Artificial Ascites and Hydrodissection in Percutaneous Thermal Ablation Cases at a Tertiary Institution: A Pictorial Essay
PICTORIAL ESSAY
Artificial Ascites and Hydrodissection in Percutaneous Thermal Ablation Cases at a Tertiary Institution: A Pictorial Essay
RK Mak, JB Chiang, HS Fung, WL Poon
Department of Radiology and Imaging, Queen Elizabeth Hospital, Hong Kong
Correspondence: Dr RK Mak, Department of Radiology and Imaging, Queen Elizabeth Hospital, Hong Kong. Email: mrk575@ha.org.hk
Submitted: 19 Feb 2022; Accepted: 7 Jul 2022.
Contributors: All authors designed the study. RKM acquired the data. RKM and JBC analysed the data. RKM drafted the manuscript. All authors critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: This study was approved by the Research Ethics Committee (Kowloon Central/Kowloon East) of the Hospital Authority (Ref No.: KC/KE-21-0228/ER-2). A waiver for written informed consent of patients was granted by the Committee as this manuscript is for pictorial review only and does not involve patientʼs treatment/procedure.
INTRODUCTION
Percutaneous thermal ablation is gaining popularity
as a curative form of treatment for many cancers.
With increasing demand for such treatment, there are
likewise increasing challenges when handling tumours
close to vital structures and unintended thermal injury
now needs to be considered. This may arise due to an
inadequate margin as the operator attempts to avoid a
critical structure. Artificial ascites and hydrodissection
are effective and economical techniques that can push
away critical structures, allowing a safe and complete
ablation. This pictorial review demonstrates the various
ways in which artificial ascites and hydrodissection
can be used in percutaneous ablation, and discusses the
different methods and mediums used at our institution
as well as the challenges and complications encountered
over a 3-year review period.
Cases with hydrodissection performed under ultrasound
(US), computed tomography (CT), and magnetic
resonance (MR) guidance from January 2018 to
September 2021 were retrieved from the Radiology
Information System under Hospital Authority of Hong Kong using keywords ‘hydrodissection’ and ‘artificial
ascites’. Images from illustrative cases have been
selected to showcase different areas in the body where
hydrodissection was performed.
Artificial ascites and/or hydrodissection was performed
in 128 patients at our centre, Queen Elizabeth Hospital,
with the majority involving hepatocellular carcinomas
(85.9%, n = 110) or renal cancers (10.2%, n = 13).
Miscellaneous target lesions included adrenal nodules,
iliac lymph nodes, pelvic collections, and breast nodules.
Examples of hydrodissection include the gallbladder
fossa, hepatic bare area, duodenum, and abdominal large
vessels. Hydrodissection of the renal pelvis and porta
hepatis have also been successfully performed.
All our percutaneous thermal ablation patients are
referred to us by the surgical department. Patients with
liver lesions first undergo a targeted US to ascertain
whether the index lesion can be well visualised on US.
The targeted US, as well as correlation with previous
cross-sectional imaging, also identifies nearby non-target
structures and determines whether artificial ascites or hydrodissection is needed. If the index lesion and the
hydrodissection pathway are suboptimally seen on US, a
CT-guided approach will be chosen.
An MR-guided approach for liver lesions, which is rare,
is reserved only for cases in which the lesion is both
US and CT occult. On the contrary, all renal tumours
are referred for MR-guided cryoablation. This is due to
departmental preference. Patients with renal tumours
are seen by our interventionists at the interventional
radiology clinic, where cross-sectional imaging of
patients is reviewed and the procedural details and
complications were discussed with the patient. Since
most of our MR-guided renal tumour ablations are
performed under local anaesthesia and/or conscious
sedation, one of the important issues debated during the
interventional radiology clinic session is the ability of
the patient to lie prone for an extended period of time as
this is pivotal to these procedures.
TECHNIQUES AND PROCEDURES
Ultrasound Guidance
Ultrasound-guided (USG) artificial ascites and
hydrodissection remain the most common technique,
especially for liver lesions, making up to 87.5%
(n = 112) of reviewed cases. For artificial ascites, two
methods are adopted at our institution. In the first,[1] [2] the
liver is directly punctured superficially with a 16-gauge
angiocatheter needle (Becton Dickinson Infusion
Therapy Systems Inc, Mexico) under US guidance.
