Successful Endovascular Management of Iatrogenic Aortic Coarctation Following Total Aortic Arch Repair in Type B Dissection: a Case Report
CASE PEPORT
Hong Kong J Radiol 2023 Jun;26(2):138-41 | Epub 15 May 2023
Successful Endovascular Management of Iatrogenic Aortic Coarctation Following Total Aortic Arch Repair in Type B
Dissection: a Case Report
PP Ng1, SCW Cheung2, CKL Ho3, KKY Man2, KKH Choi2
1 Department of Radiology and Imaging, Queen Elizabeth Hospital, Hong Kong SAR, China
2 Department of Radiology, Queen Mary Hospital, Hong Kong SAR, China
3 Department of Cardiothoracic Surgery, Queen Mary Hospital, Hong Kong SAR, China
Correspondence: Dr PP Ng, Department of Radiology and Imaging, Queen Elizabeth Hospital, Hong Kong SAR, China. Email: npp782@ha.org.hk
Submitted: 6 Oct 2021; Accepted: 26 Jan 2022.
Contributors: All authors designed the study. PPN and KKHC acquired the data. All authors analysed the data. PPN, SCWC, CKLH and KKHC drafted the manuscript. All authors critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: The authors have no conflicts of interest to declare.
Funding/Support: This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: The patients were treated in accordance with the Declaration of Helsinki and provided written informed consent for all treatments and procedures and consent for publication.
INTRODUCTION
Frozen elephant trunk (FET) technique has been
commonly used in the management of a wide variety
of thoracic aortic pathology. Postoperative kinking
of an FET stent graft resulting in clinically significant
aortic coarctation is rare. We report a challenging case
of a patient with chronic type B aortic dissection who
underwent FET repair that was complicated by clinically
significant FET graft kinking but successfully treated
with endovascular stenting.
CASE REPORT
A 47-year-old man with a history of chronic type B
aortic dissection had a dissection flap starting just distal
to the origin of the aberrant right subclavian artery
(SCA) and extending down to the aortic bifurcation.
Sequential computed tomography angiography (CTA)
showed progressive dilatation of an aortic arch dissecting
