Hysterosalpingographic Findings from Uterus to Peritoneal Cavity: A Pictorial Essay
PICTORIAL ESSAY
Hong Kong J Radiol 2023 Jun;26(2):153-60 | Epub 8 May 2023
Hysterosalpingographic Findings from Uterus to Peritoneal Cavity: A Pictorial Essay
SC Wong, KS Yung, RLS Chan, WH Luk
Department of Radiology, Princess Margaret Hospital, Hong Kong SAR, China
Correspondence: Dr Dr SC Wong, Department of Radiology, Princess Margaret Hospital, Hong Kong SAR, China. Email: wsc696@ha.org.hk
Submitted: 7 Jun 2021; Accepted: 3 Aug 2021.
Contributors: All authors designed the study, acquired the data, and analysed the data. SCW drafted the manuscript. All authors critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest: All authors have disclosed no conflicts of interest.
Funding/Support: This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability: All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics Approval: This study was approved by the Kowloon West Cluster Research Ethics Committee of Hospital Authority, Hong Kong [Ref No.: KW/EX-21-108(161-08)]. Patient consent was waived by the Committee.
BACKGROUND
With social trends of late marriage and increasing maternal age, there is ongoing demand for assisted reproduction
and subfertility investigations. Since tubal occlusion is
an important cause of female subfertility,[1] assessment of
tubal patency is a crucial part of investigations to identify
the cause of subfertility.
Hysterosalpingography (HSG) is a fluoroscopic
examination of the uterus and fallopian tubes with
contrast instillation through the cervical canal. It has
remained a popular investigation of tubal patency in
modern reproductive medicine despite being invented
more than 100 years ago.[2] It is considered a standard
first-line test for assessment of tubal patency[3] [4] in view
of its reliability, less invasiveness than laparoscopy, and
more efficient use of medical resources.[4]
With age-related fertility decline,[4] expeditious
management is essential. Severity of tubal disease
identified on HSG guides the management decision
for intervention.[5] A finding of bilateral tubal occlusion
prompts early referral for consideration of in vitro
fertilisation. Detection of uterine cavity or contour
abnormalities on HSG can guide further imaging, endoscopic investigations, and intervention. A
radiologist’s familiarity with HSG interpretation and
awareness of the spectrum of pathology is advantageous
to overall patient care and outcome.
This article presents a pictorial review of HSG cases with emphasis on a spectrum of pathologies including tubal
occlusion, tuboperitoneal pathologies, uterine contour
anomalies, and intracavity filling defects.
PERFORMING HYSTEROSALPINGOGRAPHY
Prior to instrumentation of the uterine cavity, exclusion of pregnancy and active pelvic infection are of utmost
importance.
The optimal time to perform HSG is between day 7 and
12 of the menstrual cycle.[6] This helps avoid pregnancy
and improve image interpretation with the thinner
endometrium of the early proliferative phase. Conducting
the examination during active menstruation may impair
assessment of the endometrial cavity configuration.
Contrast injection into an already distended uterine
cavity during active heavy menstruation may also cause
unnecessary patient discomfort.
The patient should be positioned in a lithotomy position
for pelvic examination. Aseptic technique with cleansing
and draping of the perineum, speculum examination to
expose the cervical os, followed by cleansing of the
ectocervix and vagina are mandatory before catheter
insertion to minimise the risk of ascending infection.
Different catheters can be used for an HSG, including
infant Foley catheter (8 Fr) or commercially available
HSG 5F catheters (CooperSurgical, Trumbull [CT],
United States). In our institution, an 8-Fr infant Foley
catheter and water-soluble contrast (such as iohexol or
iodixanol) are used.
Flushing of the Foley catheter and all extension tubes
to eliminate dead space before catheterisation will
help reduce introduction of air bubbles into the uterus.
Catheter insertion followed by slow gentle inflation of the
balloon (around 1-3 mL of water) to secure the catheter
is required. Nulliparous patients generally tolerate a
lower volume of balloon distension than patients with
prior pregnancy.
With successful catheterisation, the patient is repositioned
supine for fluoroscopic examination. The standard
views in HSG are based on the recommendations of the
American College of Radiology’s Practice Parameter for
the Performance of Hysterosalpingography.[6] [7] Chapman
& Nakielny’s Guide to Radiological Procedures[8] is also
in consensus with the above references.
Based on the above recommendations, the standard set
in each HSG study should contain four images: (1) early
uterine filling (to assess small uterine filling defects);
(2) late uterine filling and tubal filling (to assess uterine
contour and tubal abnormalities); (3) peritoneal spillage
(to document tubal patency), and (4) an image taken after
Foley balloon deflation and catheter removal (to assess
the lower uterine segment and endocervical canal).
If tubal occlusion is suspected, manoeuvres such as
delayed screening, decubitus position, and use of
spasmolytic agents (glucagon or hyoscine butylbromide)
should be performed in an attempt to determine if it is
genuine.