The needle is removed and the patient is instructed to
breath in and out making use of the relative movement
between the peritoneum and liver to allow the plastic
sheath to fall into the peritoneal space. Concomitant
gentle advancement of a 0.035-inch guidewire (Terumo
Medical Corporation, Japan) is continued until smooth
guidewire advancement is felt. CT or fluoroscopy may
be used to confirm the intraperitoneal location of the
guidewire prior to exchange for a 6-French Neo-hydro
catheter (Bioteque Corporation, Taiwan) for fluid
infusion. Another method involves a 14- to 18-gauge
Portex Tuohy epidural needle (Smiths Medical, Czech
Republic) rather than an angiocatheter. This method is
not well described in the literature but has been proven
effective and less invasive, and is popular among our
departmental interventionists. This method utilises the
epidural needle’s upward-curving tip to displace the
liver away and allow insertion into the peritoneal cavity
while avoiding puncture of the liver capsule.[3]
In hydrodissection, the target area is punctured under US guidance, usually with a 21-gauge Chiba needle (Cook
Medical, Bloomington [IN], U.S.) or a 20-to-22-gauge
spinal needle (Becton, Dickinson and Company, U.S.),
depending on operator preference. Once the needle
is perched at the intended position, 5% dextrose (D5)
solution is carefully injected under USG visualisation
until the desired effect is achieved.
Computed Tomography Guidance
CT-guided hydrodissection is usually performed for CT-guided percutaneous thermal ablations. In these cases,
an angiocatheter or Chiba needle is inserted under CT
guidance to the desired region and fluid is injected directly
to the expected site. During our review period, 4.7%
(n = 6) of hydrodissection procedures were performed
under CT guidance, mainly for renal, adrenal and ischial
bone lesion biopsies or ablation. For CT-guided ablation
cases that require artificial ascites, the ascites is usually
created under US guidance using the technique described
in the previous section; in these cases, CT serves as an
adjunct in confirming guidewire and drain catheter
positioning.
Magnetic Resonance Imaging Guidance
MR-guided hydrodissection is performed when the
thermal ablation is subsequently performed under MR
guidance. Artificial ascites is rarely induced since most
of our MR-guided ablation cases involve renal tumours.
During our review period, 7.8% (n = 10) of cases received
MR-guided hydrodissection.
Our department uses a designated 1.5T MR imaging
machine (Siemens) for MR-guided procedures with a
DORADOnova laser marking system (LAP, Germany).
The procedure begins with patients undergoing a MR
scan for planning. T2-weighted BLADE sequence (TR/TE 3000/116 ms, field of view 256 × 256, slice thickness
6 mm) is usually performed since most lesions are visible
on this sequence and motion artefacts are reduced. The
lesion is identified, and skin entry sites and pathways for
hydrodissection and cryoprobe insertion are determined.
Their respective coordinates and slice numbers are
noted, and the skin entry sites are marked and labelled
accordingly on the patient with the aid of laser marking
projections. Then, a MR-compatible spinal needle
(Innovative Tomography Products GmbH, Germany)
is inserted to the desired area under the guidance of
MR BEAT-IRTTT sequence (Siemens, Germany;
TE/TR 2.2/5.35 ms, flip angle 50°, field of view 400
mm × 400 mm, reconstructed in-plane resolution
1.8 mm × 1.8 mm, slice thickness 4 mm, acquisition time per slice 1000 ms). After needle insertion, the patient is
scanned again (using T2-weighted BLADE sequence)
to confirm needle position. The needle is re-adjusted as
needed and the patient is re-scanned until the needle tip
is at the desired location. D5 solution is then injected
carefully. The patient is re-scanned to ensure accurate
and adequate hydrodissection.
Cryoablation accounts for more than 90% of our MR-guided
ablation cases, with <1% being MR-guided
microwave ablation. The choice of ablation method is
largely by operator preference, but also rests on the fact
that the ice-ball ablation zone in cryoablation is well
depicted on MR, which ensures good coverage of the
tumour. In contrast, microwave ablation zones are not as
clear as on MR.