aneurysm (5.8 cm) and narrowing of the true lumen. In
May 2021, he underwent ascending and total aortic arch
replacement with FET graft (Thoraflex Hybrid; Terumo
Aortic, Renfrewshire, United Kingdom), aorto-right axillary artery bypass and embolisation of the aberrant right SCA with Amplatz vascular plug (Abbott Medical, Plymouth [MN], US).
Following the surgery, he developed persistent
hypertension despite multiple antihypertensive
medications and progressive respiratory distress. There
was significant upper and lower limb blood pressure
(BP) discrepancy up to 40 mmHg. Echocardiogram
demonstrated preserved left ventricular ejection fraction
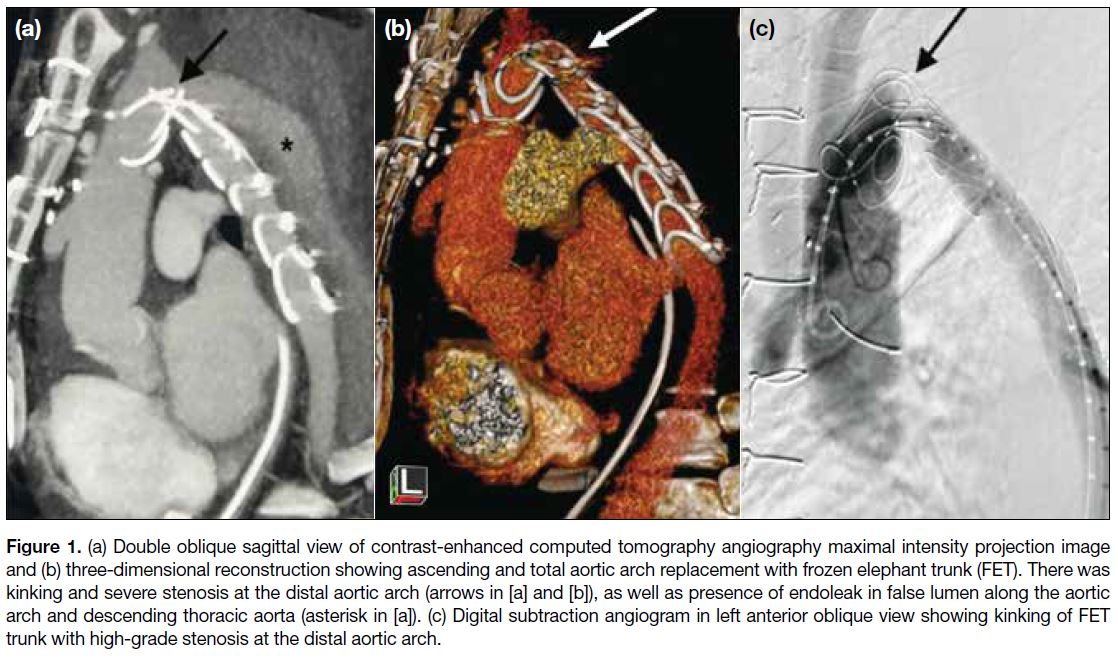
(60%). CTA on postoperative day 5 revealed kinking of
the FET stent graft at the distal aortic arch with marked
luminal stenosis (Figure 1a and b). Another finding was
an endoleak from the aberrant right SCA, later managed
by surgical ligation of the right SCA origin. Aortic
angiogram confirmed high-grade stenosis of the FET
graft at the distal aortic arch (Figure 1c). Intra-arterial
BP measurement revealed a 53-mmHg pressure gradient
across the stenosis.
Figure 1. (a) Double oblique sagittal view of contrast-enhanced computed tomography angiography maximal intensity projection image
and (b) three-dimensional reconstruction showing ascending and total aortic arch replacement with frozen elephant trunk (FET). There was
kinking and severe stenosis at the distal aortic arch (arrows in [a] and [b]), as well as presence of endoleak in false lumen along the aortic
arch and descending thoracic aorta (asterisk in [a]). (c) Digital subtraction angiogram in left anterior oblique view showing kinking of FET
trunk with high-grade stenosis at the distal aortic arch.
Endovascular stenting of the iatrogenic aortic coarctation was performed with a bare-metal stent (BMS) [Sinus-XL 34 × 100 mm; OptiMed, Ettlingen, Germany]
under local anaesthesia. Due to the acute angulation at the
aortic arch and tight stenosis, an attempt to deploy across
the stenosis within the FET was particularly challenging.
Difficulties were encountered during passage of the
