[2] [4] [6] [7]
To date, fewer than 50 cases of implant-related breast
fibromatosis have been reported.[2] [3] [4] [6] [7] [8] [9] [10] [11] [12] [13] Reported cases
are seen more often with silicone implants than saline
implants, possibly due to the higher prevalence of the
former.[6] Fibromatoses are usually reported to develop
within 2 to 3 years of implant surgery.[2] [6] The exact
causal relationship between implants and fibromatosis is nonetheless unclear.[6] [7] [10] The implant material and
trauma related to the surgery may both play a role in
the development of fibromatoses in patients with breast
implants; fibromatoses arising close to or adjacent
to the fibrous capsule of a breast implant have been
reported.[6] [10] [13]
Our literature search revealed one case with silicone
implant and intracapsular rupture.[7] To the best of our
knowledge, there has been no reported case of breast
fibromatosis associated with free silicone injection. Free
silicone injection as a means of breast augmentation is
an outdated practice and uncommon in Asia and South
America. It was introduced in the 1940s but has fallen
out of favour in view of safety issues and poor cosmetic outcomes although patients with such a clinical history are still occasionally encountered.
Our case is consistent with the literature wherein breast fibromatosis is described as a mimicker of malignancy,
both clinically and radiologically.[5]
Clinically, similar to our case, patients with breast
fibromatosis are commonly reported to present with a
unilateral solitary mass, but bilateral or even multicentric
disease has been reported.[6] [14] Non-palpable disease has
also been detected on screening mammogram.[4] The
mass is usually firm or hard and can be mobile or fixed
to the chest wall.[6] [14] Nipple retraction and skin changes
have also been reported, which are features that raise a
suspicion of malignancy.[4] [6] [14] It can be slow or rapidly
growing. Since it is not metastasising, lymphadenopathy
is not a feature.
On mammogram, breast fibromatosis has a variable appearance ranging from normal (especially for small
lesions), architectural distortion, or a circumscribed
lesion, to a high-density irregular mass with spiculated
margins. Calcifications are rare.[4] [5] [6] [12] [14] On ultrasound,
features likewise vary, ranging from a circumscribed
parallel mass to a non-parallel hypoechoic mass with
obscured, irregular or spiculated borders. More common
features include hypoechogenicity, irregular border and
posterior acoustic shadowing.[4] [5] [6] [14] Similar to its clinical
presentation, these radiological features show a lot of
overlap with breast cancer and commonly point towards
malignancy after completion of triple assessment.
Unique to our patient, mammogram and ultrasound
played a very limited role in assessment of the lesion as
the presence of free silicone largely obscured the index
lesion, but these modalities clarified the nature of the
previously unknown injected material.
MRI is reported to be useful when determining the
local extent of the disease since chest wall invasion is not uncommon. It is also superior to ultrasound and
mammogram in the detection and evaluation of a mass
in the absence of breast implants or injected materials.
On MRI, breast fibromatosis has been reported to be
T1-weighted hypo- or iso-intense and T2-weighted–hypointense, but is heterogeneously hyperintense
on fat-saturated T2-weighted images.[4] [5] [6] [14] It shows
heterogeneous contrast enhancement and all three
types of kinetic curves (types I, II and III) have been
reported. The most common pattern is a progressive
enhancement curve (type I) that may point away from
the usual presumptive diagnosis of breast cancer while
not excluding the possibility.[4] [5] The MRI findings in
our patient were consistent with the literature. We
documented additionally the progression of the lesion
on serial MRI, which was not reported previously. MRI
was also useful in determination of the nature of injected
material by silicone- and water-sensitive and suppressed
sequences.
Since breast fibromatosis commonly presents as a
malignancy mimicker, core biopsy is usually performed
for histological diagnosis. These cancer-mimicking
features of the lesion also prompted the repeated core
biopsies in our patient. The histology findings are beyond
the scope of discussion of this text.