HYSTEROSALPINGOGRAPHIC FINDINGS
Normal Anatomy
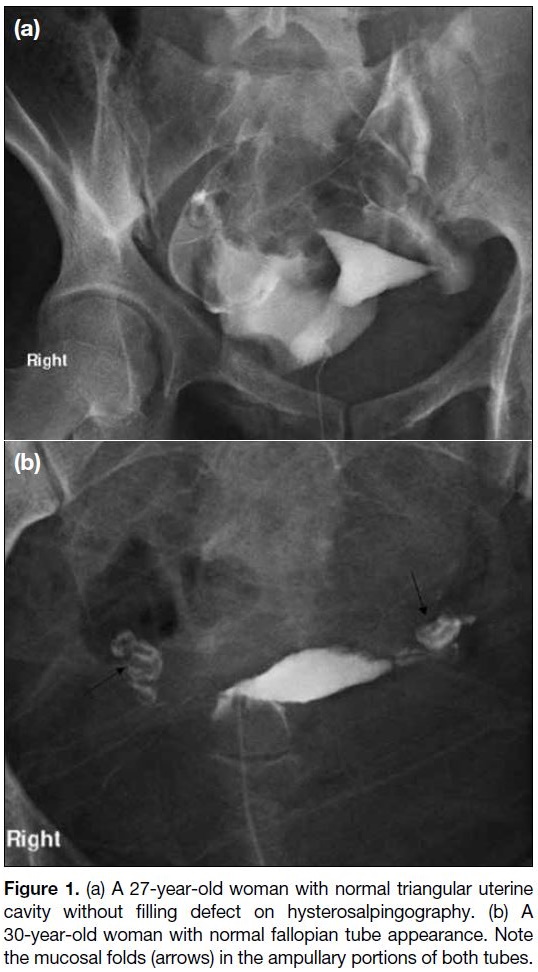
The uterine cavity has a well-defined smooth border with
inverted triangular shape and no persistent filling defect (Figure 1a). The fallopian tubes are evident as thin,
elongated smooth lines with a widening at the ampullary
portion and variable pelvic location.[6] Opacification
of the ampullary portion of the fallopian tube can be
confirmed by visualisation of mucosal folding (Figure 1b).[6] Free peritoneal contrast spillage from the fimbrial
end indicates tubal patency.
Figure 1. (a) A 27-year-old woman with normal triangular uterine
cavity without filling defect on hysterosalpingography. (b) A
30-year-old woman with normal fallopian tube appearance. Note
the mucosal folds (arrows) in the ampullary portions of both tubes.
Tubal Pathology
Non-opacification of the fallopian tube or absence of
peritoneal contrast spillage can be due to cornual smooth
muscle spasm or genuine tubal pathology. The sensitivity
and specificity of assessing bilateral tubal patency or
occlusion on HSG has been reported to be 92.1% and
85.7%, respectively.[9] There is no published consensus on
the most effective manoeuvre to relieve cornual spasm. Laparoscopy is considered as the traditional clinical
reference standard for diagnosis of tubal disease.[5] [9]
Chromotubation of the fallopian tubes with contrast dye
injection and visualisation of peritoneal dye spillage
provides assessment of tubal patency.
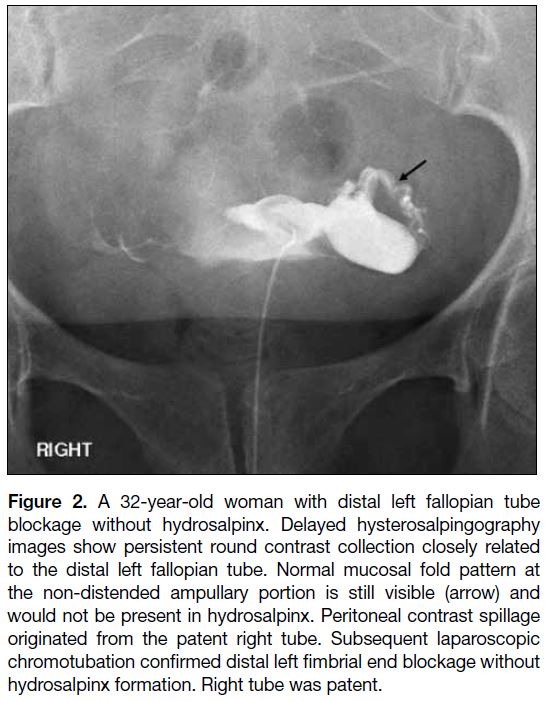
Tubal Occlusion
Tubal occlusion manifests as abrupt transition from
the contrast-filled proximal fallopian tube to a non-opacified
distal portion and absent peritoneal contrast
spillage (Figure 2). Tubal occlusion can be due to pelvic
adhesions from prior pelvic inflammatory disease,
endometriosis, or less commonly to congenital Müllerian
duct malformation.[6] [10]
Figure 2. A 32-year-old woman with distal left fallopian tube
blockage without hydrosalpinx. Delayed hysterosalpingography
images show persistent round contrast collection closely related
to the distal left fallopian tube. Normal mucosal fold pattern at
the non-distended ampullary portion is still visible (arrow) and
would not be present in hydrosalpinx. Peritoneal contrast spillage
originated from the patent right tube. Subsequent laparoscopic
chromotubation confirmed distal left fimbrial end blockage without
hydrosalpinx formation. Right tube was patent.