Anatomical Locations Requiring Hydrodissection
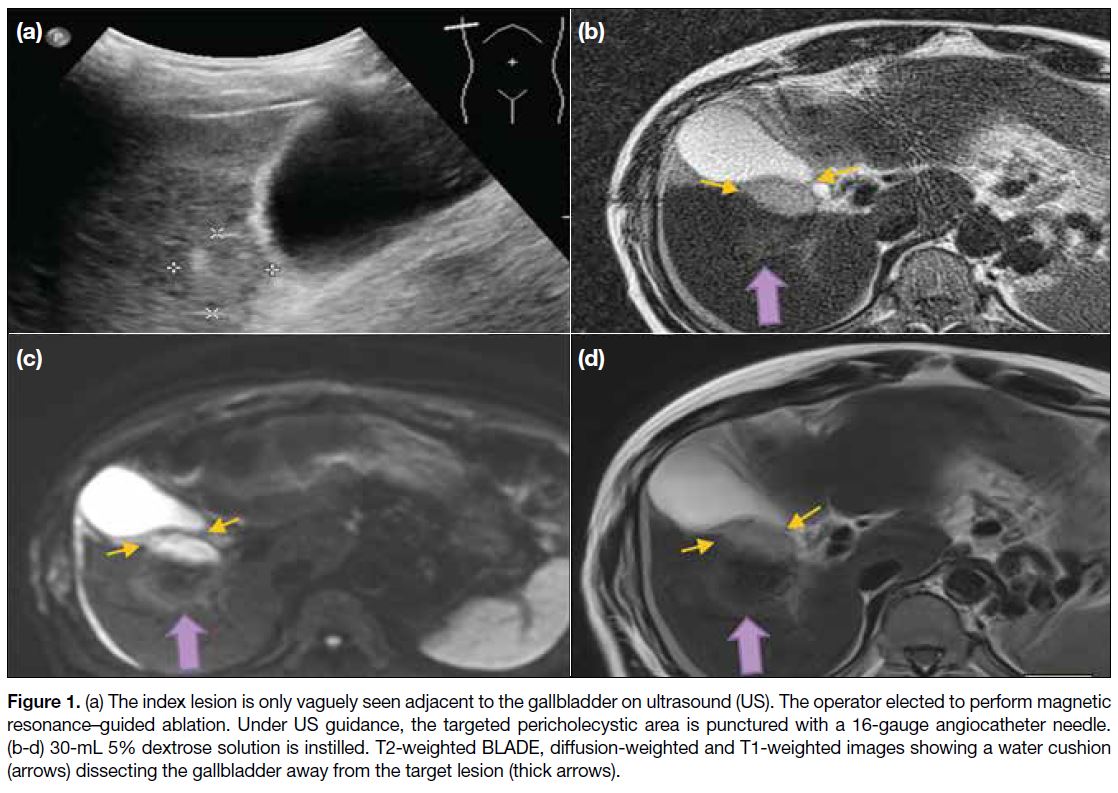
Gallbladder Fossa
Liver lesions situated in segment 5 of the liver often
require hydrodissection to dissect away the gallbladder and prevent injury and cholecystitis or rupture. This is
done by inserting a Chiba needle under US guidance
between the gallbladder and the target lesion (Figure 1).
Fluid is then injected into the gallbladder fossa under
US visualisation to cushion the gallbladder from thermal
injury.[4]
Figure 1. (a) The index lesion is only vaguely seen adjacent to the gallbladder on ultrasound (US). The operator elected to perform magnetic resonance–guided ablation. Under US guidance, the targeted pericholecystic area is punctured with a 16-gauge angiocatheter needle. (b-d) 30-mL 5% dextrose solution is instilled. T2-weighted BLADE, diffusion-weighted and T1-weighted images showing a water cushion (arrows) dissecting the gallbladder away from the target lesion (thick arrows).