The treatment of breast fibromatoses is evolving and
remains controversial, but there had been discussion
of surgery (most commonly described is wide local
excision with clear margins), and systemic therapy
with nonsteroidal anti-inflammatory drugs such as
sulindac, hormone therapy with tamoxifen, and tyrosine
kinase inhibitors have been used.[4] [5] [6] [7] [8] [10] [12] [14] Radiotherapy
is suggested to also play a role in management.[5] [6] [7] [8] [12]
It should be kept in mind that local recurrence is not
uncommon despite treatment, and follow-up is required.[4] [5] In view of the complexity of diagnosis and management,
these cases should be presented at multidisciplinary
meetings to reach a conjoint decision.
In conclusion, radiologists should be aware of the presence
of this malignancy-mimicking entity, and the limitations
of mammogram and ultrasound in patients with a history
of free silicone injection. MRI is the imaging modality of
choice for evaluation of extent of involvement of breast fibromatosis, particularly to determine the presence
and degree of chest wall invasion. Finally, the complex
diagnosis, clinically and radiologically, warrants a
multidisciplinary team discussion to facilitate optimal
management of the patient.
REFERENCES
1. Sbaraglia M, Bellan E, Dei Tos AP. The 2020 WHO classification
of soft tissue tumours: news and perspectives. Pathologica.
2020;113:70-84. Crossref
2. Hill E, Merrill A, Korourian S, Bryant-Smith G, Henry-Tillman R,
Ochoa D. Silicone breast implant associated fibromatosis. J Surg
Case Rep. 2018;2018:rjy249. Crossref
3. Balzer BL, Weiss SW. Do biomaterials cause implant-associated
mesenchymal tumors of the breast? Analysis of 8 new cases and
review of the literature. Hum Pathol. 2009;40:1564-70. Crossref
4. Lorenzen J, Cramer M, Buck N, Friedrichs K, Graubner K, Lühr CS,
et al. Desmoid type fibromatosis of the breast: ten-year institutional
results of imaging, histopathology, and surgery. Breast Care (Basel).
2021;16:77-84. Crossref
5. Guirguis MS, Adrada B, Santiago L, Candelaria R, Arribas E. Mimickers of breast malignancy: imaging findings, pathologic concordance and clinical management. Insights Imaging.
2021;12:53. Crossref
6. Alanis L, Roth R, Lerman N, Barroeta J, Germaine P. Radiologic images of an aggressive implant-associated fibromatosis of the breast and chest wall: case report and review of the literature. Radiol Case Rep. 2017;12:431-8. Crossref
7. Mátrai Z, Tóth L, Gulyás G, Szabó É, Szentirmay Z, Kásler M. A
desmoid tumor associated with a ruptured silicone breast implant.
Plast Reconstr Surg. 2011;127:1e-4e. Crossref
8. Morales RD, Mendoza AG, Luces C, Abreu EB, Romero G, Pérez G, et al. Aggressive breast fibromatosis following augmentation mastoplasty: a series of case reports. Ecancermedicalscience.
2018;12:833. Crossref
9. Silva S, Lage P, Cabral F, Alves R, Catarino A, Félix A, et al. Bilateral breast fibromatosis after silicone prosthetics in a patient with classic familial adenomatous polyposis: a case report. Oncol Lett. 2018;16:1449-54. Crossref
10. Silva Filho AF, Alves JC, Portugal EH, Fonseca RP, Almeida AC, Pereira NA, et al. Aggressive fibromatosis (desmoid tumor) associated with breast implant: literature review and presentation of three new cases. Revista Brasileira de Cirurgia Plástica.
2017;32:361-71. Crossref
11. Park JS, Lee SE, Choi JH. Desmoid-type fibromatosis associated with silicone breast implants. J Korean Soc Radiol. 2019;80:804-9. Crossref
12. Podesta C, Sukumar A, Morgan I, Vidya R. Breast implant–related fibromatosis: a rare but important adverse effect. Eur J Plast Surg. 2021;44:275-8. Crossref
13. Jewett ST, Mead JH. Extra-abdominal desmoid arising from a capsule around a silicone breast implant. Plast Reconstr Surg. 1979;63:577-9. Crossref
14. Ng WL, Teoh SY, See MH, Rahmat K, Jayalakshmi P, Ramli MT, et al. Desmoid type fibromatosis of the breast masquerading as breast carcinoma: value of dynamic magnetic resonance imaging and its correlation. Eur J Breast Health. 2021;17:197-9. Crossref