Tuboperitoneal Pathologies
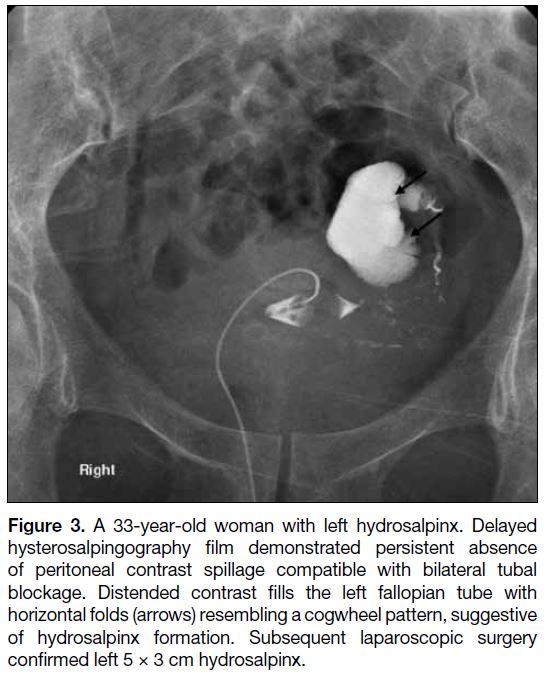
Hydrosalpinx
Hydrosalpinx represents a distended fallopian tube with
serous fluid accumulation secondary to tubal blockage
(Figure 3).[11] It is associated with pelvic inflammatory
disease, endometriosis, or prior tubal surgery (e.g.,
ligation), tubal pregnancy or rarely tubal malignancy. On
HSG, hydrosalpinx manifests as contrast-filled dilated
fallopian tubes without distal contrast spillage into the
peritoneal cavity.[6]
Figure 3. A 33-year-old woman with left hydrosalpinx. Delayed
hysterosalpingography film demonstrated persistent absence
of peritoneal contrast spillage compatible with bilateral tubal
blockage. Distended contrast fills the left fallopian tube with
horizontal folds (arrows) resembling a cogwheel pattern, suggestive
of hydrosalpinx formation. Subsequent laparoscopic surgery
confirmed left 5 × 3 cm hydrosalpinx.
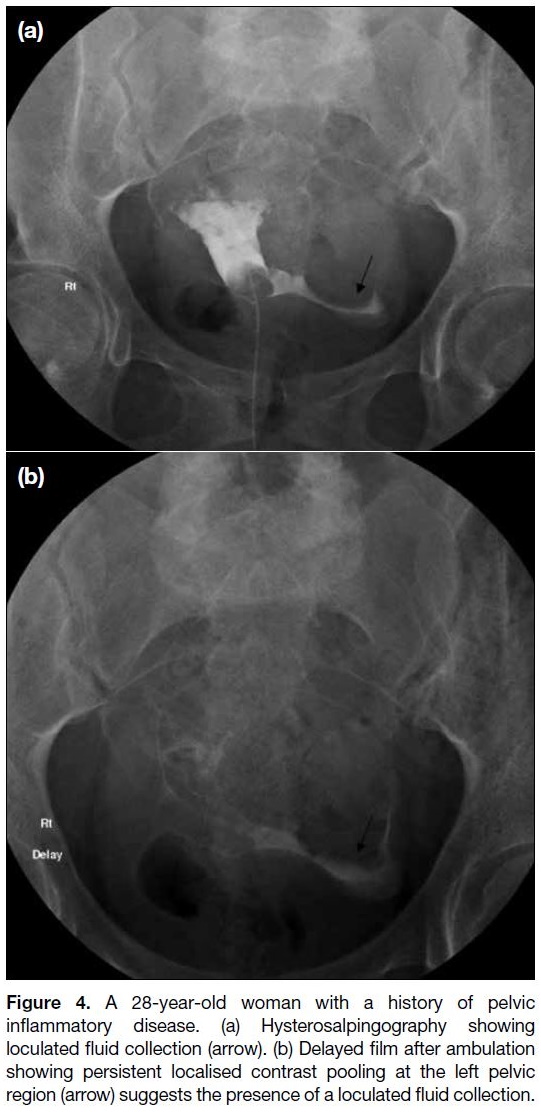
Peritubal adhesion and pelvic peritoneal loculated
collections
Pelvic inflammatory disease can result in pelvic and
peritoneal scarring and consequent adhesion bands
around the pelvic organs.[11] Adhesions around the
fallopian tubes can result in tubal blockage with loculated
contrast collections.[6] A loculated pelvic collection can
manifest as a persistent localised contrast-filled region in
delayed screening on HSG (Figure 4).
Figure 4. A 28-year-old woman with a history of pelvic
inflammatory disease. (a) Hysterosalpingography showing
loculated fluid collection (arrow). (b) Delayed film after ambulation
showing persistent localised contrast pooling at the left pelvic
region (arrow) suggests the presence of a loculated fluid collection.