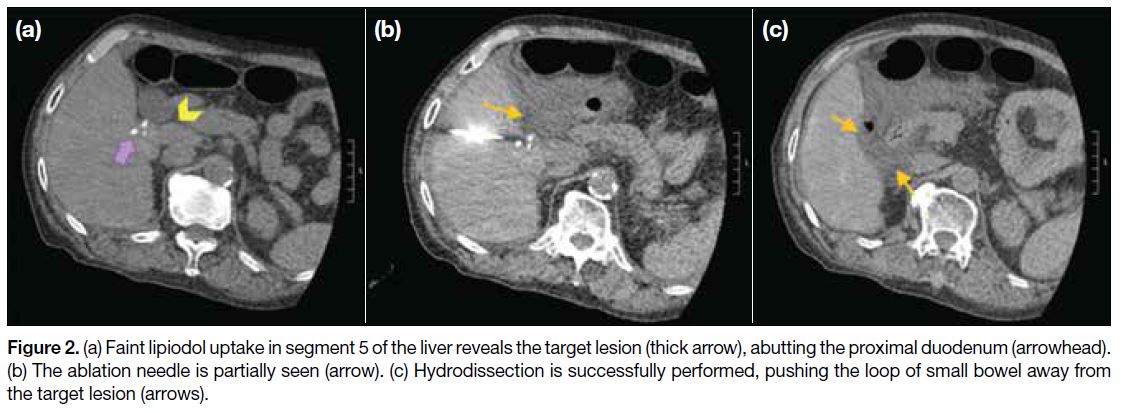
Duodenum
Often, lesions located at the caudate lobe, right inferior segment or left medial segment of the liver may abut the
proximal duodenal loop. The duodenum can be dissected
away in a similar fashion to the gallbladder (Figure 2).
Figure 2. (a) Faint lipiodol uptake in segment 5 of the liver reveals the target lesion (thick arrow), abutting the proximal duodenum (arrowhead). (b) The ablation needle is partially seen (arrow). (c) Hydrodissection is successfully performed, pushing the loop of small bowel away from the target lesion (arrows).
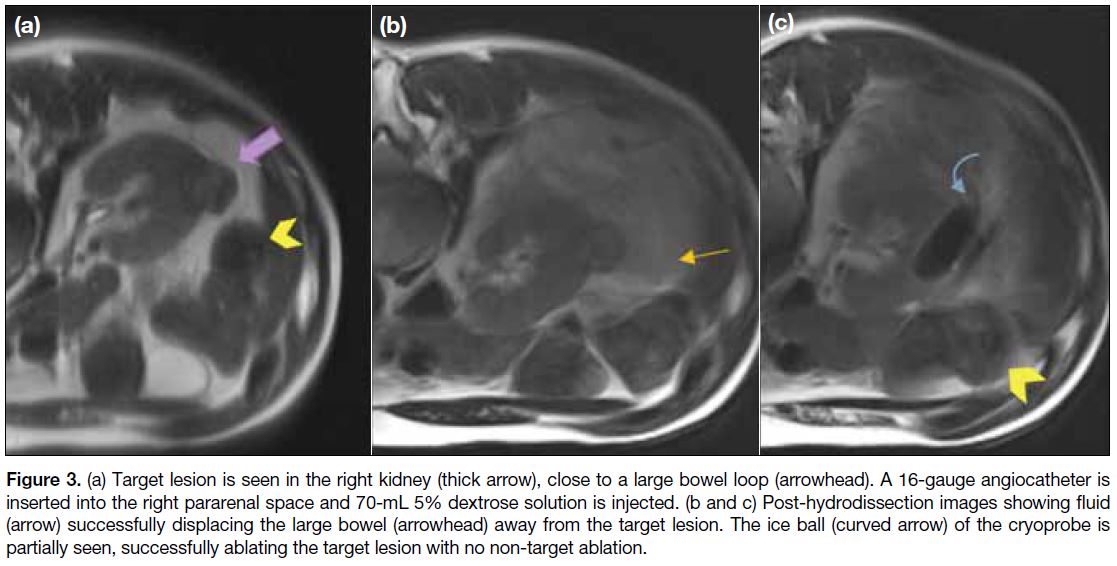
Colon
Right inferior segment liver lesions and renal lesions
are often adjacent to large bowel loops. Hydrodissection
can be performed by inserting an angiocatheter at the
pericolic space and injecting fluid under US, CT or MR
guidance (Figure 3).