delivery system across the stenosis as well as retraction
of the outer sheath for stent deployment. To overcome
these difficulties, balloon dilatation of the stenosis was
first performed. This was followed by partial deployment
of two-thirds of the stent within a 12-F sheath (Cook
Medical, Bloomington [IN], US) with the sheath tip
rested at the non-kinked portion of the FET. The entire
complex was then advanced proximally to the desirable
zone. The partially opened stent was deployed across the
stenosis by unsheathing the 12-F sheath with constant
forward pressure on the delivery system to avoid distal
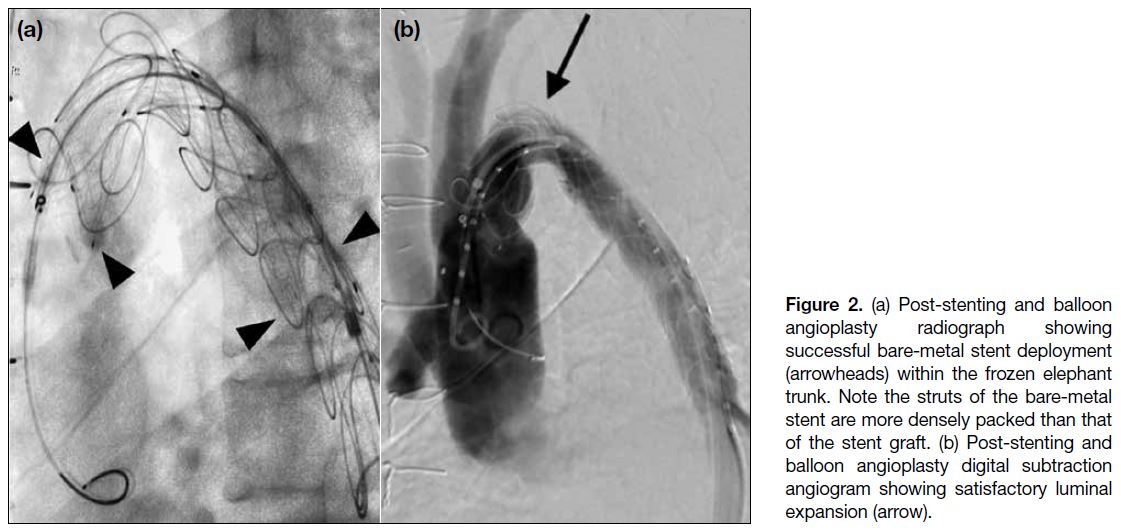
migration. Post-stenting dilatation with a 32-mm Coda
balloon catheter (Cook Medical, Bloomington [IN], US)
achieved satisfactory luminal expansion (Figure 2). Post-stenting
BP gradient improved to 20 mmHg.
Figure 2. (a) Post-stenting and balloon angioplasty radiograph showing successful bare-metal stent deployment (arrowheads) within the frozen elephant trunk. Note the struts of the bare-metal stent are more densely packed than that of the stent graft. (b) Post-stenting and balloon angioplasty digital subtraction angiogram showing satisfactory luminal expansion (arrow).
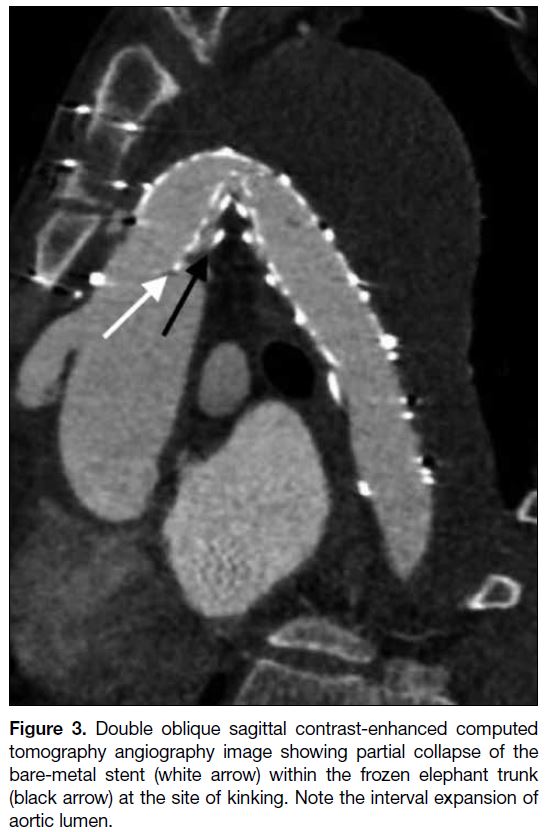
On day 3 post-stenting, the patient developed haemolytic
anaemia and syncope. Urgent CTA revealed no source
of bleeding. The BMS across the coarctation was
partially collapsed but there was interval luminal gain
when compared with preprocedural CTA (Figure 3). The haemolytic anaemia was suspected to be stent-related;
a repeat aortic angiogram was performed on
postoperative day 14 and demonstrated a small residual
pressure gradient (8 mmHg) across the kinking. Since
the stent remained partially collapsed, repeat balloon
angioplasty was performed with 32-mm Coda balloon
(Cook Medical, Bloomington [IN], US) and 26-mm
Atlas balloon (Bard Medical, New Providence [NJ],
US). Despite the lack of significant stent expansion
on fluoroscopy, the pressure gradient disappeared by
the end of procedure. The patient made an uneventful
recovery and was subsequently discharged.