Salpingitis isthmica nodosa
Salpingitis isthmica nodosa (SIN) is a fallopian tube
disease of unknown aetiology that is characterised by
proximal tubal (isthmic portion) nodular thickening,
associated diverticulosis of isthmic tubal epithelium
invading into the muscular layer with secondary smooth
muscle hypertrophy, and preserved smooth serosal
surface (Figure 5).[12] Features are more often bilateral than
unilateral. The reported incidence of SIN is 0.6% to 11%
in healthy fertile females with a strong association with
infertility and ectopic pregnancy.[13] Although SIN can
be diagnosed on HSG from its distinctive appearance,
histological proof is the gold standard.[12] The presence of
nodular outpouchings along the cornual isthmic portions
of fallopian tubes on HSG represents contrast-filled tubal
diverticula.[6] [10] The tubal outpouchings may measure up to 2 cm.[12]
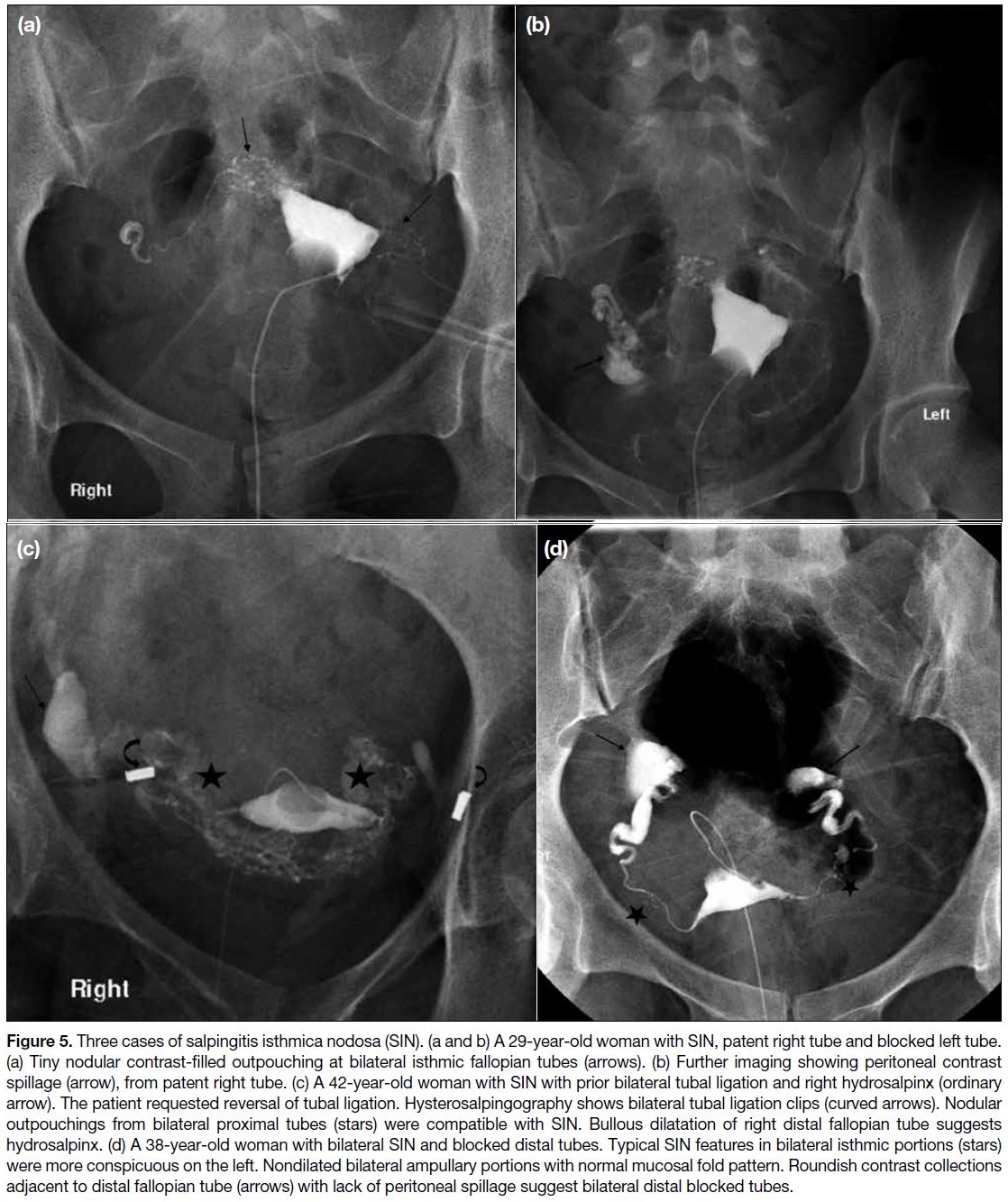
Figure 5. Three cases of salpingitis isthmica nodosa (SIN). (a and b) A 29-year-old woman with SIN, patent right tube and blocked left tube.