Figure 3. (a) Target lesion is seen in the right kidney (thick arrow), close to a large bowel loop (arrowhead). A 16-gauge angiocatheter is inserted into the right pararenal space and 70-mL 5% dextrose solution is injected. (b and c) Post-hydrodissection images showing fluid (arrow) successfully displacing the large bowel (arrowhead) away from the target lesion. The ice ball (curved arrow) of the cryoprobe is partially seen, successfully ablating the target lesion with no non-target ablation.
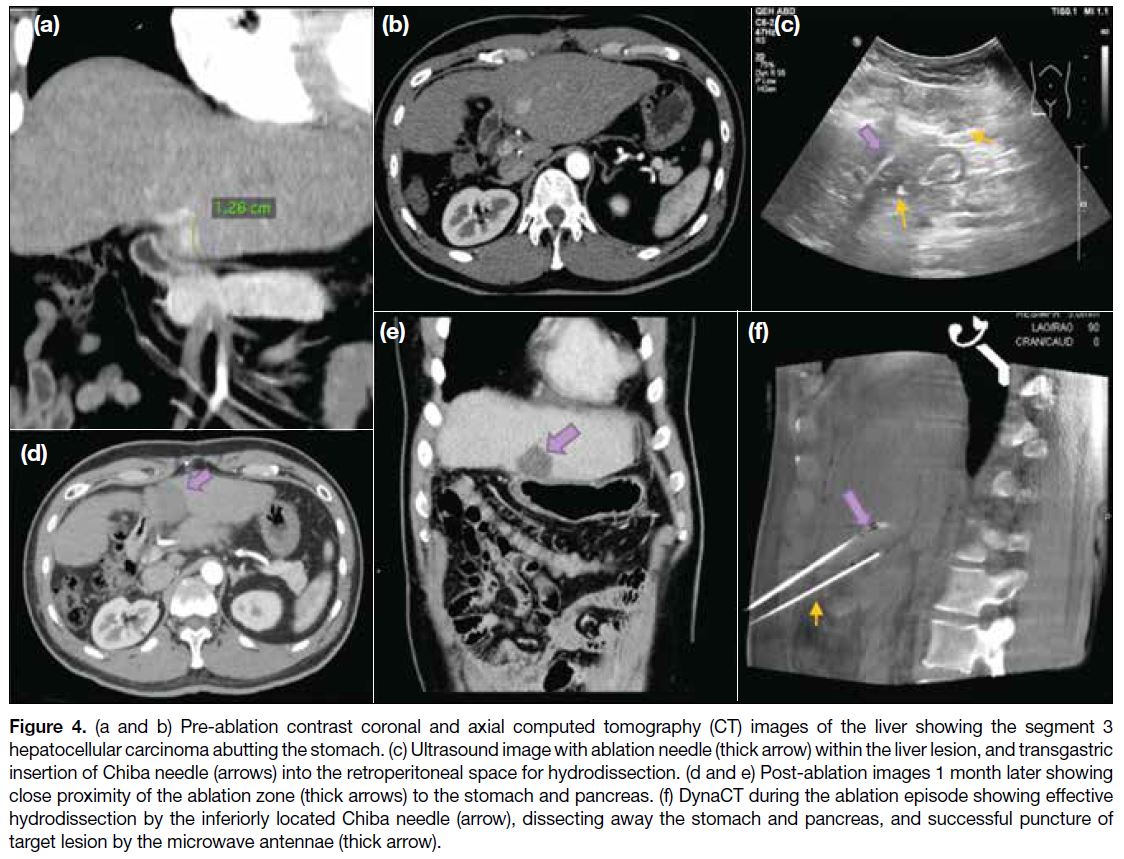
Through or Across Stomach
Lesions in deeper parts of the liver are often difficult to access as they are surrounded by various major organs,
for example the stomach and the pancreas. In two (1.6%)
of our cases during the review period, transgastric
hydrodissection was performed for ablation of liver
lesions in segment 3, aided by both US and CT. US-guided
transgastric insertion of a 21-gauge Chiba needle
into the retroperitoneal space is performed and 60- to
70-mL D5 solution is injected (Figure 4). The needle
position and hydrodissection effect is confirmed with CT.