Figure 3. Double oblique sagittal contrast-enhanced computed tomography angiography image showing partial collapse of the bare-metal stent (white arrow) within the frozen elephant trunk (black arrow) at the site of kinking. Note the interval expansion of aortic lumen.
Follow-up CTA performed 1 month post-stenting
revealed no interval collapse of the stent or luminal re-stenosis.
The patient was asymptomatic with no upper
and lower limb BP discrepancy or haemoglobin drop.
DISCUSSION
Although the FET technique is now widely used
to manage thoracic aortic dissection, postoperative
kinking of the stent graft that necessitates secondary
intervention has rarely been reported in the literature.
FET kinking often occurs at the junction of the distal
aortic arch and descending aorta.[1] Risk factors for graft
kinking include acute aortic arch angulation, marked true lumen narrowing, a rigid chronic dissection membrane,
reperfusion of false lumen, use of a low radial force
device or presence of a non-stent part in the FET graft.[2] [3] [4]
Although rare, kinking can result in coarctation, graft
thrombosis, haemolytic anaemia, and heart failure.
Secondary surgery to rectify the kinked graft can be
complex and necessitate cardiopulmonary bypass.
Endovascular repair offers a minimally invasive option
that can be performed under local anaesthesia. Realigning
a stent within the kinked graft may provide adequate
scaffolding to maintain graft patency. Complications
include stent fracture, migration, and collapse.
Graft kinking in this patient was both clinically and
radiologically significant, as evidenced by the presence
of heart failure, haemolytic anaemia, marked luminal
narrowing on imaging, and significant pressure gradient.
Possible contributing factors in this case included acute
aortic arch angulation, rigid chronic dissection flap, and
stenotic true lumen. As angioplasty alone would not
have provided adequate scaffolding, alignment of the
FET with stent was chosen.
An ideal stent in this scenario would have high radial
force without compromised flexibility. Stent grafts are
often preferred since they are available in a wide range
of sizes, up to 46 mm in diameter, and with tapering
property; they also provide additional safety in case of inadvertent aortic rupture during the procedure. In our
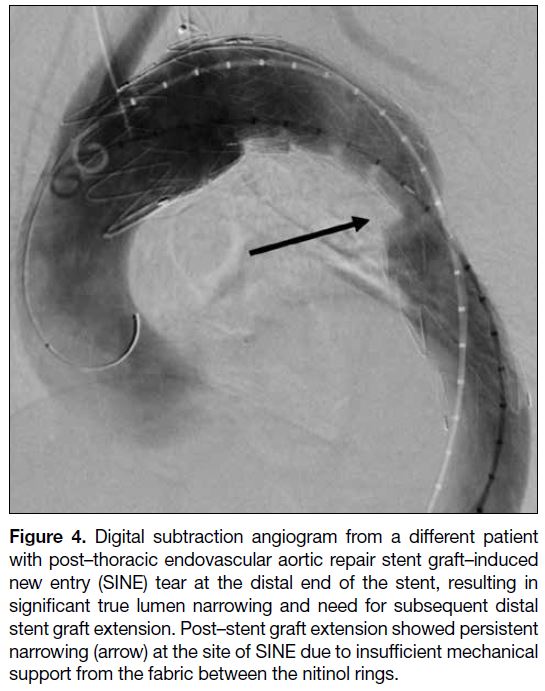
experience, better support can be achieved by positioning
the stent struts at the most stenotic point to maximise
mechanical support (Figure 4). This is more achievable
with BMS because their stent struts are more densely
packed compared with stent grafts where the nitinol
rings are typically separated by 10 to 15 mm of fabric.