(a) Tiny nodular contrast-filled outpouching at bilateral isthmic fallopian tubes (arrows). (b) Further imaging showing peritoneal contrast
spillage (arrow), from patent right tube. (c) A 42-year-old woman with SIN with prior bilateral tubal ligation and right hydrosalpinx (ordinary
arrow). The patient requested reversal of tubal ligation. Hysterosalpingography shows bilateral tubal ligation clips (curved arrows). Nodular
outpouchings from bilateral proximal tubes (stars) were compatible with SIN. Bullous dilatation of right distal fallopian tube suggests
hydrosalpinx. (d) A 38-year-old woman with bilateral SIN and blocked distal tubes. Typical SIN features in bilateral isthmic portions (stars)
were more conspicuous on the left. Nondilated bilateral ampullary portions with normal mucosal fold pattern. Roundish contrast collections
adjacent to distal fallopian tube (arrows) with lack of peritoneal spillage suggest bilateral distal blocked tubes.
Uterine Cavity Contour Abnormalities
Uterine cavity contour abnormalities can be categorised
as uterine cavity outpouching or extrinsic indentation
or irregular outline (that can be associated with prior
surgery, infection or inflammation).
Contour outpouchin
Adenomyosis is the extension of endometrial glandular
tissue into the myometrium and can be diffuse or focal
in extent (Figure 6). When regions of endometrial glands
are connected to the uterine cavity, they can become opacified on HSG study and show as diverticula or
outpouchings in the uterine cavity.[6] Further imaging
with ultrasound or magnetic resonance imaging (MRI)
allows confirmation of the diagnosis and visualisation of
the extent of adenomyotic changes. Differentiation with
irregular cavity outline may be encountered if HSG is
performed during menstruation (Figure 7).
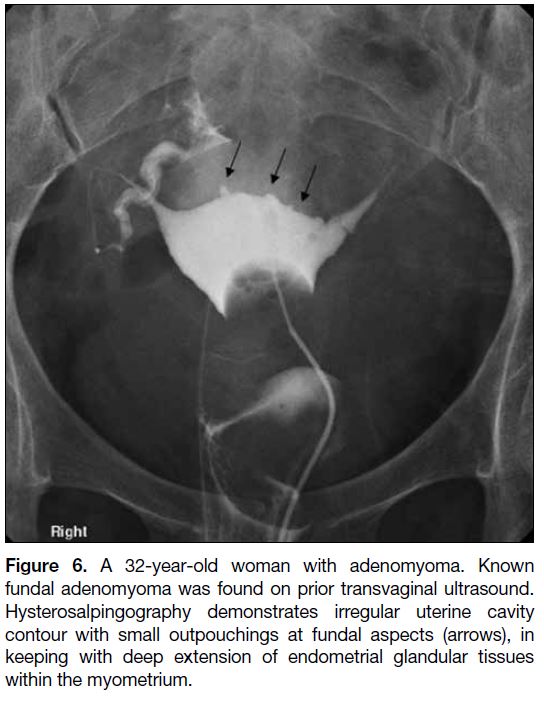
Figure 6. A 32-year-old woman with adenomyoma. Known
fundal adenomyoma was found on prior transvaginal ultrasound.
Hysterosalpingography demonstrates irregular uterine cavity
contour with small outpouchings at fundal aspects (arrows), in
keeping with deep extension of endometrial glandular tissues
within the myometrium.
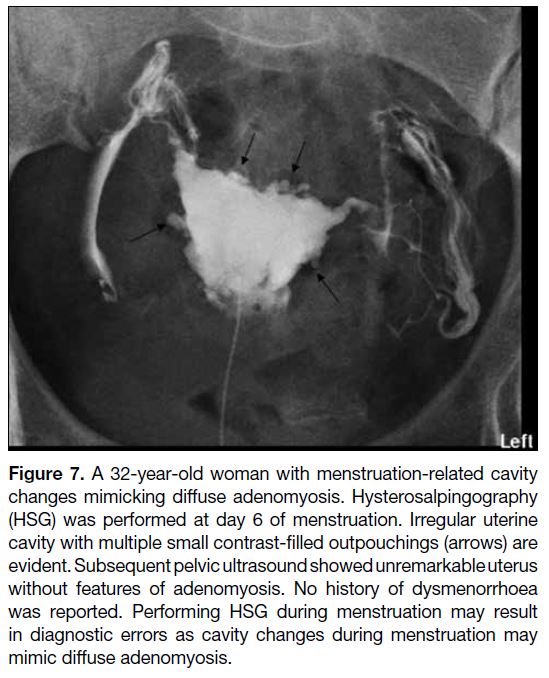
Figure 7. A 32-year-old woman with menstruation-related cavity
changes mimicking diffuse adenomyosis. Hysterosalpingography
(HSG) was performed at day 6 of menstruation. Irregular uterine
cavity with multiple small contrast-filled outpouchings (arrows) are
evident. Subsequent pelvic ultrasound showed unremarkable uterus
without features of adenomyosis. No history of dysmenorrhoea
was reported. Performing HSG during menstruation may result
in diagnostic errors as cavity changes during menstruation may
mimic diffuse adenomyosis.