Figure 4. (a and b) Pre-ablation contrast coronal and axial computed tomography (CT) images of the liver showing the segment 3
hepatocellular carcinoma abutting the stomach. (c) Ultrasound image with ablation needle (thick arrow) within the liver lesion, and transgastric
insertion of Chiba needle (arrows) into the retroperitoneal space for hydrodissection. (d and e) Post-ablation images 1 month later showing
close proximity of the ablation zone (thick arrows) to the stomach and pancreas. (f) DynaCT during the ablation episode showing effective
hydrodissection by the inferiorly located Chiba needle (arrow), dissecting away the stomach and pancreas, and successful puncture of
target lesion by the microwave antennae (thick arrow).
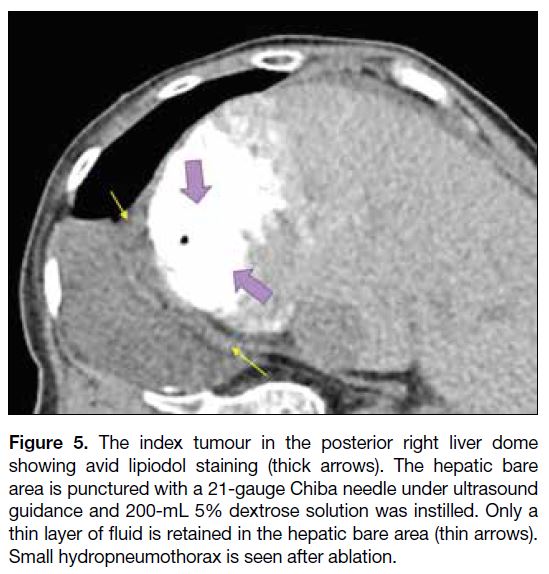
Hepatic Bare Area
Lesions can occur in the posterior liver dome and ablating
these lesions may mean injuring the diaphragm and the pleura. In these cases, hydrodissection of the hepatic bare
area is helpful.[5] [6] One case of hydrodissection of hepatic
bare area was performed during our study period. The
hepatocellular carcinoma appeared as a sizable lipiodol-stained
mass in the right liver dome, abutting the right
hemidiaphragm. Under US guidance, the hepatic bare
area was punctured with a 21-gauge Chiba needle. D5
solution was injected under USG visualisation to directly
observe the desired hydrodissection effect. In this case, the
hydrodissection effect was minimal despite instillation
of 50-mL D5 solution. Interval CT scanning showed that
the needle tip was in the correct position but that most of
the fluid had flown to the peritoneal cavity. In the end, 200-mL D5 solution was injected with a thin layer of
fluid retained at the hepatic bare area (Figure 5). The liver
dome tumour was successfully ablated. Post-ablation CT
showed a small ipsilateral hydropneumothorax that may
have been caused by inadvertent Chiba puncture or from
ablation-related minor diaphragmatic injury, and for
which a 7-French Neo-hydro drain catheter (Bioteque
Corporation, Taiwan) was inserted.
Figure 5. The index tumour in the posterior right liver dome
showing avid lipiodol staining (thick arrows). The hepatic bare
area is punctured with a 21-gauge Chiba needle under ultrasound
guidance and 200-mL 5% dextrose solution was instilled. Only a
thin layer of fluid is retained in the hepatic bare area (thin arrows).
Small hydropneumothorax is seen after ablation.
Artificial Ascites and Hydrodissection Medium
D5 solution was used as a medium in most of our cases
for artificial ascites or hydrodissection. This was due to
the high prevalence of radiofrequency ablation cases at
our centre that comprised 49.2% (n = 63) of all cases
during the review period. In these cases, D5 solution was
chosen for its reduced electrical conductivity compared
with normal saline. In non-radiofrequency ablation cases,
both D5 and normal saline solutions have been used — these choices are operator dependent or by operator
preference. In four cases (3.1%), diluted contrast was
used for hydrodissection. Diluted contrast has the added
advantage of providing stark contrast to adjacent intra-abdominal
organs in unenhanced CT-guided procedures.