Figure 4. Digital subtraction angiogram from a different patient
with post–thoracic endovascular aortic repair stent graft–induced
new entry (SINE) tear at the distal end of the stent, resulting in
significant true lumen narrowing and need for subsequent distal
stent graft extension. Post–stent graft extension showed persistent
narrowing (arrow) at the site of SINE due to insufficient mechanical
support from the fabric between the nitinol rings.
Sinus-XL is a self-expanding uncovered nitinol stent
with a closed-cell design that has been proven to be safe
and durable in treating adult aortic coarctation.[5] Despite
its low profile and the ability to deliver up to 36 mm stent
within a 10-F introducer, we experienced difficulties in
traversing the kink and stent deployment because the
acute angle prevented outer-sheath retraction by the
coaxial pull-back system. This would have been even
more challenging if a similarly sized stent graft had been
chosen since it would have required 20-F access.
Most stent manufacturers discourage operators from
deploying stents across an acute angle due to potential
entrapment of the delivery system. Nonetheless stent
deployment can be facilitated by methods that straighten the target coverage, as in our patient. Other methods
include use of buddy wires or the body flossing technique.
These techniques were less appropriate for our patient
given the recent surgical anastomosis; overcorrecting
the existing anatomy might have increased the risk of
anastomosis-related complications. Moreover, degree of
stent apposition does not necessarily correlate with the
pressure gradient across the kinking, as illustrated in this
case. Thus, in cases of tight stenosis, aggressive balloon
dilatation to pursue a ‘perfectly’ expanded stent may not
be necessary and should be avoided.
In conclusion, FET stent graft kinking is rarely
encountered but can result in significant haemodynamic
consequences. Endovascular stenting is a safe and
effective alternative to surgery. In our patient, we
illustrate the advantages of a BMS over a stent graft
in offering more focal support to the kinked portion
in a low-profile setting. Stenting across the severely
kinked graft can be technically challenging and require
additional manoeuvres, as demonstrated by our case.
To date, no study has compared BMS with covered
stents for treatment of aortic coarctation; head-to-head
bench comparison with reference to radial forces is
also lacking. Although we believe that endovascular
repair can be considered for patients with FET graft
kinking to avoid a second major operation, the decision
to use a BMS or covered stent should be tailored to the
individual and take account of the underlying pathology
and anatomy.
REFERENCES
1. Roselli EE, Bakaeen FG, Johnston DR, Soltesz EG, Tong MZ. Role of the frozen elephant trunk procedure for chronic aortic dissection. Eur J Cardiothorac Surg. 2017;51(suppl 1):i35-9. Crossref
2. Shrestha M, Bachet J, Bavaria J, Carrel TP, De Paulis R, Di Bartolomeo R, et al. Current status and recommendations for use of the frozen elephant trunk technique: a position paper by the Vascular Domain of EACTS. Eur J Cardiothorac Surg.
2015;47:759-69. Crossref
3. Morisaki A, Isomura T, Fukada Y, Yoshida M. Kinking of an open stent graft after total arch replacement with the frozen elephant technique for acute type A aortic dissection. Interact Cardiovasc Thorac Surg. 2018;26:875-7. Crossref
4. Ouzounian M, Hage A, Chung J, Stevens LM, El-Hamamsy I, Chauvette V, et al. Hybrid arch frozen elephant trunk repair: evidence from the Canadian Thoracic Aortic Collaborative. Ann
Cardiothorac Surg. 2020;9:189-96. Crossref
5. Kische S, D’Ancona G, Stoeckicht Y, Ortak J, Elsässer A, Ince H. Percutaneous treatment of adult isthmic aortic coarctation: acute and long-term clinical and imaging outcome with a self-expandable uncovered nitinol stent. Circ Cardiovasc Interv. 2015;8:e001799. Crossref