Uterine contour indentation
Leiomyoma is a benign tumour composed of uterine
smooth muscle (Figure 8). Sizable leiomyoma,
especially at a submucosal location, may be evidenced
by smooth indentation of the uterine cavity with cavity
distortion on HSG.[6] [10] Distorted endometrial cavity
contour may contribute to subfertility owing to changes
in endometrial receptivity, development, and hormone
environment.[10]
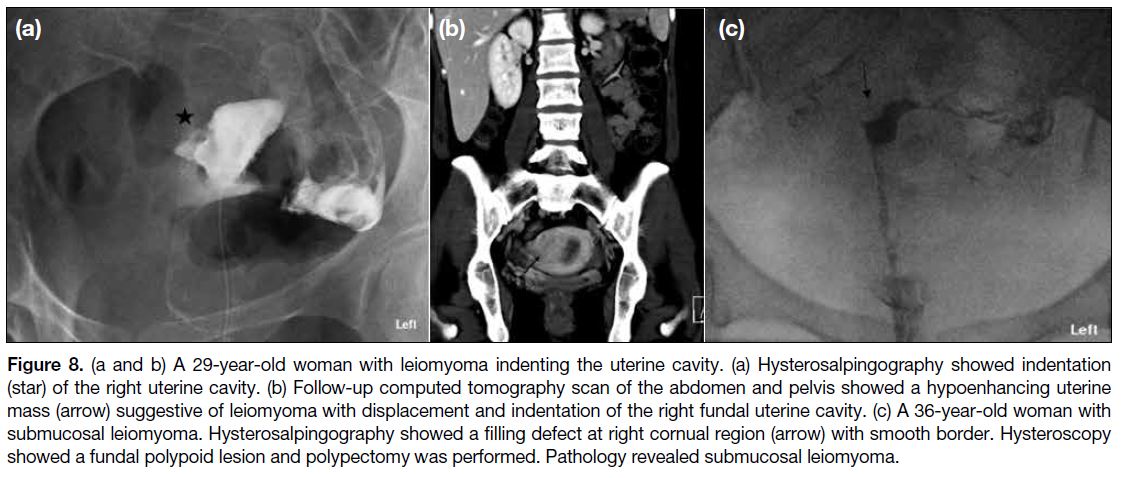
Figure 8. (a and b) A 29-year-old woman with leiomyoma indenting the uterine cavity. (a) Hysterosalpingography showed indentation
(star) of the right uterine cavity. (b) Follow-up computed tomography scan of the abdomen and pelvis showed a hypoenhancing uterine
mass (arrow) suggestive of leiomyoma with displacement and indentation of the right fundal uterine cavity. (c) A 36-year-old woman with
submucosal leiomyoma. Hysterosalpingography showed a filling defect at right cornual region (arrow) with smooth border. Hysteroscopy
showed a fundal polypoid lesion and polypectomy was performed. Pathology revealed submucosal leiomyoma.
Intracavity Filling Defects
Sensitivity of HSG in detecting intrauterine abnormalities
has been reported to be about 58.2%, compared with 82%
for ultrasonography.[10] Intracavity filling defects can be
artefactual and due to air bubbles or intracavity pathology
such as endometrial polyps, intracavity leiomyoma, or
synechiae. Persistent and stationery filling defects are
more suspicious of intracavity pathology. Preprocedural
flushing of the catheter helps minimise the chance of air
bubble contamination.
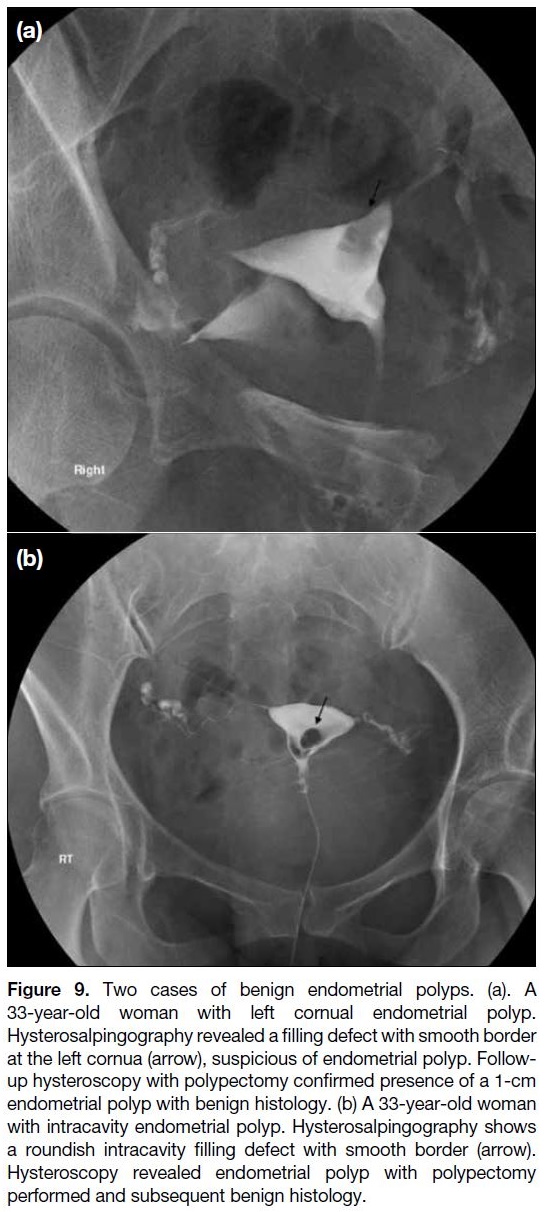
Endometrial polyps
Endometrial polyps are benign focal proliferations
of endometrial tissue, usually seen as smooth oval
to roundish filling defects on HSG (Figure 9). Saline
infusion sonohysterography with ultrasound assessment
after saline instillation into the uterine cavity can help
distinguish the causes of an intracavity filling defect
with superior accuracy for endometrial polyps than
transvaginal ultrasound or HSG.[14] Hysteroscopy is
the gold standard for diagnosis[14] and can be applied
to provide therapeutic treatment with polypectomy or
adhesiolysis.