Complications
During the review period, there were 12 cases (9.4%)
of failed hydrodissection. All were due to iatrogenic
adhesions, mostly due to previous surgeries. In one
case, ablation was still performed successfully but
with possible irritation of the diaphragm causing chest
and shoulder pain. There were seven cases (5.5%) of
hydrodissection-related complications that included
three cases of inadvertent pleural effusion, one case of
pneumothorax, one case of gallbladder puncture, one
case of hepatic venous injury, and one case of portal
venous injury. Pleural-related complications may be due
to unintentional puncture or guidewire manipulation and
subsequent catheterisation of the pleural cavity, possibly
due to puncture near the pleural recess, or due to passage
of the guidewire through diaphragmatic fenestrations. In
the same inadvertent manner, a hepatic venous branch
and a portal venous branch were respectively punctured.
In the case of hepatic venous injury, the left liver lobe was punctured with a 16-gauge angiocatheter needle.
Unopposed guidewire manipulation was felt so the
catheter-guidewire system was thought to be in the
peritoneal cavity, and a 6-French Neo-hydro drain
catheter was inserted over the guidewire. Nonetheless
active venous bleeding was noted from the drain
catheter, and angiogram through the drain catheter under
fluoroscopy showed misplacement of the drain within
a hepatic vein. A 5-French BRITE TIP sheath (Cordis,
Miami [FL], United States) was exchanged and the
segmental hepatic vein was cannulated with a 5-French
C1 catheter (Terumo Medical Corporation, Japan). This
was followed by embolisation with one 12 mm × 40 cm
Interlock coil, followed by 50% n-butyl cyanoacrylate
glue (mixed with lipiodol) embolisation of the needle
tract. No gross intra-abdominal haemorrhage was
evident on postoperative US. The ablation procedure
was aborted for this case and the patient subsequently
referred for transarterial chemo-embolisation.
The cases of portal venous injury and inadvertent gallbladder puncture were self-limiting. Other known
complications documented in the literature such as
hydro-electrolytic disorders were not encountered in our
case series. No cases of non-target ablations occurred
during the review period.
CONCLUSION
Adequate artificial ascites and hydrodissection enable
the operator to perform ablation in difficult locations by
reducing the risk of non-target ablation, hence allowing
ablation with an adequate margin. Hydrodissection also
has the benefit of aiding access to previously difficult-to-access lesions. Additionally, hydrodissection may
improve the patient’s comfort during percutaneous
intervention, for example reducing pain when the tumour
is adjacent to the diaphragm. Further investigation
into patient comfort and treatment outcomes would
be worthwhile. Comparison of the efficacy of
hydrodissection with other thermoprotective methods
such as endoluminal cooling, pneumodissection or organ
filling would also be helpful.
To conclude, artificial ascites and hydrodissection
are easy, effective and economical ways to achieve
thermal protection and improve lesion accessibility for
ablations.
REFERENCES
1. Kim JW, Shin SS, Heo SH, Hong JH, Lim HS, Seon HJ, et al. Ultrasound-guided percutaneous radiofrequency ablation of liver tumors: how we do it safely and completely. Korean J Radiol.
2015;16:1226-39. Crossref
2. Garnon J, Cazzato RL, Caudrelier J, Nouri-Neuville M, Rao P, Boatta E, et al. Adjunctive thermoprotection during percutaneous thermal ablation procedures: review of current techniques. Cardiovasc Intervent Radiol. 2019;42:344-57. Crossref
3. Hinshaw JL, Lubner MG, Ziemlewicz TJ, Lee FT Jr, Brace CL.
Percutaneous tumor ablation tools: microwave, radiofrequency,
or cryoablation — what should you use and why? Radiographics.
2014;34:1344-62. Crossref
4. Garnon J, Koch G, Caudrelier J, Ramamurthy N, Auloge P, Cazzato
RL, et al. Hydrodissection of the gallbladder bed: a technique for
ablations located close to the gallbladder. Cardiovasc Intervent
Radiol. 2019;42:1029-35. Crossref
5. Garnon J, Cazzato RL, Auloge P, Ramamurthy N, Koch G, Gangi
A. Adjunctive hydrodissection of the bare area of liver during
percutaneous thermal ablation of sub-cardiac hepatic tumours.
Abdom Radiol (NY). 2020;45:3352-60. Crossref
6. Rhim H, Lim HK. Radiofrequency ablation for hepatocellular carcinoma abutting the diaphragm: the value of artificial ascites. Abdom Imaging. 2009;34:371-80. Crossref