Figure 9. Two cases of benign endometrial polyps. (a). A
33-year-old woman with left cornual endometrial polyp.
Hysterosalpingography revealed a filling defect with smooth border
at the left cornua (arrow), suspicious of endometrial polyp. Follow-up
hysteroscopy with polypectomy confirmed presence of a 1-cm
endometrial polyp with benign histology. (b) A 33-year-old woman
with intracavity endometrial polyp. Hysterosalpingography shows
a roundish intracavity filling defect with smooth border (arrow).
Hysteroscopy revealed endometrial polyp with polypectomy
performed and subsequent benign histology.
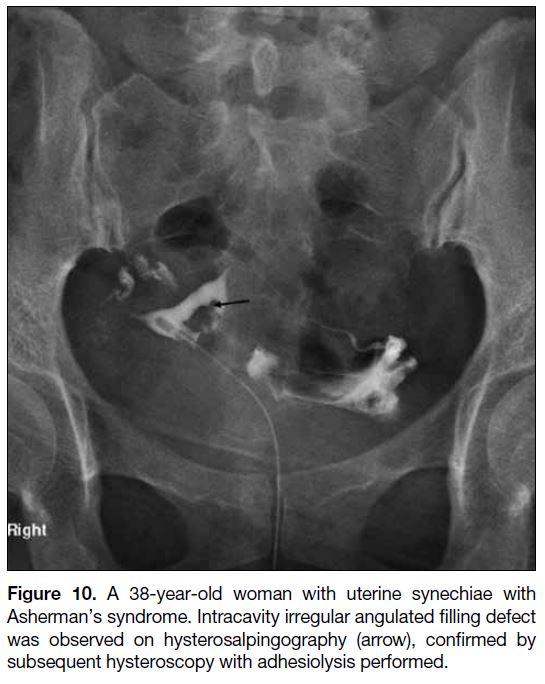
Synechiae
Intrauterine adhesions or synechiae result from insult
to the basal endometrial lining, such as prior surgery
(especially dilatation and curettage) or endometritis
(Figure 10). Asherman’s syndrome is the presence
of intrauterine adhesions with clinical manifestations
of abnormal menstruation (e.g., amenorrhoea/hypomenorrhoea) and subfertility.[6] On HSG, adhesions
are more often linear, irregular or angulated.
Figure 10. A 38-year-old woman with uterine synechiae with
Asherman’s syndrome. Intracavity irregular angulated filling defect
was observed on hysterosalpingography (arrow), confirmed by
subsequent hysteroscopy with adhesiolysis performed.
Variant Anatomy or Congenital Malformation
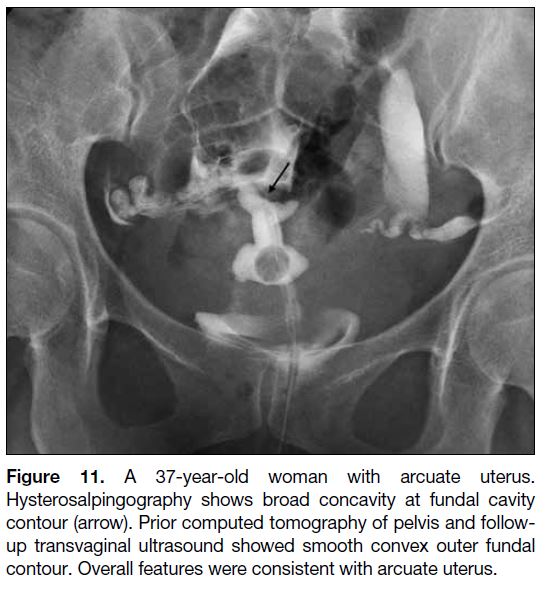
Arcuate uterus
Arcuate uterus is classified as class VI Müllerian duct
abnormality according to the American Fertility Society scheme.[15] Arcuate uterus arises from incomplete septal
resorption at the level of the uterine fundus resulting
in mild focal bulging. On HSG it manifests as broad
mild concavity of the fundal cavity contour (Figure 11). Further imaging with three-dimensional ultrasound or MRI to visualise the normal outer uterine contour is
helpful. It has been regarded as a normal uterine variant
without significant adverse impact on fertility.[15] [16]
Figure 11. A 37-year-old woman with arcuate uterus.
Hysterosalpingography shows broad concavity at fundal cavity
contour (arrow). Prior computed tomography of pelvis and follow-up
transvaginal ultrasound showed smooth convex outer fundal
contour. Overall features were consistent with arcuate uterus.
Congenital uterine anomalies
The wide spectrum and complex pathogenesis of
congenital uterine anomalies are beyond the scope
of this pictorial review. The developmental stages of the uterus include organogenesis of paired Müllerian
ducts, fusion, and septal resorption. Disorders in these
developmental stages result in congenital uterine
anomalies with varying severity and implications for
fertility.[10] HSG offers valuable information regarding
uterine cavity morphology and can suggest the presence
of a congenital uterine anomaly. Further evaluation with
three-dimensional pelvic ultrasound or MRI is warranted for assessment of outer or fundal uterine contour for
accurate diagnosis.
CONCLUSION
HSG is vital in reproductive medicine given its reliability in diagnosing tubal occlusion with relatively low
invasiveness. Systematic interpretation of HSG findings
and understanding of the spectrum of pathologies, including uterine contour changes, intracavity filling
defects, tubal patency, and tuboperitoneal pathology will
facilitate identification of the cause of female subfertility
and expedite management to improve reproductive
outcome.
REFERENCES
1. Dun EC, Nezhat CH. Tubal factor infertility: diagnosis and
management in the era of assisted reproductive technology. Obstet
Gynecol Clin North Am. 2012;39:551-66. Crossref
2. Omidiji OA, Toyobo OO, Adegbola O, Fatade A, Olowoyeye OA. Hysterosalpingographic findings in infertility—what has changed over the years? Afr Health Sci. 2019;19:1866-74. Crossref
3. Infertility workup for the women’s health specialist: ACOG Committee Opinion, Number 781. Obstet Gynaecol. 2019;133:e377-84. Crossref
4. Fertility problems: assessment and treatment. London: National
Institute for Health and Care Excellence (NICE); Sep 2017.
5. Practice Committee of the American Society for Reproductive
Medicine. Role of tubal surgery in the era of assisted reproductive
technology: a committee opinion. Fertil Steril. 2021;115:1143-50. Crossref
6. Simpson WL Jr, Beitia LG, Mester J. Hysterosalpingography: a reemerging study. Radiographics. 2006;26:419-31. Crossref
7. American College of Radiology. ACR Practice Parameter for the Performance of Hysterosalpingography. Available from: https://www.acr.org/-/media/ACR/Files/Practice-Parameters/HSG.pdf.
Accessed 1 Apr 2021.
8. Watson N. Reproductive system. In: Watson N, Jones H, editors.
Chapman & Nakielny’s Guide to Radiological Procedures. 6th ed.
Edinburgh, UK: Saunders; 2014. p 159-62.
9. Foroozanfard F, Sadat Z. Diagnostic value of hysterosalpingography
and laparoscopy for tubal patency in infertile women. Nurs Midwifery Stud. 2013;2:188-92. Crossref
10. Vickramarajah S, Stewart V, van Ree K, Hemingway AP, Crofton ME,
Bharwani N. Subfertility: what the radiologist needs to know.
Radiographics. 2017;37:1587-602. Crossref
11. Revzin MV, Mathur M, Dave HB, Macer ML, Spektor M. Pelvic
inflammatory disease: multimodality imaging approach with
clinical-pathologic correlation. Radiographics. 2016;36:1579-96. Crossref
12. Jenkins CS, Williams SR, Schmidt GE. Salpingitis isthmica nodosa:
a review of the literature, discussion of clinical significance, and
consideration of patient management. Fertil Steril. 1993;60:599-607. Crossref
13. Majmudar B, Henderson PH 3rd, Semple E. Salpingitis isthmica nodosa: a high-risk factor for tubal pregnancy. Obstet Gynecol. 1983;62:73-8.
14. Soares SR, Barbosa dos Reis MM, Camargos AF. Diagnostic
accuracy of sonohysterography, transvaginal sonography, and
hysterosalpingography in patients with uterine cavity diseases.
Fertil Steril. 2000;73:406-11. Crossref
15. The American Fertility Society classifications of adnexal adhesions,
distal tubal occlusion, tubal occlusion secondary to tubal ligation,
tubal pregnancies, Müllerian anomalies and intrauterine adhesions.
Fertil Steril. 1988;49:944-55. Crossref
16. Ubeda B, Paraira M, Alert E, Abuin RA. Hysterosalpingography:
spectrum of normal variants and nonpathologic findings. AJR Am
J Roentgenol. 2001;177:131-5. Crossref